Abstract
We investigated the curative effect of Pheretima aspergillum (earthworm, PA) on rats with middle cerebral artery occlusion (MCAo). The MCAo-induced cerebral infarction was established and its underlying mechanisms by counting the infarction areas and evaluating the rats' neurological status. Immunostaining was used to test the expression of NeuN, and glial fibrillary acidic (GFAP), S100B, and brain-derived neurotrophic factor (BDNF) proteins. Our results showed that oral administration of PA for two weeks to rats with MCAo successfully reduced cerebral infarction areas in the cortex and striatum, and also reduced scores of neurological deficit. The PA-treated MCAo rats showed greatly decreased neuronal death, glial proliferation, and S100B proteins in the penumbra area of the cortex and in the ischemic core area of the cortex, but BDNF did not changed. These results demonstrated novel and detailed cellular mechanisms underlying the neuroprotective effects of PA in MCAo rats
Keywords: Cerebral infarction, Middle cerebral occlusion, Neuroprotective effect, Pheretima aspergillum
Introduction
“Stroke” refers to a sudden loss of neurological function induced by an acute interruption in the blood supply to the brain. Either the entire brain or specific local tissues may be affected. The economic cost of stroke ranks high on the list of health insurance spending in both the United States and Taiwan. Stroke patients encounter a variety of functional deficits, such as unconsciousness or the impairment of motor, sensory, cognitive, and language abilities. The etiological classification of stroke is subgrouped according to the occlusive and hemorrhagic types. In clinical practice, patients with ischemic stroke are usually treated with tissue plasminogen activity (tPA) to reduce thrombosis in brain arteries. This treatment is highly effective in restoring consciousness, but may induce serious side effects (Krakovsky et al., 2011). Intracerebral hemorrhage is one of the major symptoms associated with morbidity and mortality in stroke patients (Patil et al., 2012).
Glial fibrillary acidic protein (GFAP) is a marker of astrocytes, which are potentiated by epilepsy and stroke processes (Foerch et al., 2006; Lin and Hsieh, 2011). Glial cells and astrocytes may be implicated in pain sensation, stroke, and epilepsy processes, and astrocytes have been found to release neurotransmitters to modulate neuron excitability (Pasti et al., 1997). The S100B proteins are known to increase the concentration of intracellular free calcium (Barger and Van Eldik, 1992); S100B proteins have a low molecular weight and possess calcium-binding properties (Donato, 1999). These proteins are distributed in many tissues and affect protein phosphorylation, cytoskeletal function, and intracellular calcium homeostasis (Donato, 1999). S100B proteins are highly expressed in the brain and primary sensory neurons, including the trigeminal and dorsal root ganglia (Rickmann and Wolff, 1995; Ichikawa et al., 1997). S100B has several regulatory effects on cell proliferation, division, and metabolism, and may be upregulated in kainic acid (KA)-induced epilepsy (Lin and Hsieh, 2011; Liu et al., 2012). S100B protein levels may also increase during stroke-induced ischemia, and may therefore serve as a marker of neuronal injury (Hu et al., 2010).
Nerve growth factor (NGF) was the first nervous system growth factor to be identified in the 1950s; since then, more than 50 similar growth factors that influence neural functioning have been discovered. NGF affects neuronal survival, growth, and maturation, as well as neurite outgrowth and branches (Levi-Montalcini and Hamburger, 1951; Hyman et al., 1991). Other nervous system growth factors that have been suggested as potential neuroprotective drugs for neurological and psychiatric disorders include brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF), and neurturin. BDNF is used to treat many neurological disorders with associated dementia, including Parkinson's disease and Alzheimer's disease (Tuszynski et al., 2005; Nagahara and Tuszynski, 2011). One study found that BDNF bound with tropomyosin-related kinase receptor type B (TRKB) and further induced mitogen-activated protein kinases (MEKs), extracellular signal-regulated kinases 1/2 (ERK1/2), or alternatively PI3K-AKT pathways (Kordower et al., 1999).
Traditional Chinese Medicine (TCM) has been used in China for thousands of years. One of the medicines administered by TCM doctors is Pheretima Aspergillum (PA), a cold and salty material derived from the earthworm. The body of the PA is filled with fluid containing potent biological agents such as growth factor, adhesion, and hemoglutination molecules. Wei et al reported that the extract of PA promoted the regeneration of injured peripheral nerves (Wei et al., 2009). In clinical practice, PA has long been recognized for its curative effects in the treatment of epilepsy, and the therapeutic effects of PA in stroke and epilepsy patients have been well documented, as have its anti-coagulative properties. However, limited data exist on the detailed mechanisms of PA. Recent research demonstrated that PA induces anti-coagulative activity (Hrzenjak et al., 1998) and anti-oxidative effects (Grdisa et al., 2001). In addition, PA can enhance Schwann cell migration and proliferation as a nerve regeneration medication (Chang et al., 2011a).
We investigated the curative effects and cellular mechanisms of PA when used to treat cerebral infarction induced by middle cerebral artery occlusion (MCAo). We tested whether PA could protect against MCAo-induced cerebral infarction and its associated cortex neuronal death, using immunohistochemistry to verify the role of PA in MCAo-induced cerebral infarction. The expression of GFAP, S100B protein, and BDNF were compared between control, MCAo, and PA-treated groups. Overall, oral administration of PA attenuated the infarction areas by attenuating GFAP and S100B protein levels.
Material and methods
Animals
Male Sprague-Dawley (SD) rats, each weighing 300 g to 350 g, were used in this study. Use of these animals was approved by the Institute of Animal Care and Use Committee of China Medical University, and our research followed the Guide for the Use of Laboratory Animals (National Academy Press). Our research comprised 2 experiments, namely, Experiments A and B, as described in Section 2.3 below. Experiment A included 30 rats divided into 5 groups, each containing 6 rats. Eighteen rats were studied in Experiment B, divided into 3 groups, each containing 6 rats. During the experiment, the rats were denied food overnight but had free access to water.
Preparation of PA
The PA [Megascolecidae, Pheretima aspergillum (E. perrier)] used in this study was produced in the Guangxi province of China. The origin of the PA was authenticated, and the material was examined by Sun Ten Pharmaceutical Co. Ltd. in Taiwan for microorganisms, heavy metals, and pesticide, according to the accepted standards of good manufacturing practice in Taiwan. The extract of PA was produced by boiling 1 kg of dried PA with 14 L of distilled water for 1 h. The mixture was then concentrated into 1.33 kg/kg. The concentration of the PA solution was identified by sodium dodecyl sulfate polyacryamide gel electrophoresis using lumbrokinase (BOLUCKE, Canada) as a standard. These assessments were performed by Sun Ten Pharmaceutical Co. Ltd. (Taiwan). The final solution of PA was prepared as 1.0 g/ml.
Establishment of MCAo model
The MCAo animal model was established in SD rats through an intraluminal suture method, as previously described (Cheng et al., 2008; Lin and Hsieh, 2010). Rats were anesthetized with chloral hydrate (400 mg/kg, i.p.), and the right common carotid artery (CCA) and the internal carotid artery (ICA) were exposed through a neck midline incision. This was followed by ligation of the pterygopalatine artery close to its branch. A 3-0 nylon filament suture, blunted at the tip by a flame and coated with poly-L-lysine (Sigma, USA), was inserted into the right external carotid artery through the CCA and up to the ICA, a distance of 20 mm to 25 mm, to block the origin of the right middle cerebral artery (MCA). The suture was removed slowly 15 min after the occlusion, to re-establish blood flow.
Our study consisted of two experiments. Experiment A focused on the curative effects of PA on cerebral infarction and neurological status, and experiment B used immunohistochemistry staining to examine the precise curative mechanism of PA.
Grouping of rats, and evaluation of neurological status
At the start of the research, the rats were randomly divided into 5 groups as follows: 1) the sham group, which received neither MCAo nor PA treatment; 2) the control group, which underwent MCAo without treatment by PA; 3) the PA 0.25 group, which underwent MCAo and received daily oral treatment with 0.25 g PA per kg of body weight; 4) the PA 0.5 group, which underwent MCAo and received 0.5 g PA/kg daily; and 5) the PA 1.0 group, which underwent MCAo and received 1.0 g PA/kg daily. Groups when the scores ≧ 7 except sham groups (without MCAo and without PA). The rats' neurological status was evaluated at three separate time points after MCAo manipulation, namely 24 h, day 8, and day 15. The PA was orally administered immediately after the first evaluation and treatment was continued for 2 weeks, 5 days per week. At the end of this period, on day 15, the rats' neurological statuses were evaluated and the animals were then sacrificed and their brains were removed. Neurological status was evaluated in each rat by assessing the motor, sensory, balancing, and reflex functions. The maximum possible neurological score was 18, as described in our previous studies (Lin and Hsieh, 2010).
Measurement of infarction area
Each rat brain was sectioned into 2 mm slices according to a brain matrix, immediately after removal. The samples were placed in a 2% solution of 2,3,5-triphenyl tetrazolium chloride (TTC, Merk, Germany) for 15 min at 37 °C, as described in our previous report (Lin and Hsieh, 2011). The results produced a white area representing infarction, and red-purple, indicating areas not affected by infarction. We also calculated a brain tissue ratio to represent the degree of shrinkage, using an Image analysis system (Image J 1.42a, National Institute of Health, USA). This ratio was calculated as total area (infarction plus non-infarction areas) of the right hemisphere divided by total area of the left hemisphere, and was presented as a percentage (%).
Immunohistochemistry staining
The rats were randomly divided into the control, PA 0.5 groups when the scores of neurological deficit ≧ 7 except sham group at 24 h after MCAo manipulation. On day 15 after MCAo manipulation, the animals were deeply anesthetized with chloral hydrate (400 mg/kg, i.p.), and perfused with normal saline through the cardiac vascular system, followed by infusion with 4% paraformaldehyde (Merck, Frankfurt, Germany) in 0.1M phosphate buffered saline (PBS, pH = 7.4). The brains were removed and placed in the same fixative and stored overnight at 4 °C. After being washed briefly with PBS, the brains were transferred to 30% sucrose in 0.01 M PBS for cryoprotection. Thereafter, coronal sections containing the hippocampal area were cut into 20 µm slices using a cryosectioning technique. The sections were pre-incubated (for 2 h at 25 °C) with a mixture of 10% horse serum and 0.3% Triton X-100 in PBS, to avoid non-specific binding. Sections were then incubated overnight at 4 °C with a mixture of rat monoclonal antibody against GFAP (1:200; Oncogene, USA), NeuN (1:1000; Chemicon, USA), and BDNF (1:1000; Millipore, Temecula, USA) in a PBS with 0.1% horse serum and 0.1% Triton X-100. Sections were subsequently incubated (for 2 h at 25 °C) with biotinylated-conjugated secondary antibody (1:200 diluted; Vector, Burlingame, CA, USA), and were then further incubated with avidin-horseradish peroxidase complex (ABC-Elite, Vector). The sections were washed three times with PBS between each incubation step, for 10 min per wash. Finally, the sections were visualized using 3,3'-diaminobenzidine as the chromogen.
Statistical analysis
All statistic data are presented as the mean ± standard error. Statistical significance between sham, control, and PA group was tested using the ANOVA test, followed by a post hoc Tukey's test (p < 0.05 was considered statistically significant).
Results
Curative effects of PA on MCAo-induced cerebral infarction
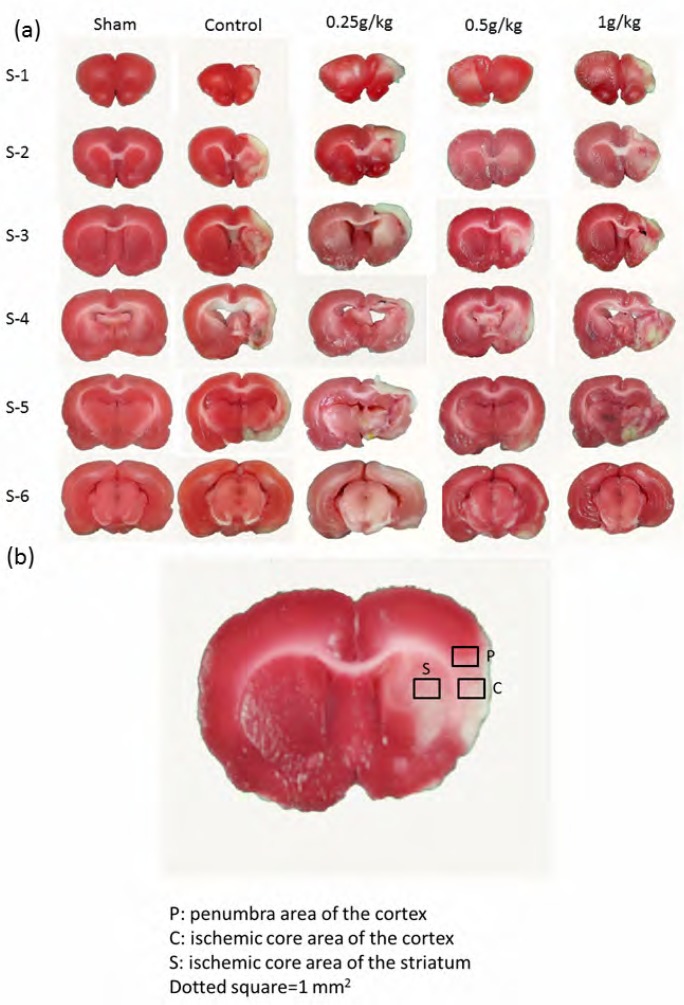
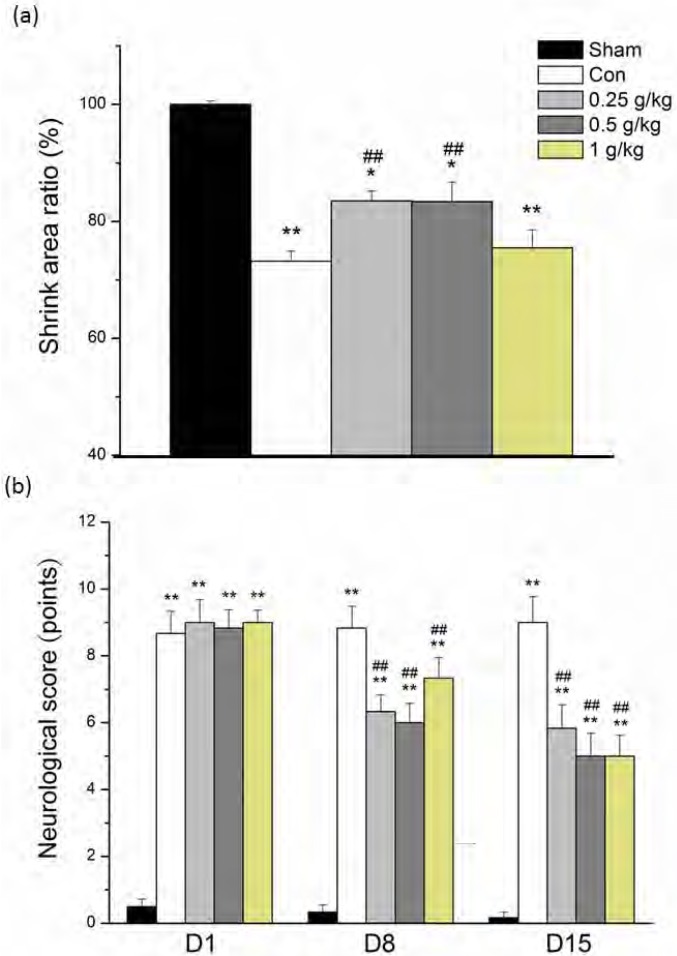
Our results revealed that the curative effects of PA on MCAo-induced stroke in rats were the result of reduced infarction areas, with an associated decrease in the scores of neurological deficit. Stroke is a major clinical disease resulting from inadequate blood supply to the brain. We induced an in vivo animal model of stroke in SD rats, as previously described (Lin and Hsieh, 2010). Substantial neurological malfunctioning which resembled that of stroke was successfully induced in the rats. Each experimental group contained 6 animals, namely, S-1 to S-6, with brain sections derived as shown inFigure 1a. In the sham group, no significant infarction volume was found across whole brain tissue. In the groups receiving MCAo manipulation, the rat brains were severely damaged, with severe ischemia-induced infarction areas.Figure 1b illustrates the quantification of the infarction areas in the penumbra and ischemic core areas of the cortex, and the ischemic core area of the striatum. Oral administration of PA significantly reduced the infarction areas, with lower PA concentrations of 0.25 g/kg to 0.5 g/kg being more effective than 1 g/kg PA (83.5±1.7%, 83.4±3.3%, and 75.5±3.1% compared with the sham group, for doses of 0.25 g/kg, 0.5 g/kg, and 1 g/kg PA, respectively), whereas control group was 73.3±4.1%. All data were statistically analyzed and the results are presented inFigure 2a. Oral PA significantly reduced MCAo-induced neurological deficits, as shown inFigure 2b.
Figure 1.
MCAo-induced cerebral infarction areas in whole brains, illustrating the quantity of proteins in cortex and striatum. (a) MCAo-induced ischemia areas within six individual rat brain slices in the sham, control, and PA treatment groups. (b) Illustration of investigation of selected proteins in penumbra areas of the cortex, ischemia core areas of the cortex, and ischemia core of the striatum. Dotted areas= 1.0 mm2.
Figure 2.
The cerebral infarction areas and scores for neurological deficit in the sham, control, and PA treatment groups (0.25 g/kg, 0.5 g/kg, and 1g/kg). (a) The ratio of shrunken area compared with the sham group (Sham). (b) MCAo-induced neurological deficit scores for each group were recorded on days 1 (D1), 8 (D8), and 15 (D15) after MCAo. * P < .05 compared with the sham group. ** P < .01 compared with sham group. ## P < .01 compared with control group (Con).
Oral administration of PA modulated neuron survival of GFAP and S100B
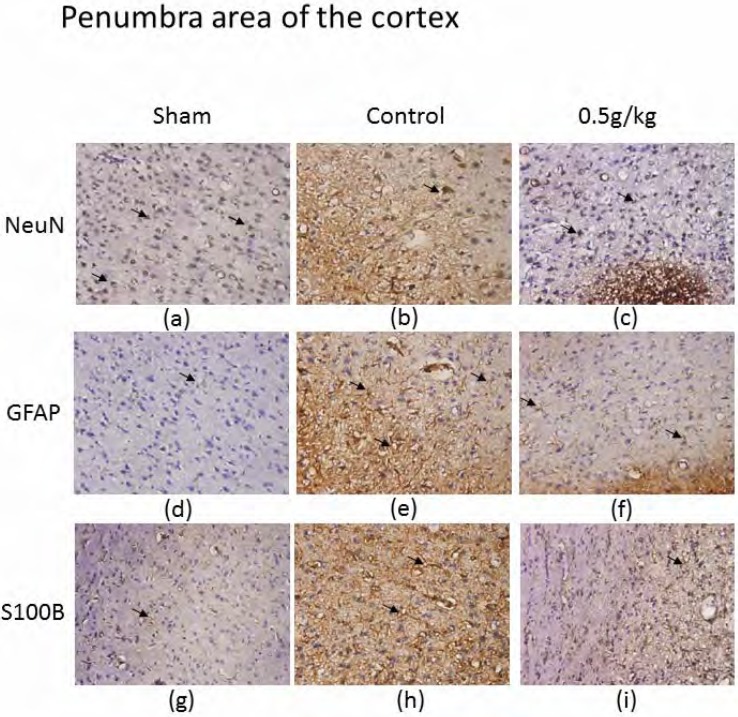
Our results indicated that MCAo-induced cerebral infarction was present in the penumbra area of the cortex. Oral administration of PA reversed MCAo-induced ischemia, and modulated neuron survival of GFAP and S100B in the penumbra area of the cortex. We attempted to identify MCAo-mediated stroke and the curative effect of PA and its underlying mechanisms. We found that neurons in the penumbra area of the cortex survived in a healthy state in rats receiving a sham operation (637.2±11.4 neurons per field, n=6) (Figure 3a). Notably, in the MCAo group, NeuN-positive immunostaining was decreased, as was the infarction area (155.3±5.9 neurons/field, n=6) (Figure 3b). Our results showed that NeuN immunostaining was significantly recovered with the administration of 0.5 g/kg PA (321.2±40.1 neurons/field, n=6) (Figure 3c).
Figure 3.
Immunostaining of hematoxylin and eosin (HE), NeuN, GFAP, and S100B in penumbra area of the cortex of sham, control, and 0.5 g/kg PA-treated groups. HE (blue) and NeuN (brown) positive staining in penumbra area of the cortex in sham (a), control (b), and 0.5 g/kg PA (c) groups. HE (blue) and GFAP (brown) positive stained cells in sham (d), control (e), and 0.5 g/kg PA (f) groups. HE (blue) and S100B (brown) positive stained cells in sham (g), control (h), and 0.5 g/kg PA (i) groups. The arrows indicate positive staining. The pictures were captured at 100 × magnification.
GFAP is a marker of astrocytes, which increase during epileptogenesis and stroke. We investigated the role of GFAP in PA treatment. Importantly, MACo-induced ischemia dramatically increased the expression of GFAP in the penumbra area of the cortex (from 5.3±1.4 to 163.5±21.7 astrocyte/field, n=6) (Figures 3d and 3e). The increase in GFAP was ameliorated after 0.5 g/kg PA administration for 2 weeks (35.0±5.7 astrocyte/field, n=6) (Figure 3f). We also attempted to verify the role of S100B in PA-treated rats with MCAo, because S100B is a marker of brain injury, including acute ischemic stroke. Our results showed that S100B was highly augmented under MCAo-induced stroke (from 9.3±3.0 to 152.7±22.4 cells/field, n=6) (Figures 3g and 3h). This phenomenon was reversed by the oral administration of PA for 2 weeks, implying a pathway of PA mechanism (108.2±21.2 cells/field, n=6) (Figure 3i). We next tested the possible involvement of BDNF in MCAo-induced stroke.
Oral administration of PA modulated BDNF proteins in the penumbra and ischemic core of the cortex.
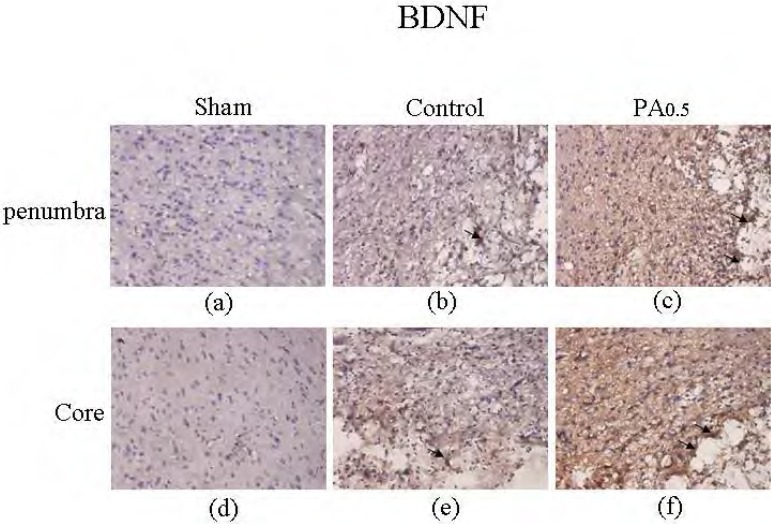
Our results revealed that oral administration of PA modulated BDNF proteins in the penumbra area and the ischemic core area of the cortex. The BDNF in the penumbra was slightly increased in the MCAo rats compared with the sham group (from 1.3±0.8 to 33.7±8.8 cells/field, n=6) (Figures 4a and 4b). The expression of BDNF was not significantly upregulated after PA administration (33.5±12.1 cells/field, n=6) (Figure 4c). The appearance of BDNF in the ischemic core of the cortex was slightly increased in the MCAo group (from 0.8±0.5 to 40.0±7.2 cells/field, n=6) (Figures 4d and 4e), but was not altered by 2 weeks of oral PA treatment (32.2±11.1 cells/field, n=6) (Figure 4f).
Figure 4.
Immunostaining of hematoxylin and rosin (HE) and BDNF in the penumbra area and ischemic core of the cortex of sham, control, and 0.5 g/kg PA-treated groups. HE (blue) and BDNF (brown) positive staining in penumbra area of the cortex in sham (a), control (b), and 0.5 g/kg PA (c) groups. HE (blue) and BDNF (brown) positive staining in ischemic core of the cortex in sham (d), control (e), and 0.5 g/kg PA (f) groups. The arrows indicate positive staining. The pictures were captured at 100 × magnification.
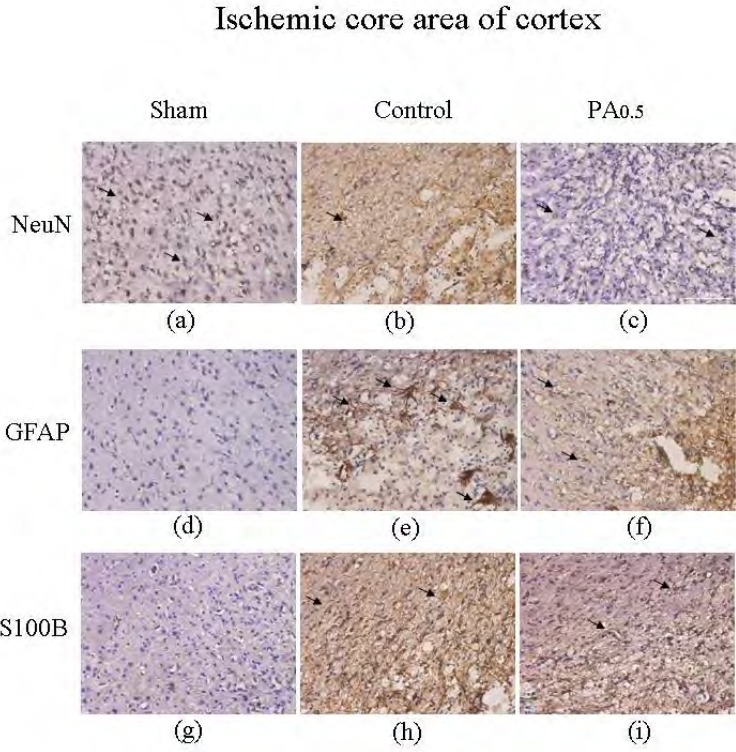
Oral PA decreased GFAP and S100B in the ischemic core area of the cortex
We then tested the effect of PA on MCAo-mediated stroke and its related signaling mechanisms. Neuron survival in the cortex was downregulated after MCAo treatment, indicating damage to cortex neurons (from 630.8±9.2 to 61.7±14.3 neurons/field, n=6) (Figures 5a and 5b). Similarly, curative effects were visible in the cortex after 2 weeks of PA administration (208.7±51.2 neurons/field, n=6) (Figure 5c). The GFAP expression patterns in the cortex were similar to that in the penumbra area of the cortex, with the MCAo group showing increased expression compared with the sham group (from 7.8±2.3 to 79.2±14.6 astrocyte/field, n=6) (Figure 5d and 5e). The phenomenon was reversed with 2 weeks of PA treatment, as shown in Figure 5f (54.7±5.6 cells/field, n=6).Figure 5g shows the normal distribution of S100B expression, which was upregulated in MCAo-induced ischemia as illustrated inFigure 5h (from 10.7±3.1 to 111.2±17.5 cells/field, n=6).Figure 5i shows the role of PA in reversing MCAo-induced ischemia (75.7±10.3 cells/field).
Figure 5.
Immunostaining of hematoxylin and eosin (HE), NeuN, GFAP, and S100B in the ischemic core of the cortex in sham, control, and 0.5 g/kg PA-treated groups. HE (blue) and NeuN (brown) positive stained cells in ischemic core area of the cortex in sham (a), control (b), and 0.5 g/kg PA (c) groups. HE (blue) and GFAP (brown) positive stained cells in sham (d), control (e), and 0.5 g/kg PA (f) groups. HE (blue) and S100B (brown) positive stained cells in sham (g), control (h), and 0.5 g/kg PA (i) groups. The arrows indicate positive staining of cells. The pictures were captured at 100 × magnification.
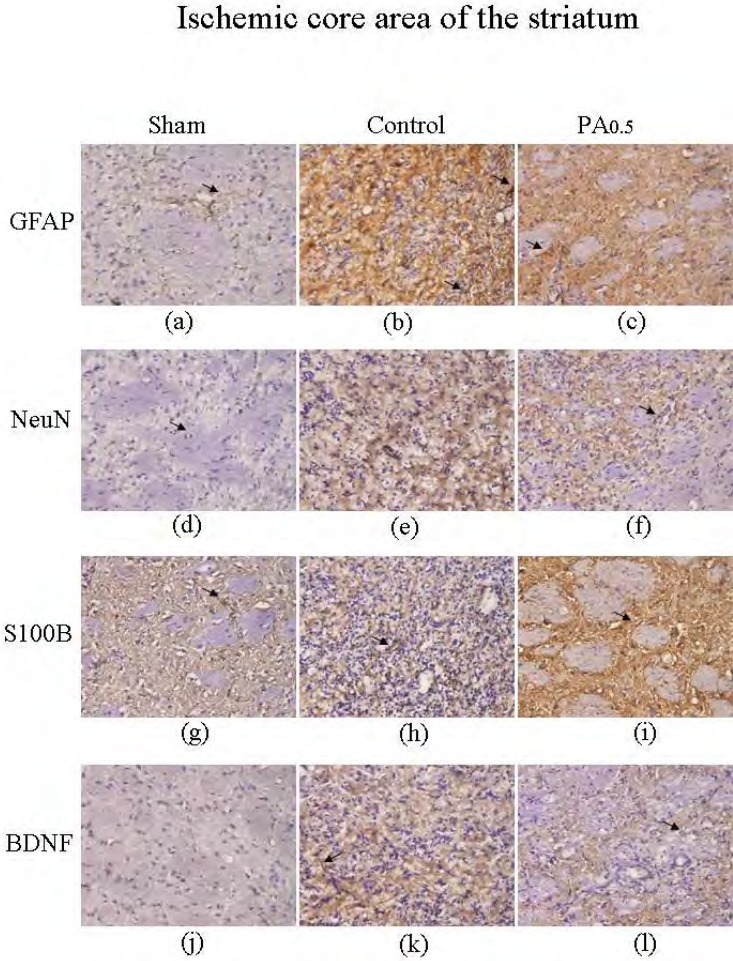
The effect of PA treatment on neuron survival, GFAP and S100B in the ischemic core area of the striatum.
We investigated whether oral PA could increase neuron survival and BDNF expression while simultaneously decreasing GFAP and S100B overexpression. Our results demonstrated that the number of striatum neurons was from 7.2±0.9 to 11.8±2.2 (cells/field, n=6) after MCAo (Figures 6a and 6b). This pattern was not altered by oral PA treatment (13.8±3.6 cells/field, n=6) (Figure 6c). Similar results were obtained for GFAP expression, which increased as a result of MCAo (from 5.3±1.1 to 52.0±25.6 cells/field, n=6) (Figures 6d and 6e), but the basic pattern did not appear to be affected by PA treatment (73.3±23.0 cells/field, n=6) (Figure 6f). The expression phenomena of S100B were coordinative to GFAP as increasing in MCAo group (from 7.3±1.4 to 49.3±14.2 cells/field, n=6) (Figures 6g and 6h), with no significant changes being found after PA administration (46.2±11.3 cells/field, n=6) (Figure 6i). Furthermore, the density of BDNF was higher in the MCAo group (from 0.5±0.5 to 7.8±1.1 cells/field, n=6) in the striatum areas (Figures 6j and 6k), but the results were similar for the control and PA groups (10.8±2.6 cells/field, n=6) (Figure 6).
Figure 6.
Immunostaining of hematoxylin and eosin (HE), NeuN, GFAP, S100B, and BDNF in ischemic core of the striatum in sham, control, and 0.5 g/kg PA-treated groups. HE (blue) and NeuN (brown) positive stained cells in ischemic core of the striatum in sham (a), control (b), and 0.5 g/kg PA (c) groups. HE (blue) and GFAP (brown) positive stained cells in sham (d), control (e), and 0.5 g/kg PA (f) groups. HE (blue) and S100B (brown) positive stained cells in sham (g), control (h), and 0.5 g/kg PA (i) groups. HE (blue) and BDNF (brown) positive staining cells in sham (j), control (k), and 0.5 g/kg PA (l) groups. The arrows indicate positive staining. The pictures were captured at 100 × magnification.
Discussion
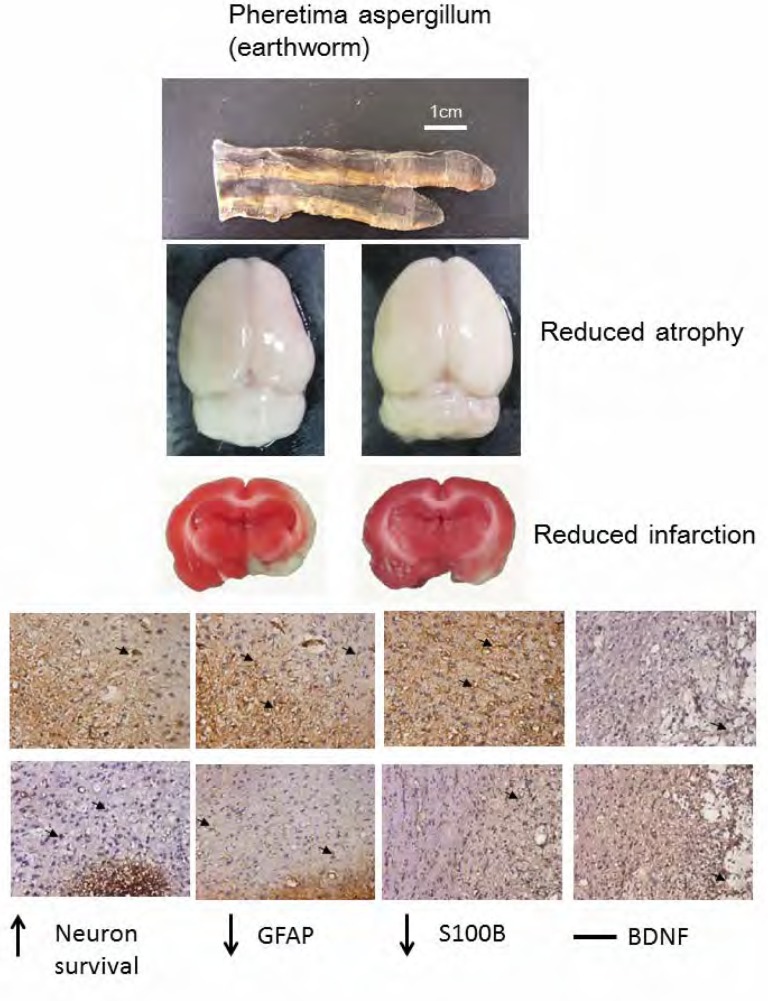
We first established a MCAo animal model with ischemia mainly in the penumbra and ischemic core areas of the cortex, and the ischemic core area of the striatum. We found that the effect of PA treatment on MCAo-induced ischemia was dose-dependent, with the most effective dose being 0.25 to 0.5 g/kg rather than 1g/kg. This finding provides evidence for the curative effect of Chinese herbal PA in reducing infarction volume. We further examined the detailed mechanisms underlying the effect of PA. We provide novel evidence that neuronal survival was downregulated by MCAo manipulation, but was reversed to the normal state after oral PA administration. GFAP and S100B were overexpressed after MCAo but were ameliorated by PA administration (summarized in Figure 7).
Figure 7.
Interpretive Diagram. Pheretima aspergillum (PA) reduced brain atrophy and cerebral infarction in middle cerebral artery occlusion rats. The PA increased neuron survival, and reduced glial fibrillary acid protein (GFAP) and S100B protein, but PA did not change brain-derived neurotrophic factor (BDNF).
Stroke is one of the major syndromes observed in clinical emergencies, and carries a high cost for both patients and medical staff. Many physical and physiological markers are involved in the diagnosis of stroke, including GFAP and S100B levels. S100B is a calcium-binding protein mainly found in glial cells. The overexpression of S100B has been shown to have neurotoxicity with calcium toxicity, and an increase in S100B is highly associated with hematoma volume (Delgado et al., 2006). Recent studies have suggested that GFAP and S100B could be biomarkers for acute stroke (Foerch et al., 2012). Our previous study demonstrated an increment in GFAP and S100B in hippocampal CA1 and CA3 due to kainic acid-induced epilepsy (Lin and Hsieh, 2011; Liu et al., 2012). GFAP is mainly a brain-specific intermediate filament protein involved in glial cell morphology and migration. GFAP is rarely secreted by cells under normal physiological conditions, and is not detectable in the plasma of healthy humans (Missler et al., 1999). However, large amounts of GFAP are released in acute ischemia stroke patients (Wunderlich et al., 2004). Datta et al reported that GFAP was increased at 24 h after ischemic reperfusion injury, indicating the negative effect of GFAP (Datta et al., 2011). The current study found that oral administration of 0.5 g/kg PA successfully reduced the ischemic areas and neurological deficit in MCAo rats. This curative effect was accompanied by attenuating the overexpression of GFAP and S100B proteins. These novel mechanisms of PA have potential for both experimental and clinical applications. PA can increase growth associated protein 43 (GFP 43) and synapsin I in PC12 cell, and also can increase success percentage in rat with a 10 mm sciatic nerve defect. Therefore, PA can enhance the regeneration of peripheral nerve (Chen et al., 2010). The PA mediates through extracellular signal regular protein (ERK1/2) and p38 of MAPK signaling pathway to induce the expression of matrix degrading proteolytic enzymes, and metalloproteinase (MMP) 2 and MMP 9 to promote the migration and regeneration of Schwann cells (Chang et al., 2011b). The main mechanism of PA enhancing Schwann regeneration is depending on P13K protein (Change et al., 2011c).
Recently, BDNF has been suggested as having therapeutic potential for several diseases, including Alzheimer's disease and Parkinson's disease. Several studies have demonstrated that BDNF ameliorated cell death and potentiated neuronal functioning. Hyman et al reported that BDNF supported the survival of dopaminergic neurons in the substantia nigra (Hyman et al., 1991). In patients with Parkinson's disease, BDNF expression was downregulated in the substantia nigra, indicating a key role of BDNF in the disease (Hyman et al., 1991). BDNF may also promote neuron survival and neurite outgrowth (Mogi et al., 1999). The current study investigated the curative effect of oral PA in MCAo-induced ischemic rats. Our results indicated that the therapeutic function was not due to augmenting the density of BDNF in the penumbra area of the cortex, the ischemic core area of the cortex, and the ischemic core area of the striatum. These novel results are dramatic and interesting, and illustrate the important therapeutic effects of PA as administered by traditional Chinese doctors.
Conclusions
We found that treatment with PA significantly improved rats' neurological and functional recovery after MCAo. Treatment with PA was also associated with increased density of neurons, and reduced GFAP and S100B astrocyte activation.
Acknowledgments
This study was supported by a grant from China Medical University (CMU99-EW-02). It was also supported in part by the Taiwanese Department of Health, Clinical Trials and Research Center of Excellence (DOH101-TD-B-111-004).
Glossary
- UR
Uncaria Rhynchophylla
- PA
Pheretima aspergillum
- MCAo
middle cerebral artery occlusion
- GFAP
glial fibrillary acidic protein
- BDNF
brain-derived neurotrophic factor
- tPA
tissue plasminogen activity
- KA
kainic acid
- NGF
Nerve growth factor
- TRKB
tropomyosin-related kinase receptor type B
- MEKs
mitogen-activated protein kinases
- ERK1/2
extracellular signal-regulated kinases 1/2
- TCM
Traditional Chinese Medicine
- SD
Sprague-Dawley
References
- 1.Al Sabti H. Therapeutic angiogenesis in cardiovascular disease. J Cardiothorac Surg. 2007;2:49. doi: 10.1186/1749-8090-2-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barger SW, Van Eldik LJ. S100 beta stimulates calcium fluxes in glial and neuronal cells. J Biol Chem. 1992;267:9689–9694. [PubMed] [Google Scholar]
- 3.Chang YM, Chi WY, Lai TY, Chen YS, Tsai FJ, Tsai CH, Kuo WW, Cheng YC, Lin CC, Huang CY. Dilong: role in peripheral nerve regeneration. Evidence-Based Complementary and Alternative Medicine. 2011a;2011 doi: 10.1093/ecam/nep079. Article ID 380809, 9 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang YM, Shih YT, Chen YS, Liu CL, Fang WK, Tsai CH, Kuo WW, Lai TY, Huang CY. Schwann cell migration induced by earthworm extract via activation of PAs and MMP2/9 mediated through ERK1/2 and p38. Evidence-Based Complementary and Alternative Medicine. 2011b;2011 doi: 10.1093/ecam/nep131. Article ID 395458, 12 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang YM, Kuo WH, Lai TY, Shih YT, Tsai FJ, Tsai CH, Shu WT, Chen YY, Chen YS, Kuo WW, Huang CY. RSC96 Schwann Cell Proliferation and survival induced by dilong through P13K/Akt signaling mediated by IGF-I. Evidence-Based Complementary and Alternative Medicine. 2011c;2011 doi: 10.1093/ecam/nep216. Article ID 216148, 9 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen CT, Lin JG, Lu TW, Tsai FJ, Huang CY, Yao CH, Chen YS. Earthworm extracts facilitate PC12 cell differentiation and promote axonal sprouting in peripheral nerve injury. Amer J Chin Med. 2010;38:547–560. doi: 10.1142/S0192415X10008044. [DOI] [PubMed] [Google Scholar]
- 7.Datta A, Jingru Q, Khor TH, Teo MT, Heese K, Sze SK. Quantitative neuroproteomics of an in vivo rodent model of focal cerebral ischemia/reperfusion injury reveals a temporal regulation of novel pathophysiological molecular markers. J Proteome Res. 2011;10:5199–5213. doi: 10.1021/pr200673y. [DOI] [PubMed] [Google Scholar]
- 8.Delgado P, Alvarez Sabin J, Santamarina E, Molina CA, Quintana M, Rosell A, Montaner J. Plasma S100B level after acute spontaneous intracerebral hemorrhage. Stroke. 2006;37:2837–2839. doi: 10.1161/01.STR.0000245085.58807.ad. [DOI] [PubMed] [Google Scholar]
- 9.Donato R. Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim Biophys Acta. 1999;1450:191–231. doi: 10.1016/s0167-4889(99)00058-0. [DOI] [PubMed] [Google Scholar]
- 10.Foerch C, Curdt I, Yan B, Dvorak F, Hermans M, Berkefeld J, Raabe A, Neumann-Haefelin T, Steinmetz H, Sitzer M. Serum glial fibrillary acidic protein as a biomarker for intracerebral haemorrhage in patients with acute stroke. J Neurol Neurosurg Psychiatry. 2006;77:181–184. doi: 10.1136/jnnp.2005.074823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foerch C, Niessner M, Back T, De Marchis GM, Ferbert A, Grehl H, Hamann GF, Jacobs A, Kastrup A, Klimpe S, Palm F, Thomalla G, Worthmann H, Sitzer M. Diagnostic accuracy of plasma glial fibrillary acidic protein for differentiating intracerebral hemorrhage and cerebral ischemia in patients with symptoms of acute stroke. Clin Chem. 2012;58:237–245. doi: 10.1373/clinchem.2011.172676. [DOI] [PubMed] [Google Scholar]
- 12.Grdisa M, Popovic M, Hrzenjak T. Glycolipoprotein extract (G-90) from earthworm Eisenia foetida exerts some antioxidative activity. Comp Comp Biochem Physiol A Mol Integr Physiol. 2001;128:821–825. doi: 10.1016/s1095-6433(00)00323-8. [DOI] [PubMed] [Google Scholar]
- 13.Hrzenjak T, Popovic M, Bozic T, Grdisa M, Kobrehel D, Tiska-Rudman L. Fibrinolytic and anticoagulative activities from the earthworm Eisenia foetida. Comp Biochem Physiol B Biochem Mol Biol. 1998;119:825–832. doi: 10.1016/s0305-0491(98)00060-1. [DOI] [PubMed] [Google Scholar]
- 14.Hu YY, Dong XQ, Yu WH, Zhang ZY. Change in plasma S100B level after acute spontaneous basal ganglia hemorrhage. Shock. 2010;33:134–140. doi: 10.1097/SHK.0b013e3181ad5c88. [DOI] [PubMed] [Google Scholar]
- 15.Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto SP, Lindsay RM. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350:230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- 16.Ichikawa H, Jacobowitz DM, Sugimoto T. S100 protein-immunoreactive primary sensory neurons in the trigeminal and dorsal root ganglia of the rat. Brain Res. 1997;748:253–257. doi: 10.1016/s0006-8993(96)01364-9. [DOI] [PubMed] [Google Scholar]
- 17.Kordower JH, Palfi S, Chen EY, Ma SY, Sendera T, Cochran EJ, Mufson EJ, Penn R, Goetz CG, Comella CD. Clinicopathological findings following intraventricular glial-derived neurotrophic factor treatment in a patient with Parkinson's disease. Ann Neurol. 1999;46:419–424. doi: 10.1002/1531-8249(199909)46:3<419::aid-ana21>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 18.Krakovsky M, Polianski V, Nimrod A, Higazi A, Leker RR, Lamensdorf I. THR-18, a 18-mer peptide derived from PAI-1, is neuroprotective and improves thrombolysis by tPA in rat stroke models. Neurol Res. 2011;33:983–990. doi: 10.1179/1743132811Y.0000000018. [DOI] [PubMed] [Google Scholar]
- 19.Levi-Montalcini R, Hamburger V. Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J Exp Zool. 1951;116:321–361. doi: 10.1002/jez.1401160206. [DOI] [PubMed] [Google Scholar]
- 20.Lin YW, Hsieh CL. Electroacupuncture at Baihui acupoint (GV20) reverses behavior deficit and long-term potentiation through N-methyl-d-aspartate and transient receptor potential vanilloid subtype 1 receptors in middle cerebral artery occlusion rats. J Integr Neurosci. 2010;9:269–282. doi: 10.1142/s0219635210002433. [DOI] [PubMed] [Google Scholar]
- 21.Lin YW, Hsieh CL. Oral Uncaria rhynchophylla (UR) reduces kainic acid-induced epileptic seizures and neuronal death accompanied by attenuating glial cell proliferation and S100B proteins in rats. J Ethnopharmacol. 2011;135:313–320. doi: 10.1016/j.jep.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Liu CH, Lin YW, Tang NY, Liu HJ, Hsieh CL. Neuroprotective effect of Uncaria rhynchophylla in kainic acid-induced epileptic seizures by modulating hippocampal mossy fiber sprouting, neuron survival, astrocyte proliferation, and S100B expression. Evid Based Complement Alternat Med. 2012;2012:194790. doi: 10.1155/2012/194790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Missler U, Wiesmann M, Wittmann G, Magerkurth O, Hagenstrom H. Measurement of glial fibrillary acidic protein in human blood: analytical method and preliminary clinical results. Clin Chem. 1999;45:138–141. [PubMed] [Google Scholar]
- 24.Mogi M, Togari A, Kondo T, Mizuno Y, Komure O, Kuno S, Ichinose H, Nagatsu T. Brain-derived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson's disease. Neurosci Lett. 1999;270:45–48. doi: 10.1016/s0304-3940(99)00463-2. [DOI] [PubMed] [Google Scholar]
- 25.Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. 2011;10:209–219. doi: 10.1038/nrd3366. [DOI] [PubMed] [Google Scholar]
- 26.Pasti L, Volterra A, Pozzan T, Carmignoto G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci. 1997;17:7817–7830. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patil CG, Alexander AL, Gephart MG, Lad SP, Arrigo RT, Boakye MA. Population based study of inpatient outcomes after operative management of non-traumatic intracerebral hemorrhage in the United States. World Neurosurg. doi: 10.1016/j.wneu.2011.10.042. In press. [DOI] [PubMed] [Google Scholar]
- 28.Rickmann M, Wolff JR. S100 protein expression in subpopulations of neurons of rat brain. Neuroscience. 1995;67:977–991. doi: 10.1016/0306-4522(94)00615-c. [DOI] [PubMed] [Google Scholar]
- 29.Tuszynski MH, Thal L, Pay M, Salmon DP, U HS, Bakay R, Patel P, Blesch A, Vahlsing HL, Ho G, Tong G, Potkin SG, Fallon J, Hansen L, Mufson EJ, Kordower JH, Gall C, Conner J. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;11:551–555. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- 30.Wei S, Yin X, Kou Y, Jiang B. Lumbricus extract promotes the regeneration of injured peripheral nerve in rats. J Ethnopharmacol. 2009;123:51–54. doi: 10.1016/j.jep.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 31.Wunderlich MT, Wallesch CW, Goertler M. Release of neurobiochemical markers of brain damage is related to the neurovascular status on admission and the site of arterial occlusion in acute ischemic stroke. J Neurol Sci. 2004;227:49–53. doi: 10.1016/j.jns.2004.08.005. [DOI] [PubMed] [Google Scholar]