Abstract
The present study has investigated the modulating effect of carnosic acid on the expression pattern of cell proliferative (proliferating cell nuclear antigen (PCNA) cyclin D1 and a transcription factor c-fos), apoptotic (p53, Bcl-2, Bax caspase -3 and 9), inflammatory (Nuclear factor kappa B (NFκB) and cyclooxygenase-2 (COX- 2) and angiogenic (vascular endothelial growth factor (VEGF) markers during 7,12-dimethylbenz[a]anthracene (DMBA)-induced hamster buccal pouch carcinogenesis. Oral tumors were developed in the hamsters buccal pouches by painting with 0.5% DMBA in liquid paraffin three times a week for 14 weeks. Hundred per cent tumour formation (well-differentiated squamous cell carcinoma) accompanied by deregulation in the above mentioned molecular markers was noticed in hamsters treated with DMBA alone (tumour bearing hamsters). Oral administration of carnosic acid at dose of 10mg/kg bw to hamsters treated with DMBA not only completely prevented the tumour formation, but also corrected the abnormalities in the expression pattern of molecular markers. The present study suggests that carnosic acid might have inhibited the tumour formation by exerting anti-cell-proliferative, anti-inflammatory, anti-angiogenic and apoptotic potential during DMBA-induced hamster buccal pouch carcinogenesis.
Keywords: oral cancer, apoptosis, angiogenesis, inflammation, cell proliferation
Introduction
Oral cancer, the fifth most common malignancy worldwide, causes significant morbidity and mortality worldwide with substantial economic, physiological and psychological impacts. Globally, around 5 million oral cancers are diagnosed annually and of these two third are reported from developing countries including India (Seki et al., 2011). While oral cancer accounts for 3–4% of all cancers in USA, this form of cancer comprises about 40–50% of all cancers in India. Tobacco and alcohol consumption, in various forms, are strongly attributed to the pathogenesis of oral cancer (Saba et al., 2011). Despite significant improvements have been made for oral cancer treatment with surgical therapy, radiation therapy and chemotherapy, the overall five year survival rate of oral cancer patients remains at about 50% and has not significantly improved for the last three to four decades (Schwartz, 2000). Oral squamous cell carcinoma (SCC) often has a poor prognosis, owing to local tumour invasion and frequent lymph node metastasis.
Oral carcinogenesis is a multistep process and requires accumulation of carcinogen-induced genetic changes throughout the carcinogen treated tissues (Neville and Day, 2002). 7,12-dimethylbenz(a)anthracene (DMBA) induced oral carcinogenesis in golden Syrian hamsters is an accepted and well recognized experimental model for studying biochemical, histopathological and molecular alterations occurring in oral carcinogenesis. DMBA induced molecular changes in the buccal mucosa of golden Syrian hamsters closely mimics or resembles to that of human oral tumour (Balasenthil et al., 2000; Manoharan et al., 2010a). Investigation of biomarkers' presence, quantity and expression pattern would help to correlate to the probability of malignant transformation of a cell or tissue. Evaluation of biomarkers would also help to identify patients who require more aggressive management (Agra et al., 2008). Immunohistochemical analysis of biomarkers could help to establish a direct association between the morphology and the biomarkers, which can aid in determining their functional relevance (Garcıa-Montesinos-Perea et al., 2005). Extensive studies suggested that neoplastic transformation has been associated with abnormalities in oncogenes and tumour suppressor genes that control cell cycle (Garg et al., 2008; Levine and Puzio-Kuter, 2010). Molecular markers of cell proliferation, apoptosis, angiogenesis and inflammation have been studied as potential tools to predict the prognosis of patients with oral squamous cell carcinoma (Letchoumy et al., 2007; Lindstrom et al., 2007).
In recent years, terpenoids received much attention due to their diverse pharmacological properties including anticancer potential. Carnosic acid, a phenolic diterpene, is abundant in Rosmarinus officinalis (rosemary). Carnosic acid possesses diverse biological and pharmacological activities, which include anti-inflammatory, antigenotoxic, antioxidant and anticarcinogenic effects (Manoharan et al., 2010b; Tsai et al., 2011). Offord et al. (2002) reported the photoprotective potential of carnosic acid in UVA-irradiated human skin fibroblasts. Previous studies from our laboratory demonstrated the chemopreventive potential of carnosic acid by analysing the status of biochemical markers that are related to carcinogenesis in DMBA induced hamster buccal pouch carcinogenesis (Manoharan et al.,2010a; Manoharan et al., 2010b; Manoharan et al., 2011). To provide further scientific validity to the chemopreventive potential of carnosic acid, the present study has investigated its modulating effect on the expression pattern of cell proliferative (PCNA, cyclin D1 and c-fos), apoptotic (p53, Bcl-2, Bax caspase -3 and 9), inflammatory (NFκB and COX-2) and angiogenic (VEGF) markers during 7,12-dimethylbenz[a]anthracene (DMBA)-induced hamster buccal pouch carcinogenesis.
Materials and Methods
Animals
Male golden Syrian hamsters, aged 8–10 weeks, weighing 80–120 g, were purchased from the National Institute of Nutrition, Hyderabad, India and were maintained in the Central Animal House, Rajah Muthaiah Medical College and Hospital, Annamalai University. The animals were housed five in a polypropylene cage and provided with a standard pellet diet (Agro Corporation Pvt. Ltd., Bangalore, India) and water ad libitum. The animals were maintained under controlled conditions of temperature (27±2°C) and humidity (55±5%) with a 12 h light/dark cycle.
Chemicals
7,12-dimethylbenz(a)anthracene and carnosic acid were obtained from Sigma-Aldrich Chemical Pvt. Ltd., Bangalore, India. PCNA, Bcl-2, Bax, VEGF and p53 primary antibodies were purchased from Dako, Carprinteria, CA, USA. Power Block™ reagent and secondary antibody conjugated with horseradish peroxidase were purchased from BioGenex, San Ramon, CA, USA. c-fos ELISA kit was purchased from Uscn Life Science Inc. Wuhan, China. The caspase-3 and -9 colorimetric assay kits were purchased from Biovision, Mountain View, CA, USA. COX activity assay kit was purchased from Cayman Chemical Co., USA. Trizol reagent was purchased from Invitrogen, CA, USA. cDNA reverse transcriptase kit and SYBR green fluorophore assay reagents were purchased from Applied Biosystems, Foster City, CA. Oligo nucleotide primers were purchased from Bangalore Genei.
Experimental design
The institutional animal ethics committee (Register number 160/1999/CPCSEA), Annamalai University, Annamalainagar, India, approved the experimental design. The animals were maintained as per the principles and guidelines of the ethical committee for animal care of Annamalai University in accordance with Indian National Law on animal care and use. A total number of 40 hamsters were randomized into four groups of ten animals in each. Group I animals served as control and were painted with liquid paraffin alone three times a week for 14 weeks on their left buccal pouches. Groups II and III animals were painted with 0.5% DMBA in liquid paraffin three times a week for 14 weeks on their left buccal pouches. Group III animals were orally given carnosic acid at a dose of 10mg/kg body weight /day, starting one week before exposure to the carcinogen and continued on days alternate to DMBA painting, until the end of the experiment. Group IV animals received oral administration of carnosic acid10mg/kg body weight/day alone throughout the experimental period. The experiment was terminated at the end of 16 weeks and all animals were sacrificed by cervical dislocation.
Immunohistochemical staining
Paraffin embedded tissue sections were dewaxed and rehydrated through grade ethanol to distilled water. Endogenous peroxidase was blocked by incubation with 3% H2O2 in methanol for 10 minutes. The antigen retrieval was achieved by microwave in citrate buffer solution (2.1 g citric acid/L D.H2O; 0.37g EDTA/L D.H2O; 0.2g Trypsin) (pH 6.0) for 10 minutes, followed by washing step with Tris-buffered saline (8g Nacl; 0.605g Tris) (pH 7.6). The tissue section was then incubated with power BlockTM reagent (BioGenex, San Ramon, CA, USA), universal proteinaceous blocking reagent, for 15 minutes at room temperature to block non-specific binding sites. The tissue sections were then incubated with the respective primary antibody (p53, Bcl-2, Bax, PCNA and VEGF - Dako, Carprinteria, CA, USA) overnight at 4°C. The bound primary antibody was detected by incubation with the secondary antibody conjugated with horseradish peroxidase (BioGenex, San Ramon, CA, USA) for 30 minutes at room temperature. After rinsing with Tris-buffered saline, the antigen-antibody complex was detected using 3,3′-diamminobenzidine, the substrate of horseradish peroxidase. When acceptable color intensity was reached, the slides were washed, counter stained with hematoxylin and covered with a mounting medium. Each slide was microscopically analyzed and enumerated the percentage of the positively stained cells semi-quantitatively. The percentage of positive cells was scored according to the method of Nakagawa et al. (1994) as follows: 3+ = strong staining, more than 50% of cells were stained; 2+ = moderate staining, between 20 and 50% of cells were stained; 1+ = week staining, between 1 and 20% of cells were stained; 0 = negative, less than 1% of cell staining.
Estimation of caspase- 3 and 9, COX-2 and c-fos activities by enzyme linked immunosorbent assay [ELISA]
The activities of c-fos, COX-2, caspase-3 and -9 were assayed in the buccal mucosa using ELISA kit for c-fos, Cayman's COX activity assay kit for COX-2, colorimetric assay kits for caspase-3 and caspase-9 according to the manufacturer's instructions respectively. In c-fos assay, the buccal mucosa tissues were homogenized in 1X PBS and the supernatant obtained was added to the microtiter plate wells precoated with biotin-conjugated antibody preparation specific to c-fos. Then, Avidin conjugated Horseradish peroxidase (HRP) followed by TMB substrate was added to each well. The enzyme — substrate reaction was terminated by the addition of a sulphuric acid and the color change was measured at 450 nm in a microtiter plate reader. The peroxidase activity of COX-2 activity was assayed colorimetrically by monitoring the appearance of oxidized N, N, N′, N′ - tetramethyl- P - phenylenediamine (TMPD) at 590 nm. The caspase-3 and -9 assays are based on spectrophotometric detection of the chromophore p-nitroanilide (pNA) after cleavage from the labeled substrate DEVD - pNA and LEHD-pNA respectively at 405nm in a microtiter plate reader.
Expression of NFκB and Cyclin D1 using Real Time PCR
Total RNA from the buccal mucosa was extracted with Trizol reagent. The RNA integrity and concentration was determined by electrophoresis on agarose gel and nanodrop analysis at 260 nm. Isolated total RNA (1µg) was reverse transcribed to cDNA with random primers from the High cDNA Reverse Transcriptase Kit. cDNA was amplified in duplicates using a thermal cycler (9700 HT RT - PCR, Applied Biosystem, UK) for the expression of NFκB, Cyclin D1 and β-actin with SYBR green fluorophore following the manufacturer's recommended amplification procedure. List of primers used for Real-time PCR analysis was given in Table 1. The relative quantification of target gene expression was determined using the comparative CT method. The ΔCt was calculated as the difference between the average Ct values of the endogenous control (β-actin) from the average Ct value of test gene. The ΔΔCt was determined by subtracting the ΔCt of the control from the ΔCt of the test sample. Relative expression of the target gene was calculated by the formula, 2−ΔΔCt, which was the amount of gene product, normalised to the endogenous control and relative to the control sample.
Table 1.
List of Primers Used for Real-time PCR Analysis
| Genes | Primers | Sequences |
| NFκB | forward reverse |
5′-ATGGACGATCTGTTTCCCCT-3′ 5′- CGGTTTACTCGGCAGATCTT-3′ |
| Cyclin D1 | forward reverse |
5′-CGGAGGACAACAAACAGATC-3′; 5′-GGGTGTGCAAGCCAGGTCCA-3′ |
| β-actin | forward reverse |
5′-AACCGCGAGAAGATGACCCAGATCATGTTT-3′ 5′-AGCAGCCGTGGCCATC TCTTGCTCGAAGTC-3′ |
Statistical analysis
The data is expressed as mean ± standard deviation (S.D.) Statistical comparisons were performed by one-way analysis of variance followed by Duncan's Multiple Range Test. The results were considered statistically significant if the P values were less than 0.05.
Results
The tumor occurence, tumor volume, tumor burden and histological changes in the buccal mucosa of control and experimental animals are shown in Table 2. We have observed 100% tumor formation with mean tumor volume (239.98mm3) and tumor burden (623.94mm3) in DMBA alone-painted animals (Group II). The tumor was histopathologically confirmed as well differentiated squamous cell carcinoma. Oral administration of carnosic acid at a dose of 10mg/kg body weight completely prevented the tumor occurence and reduced the severities of hyperplasia, dysplasia and hyperkeratosis in DMBA-painted hamsters (Group III). No tumor was observed in control animals painted with liquid paraffin alone (Group I) as well as carnosic acid alone administered animals (Group IV).
Table 2.
Incidence of oral neoplasm and histopathological changes in control and experimental animals in each group (n=10)
| Parameter | Group I | Group II | Group III | Group VI |
| Control | DMBA alone | DMBA + Carnosic acid | Carnosic acid alone | |
|
Tumour incidence (oral squamous cell carcinoma) |
0 | 100% | 0 | 0 |
| Total number of tumours/animals | 0 | 26/10 | 0 | 0 |
| Tumour volume (mm3)/ animals | 0 | 239.98 ± 11.09 | 0 | 0 |
| Tumour burden (mm3)/ animals | 0 | 623.94 ± 48.20 | 0 | 0 |
| Hyperkeratosis | Absent | +++ | + | Absent |
| Hyperplasia | Absent | +++ | + | Absent |
| Dysplasia | Absent | +++ | + | Absent |
| Squamous cell carcinoma | Absent | Well differentiated |
Absent | Absent |
Values are expressed as mean± SD for 10 hamsters in each group Tumour volume was measured using the formula, ν = (4/3)π [D1/2][D2/2][D3/2] where D1, D2 and D3 are the three diameters (mm) of the tumour. Tumour burden was calculated by multiplying tumour volume and the number of tumours/animal. +++ - Severe; + - Mild.
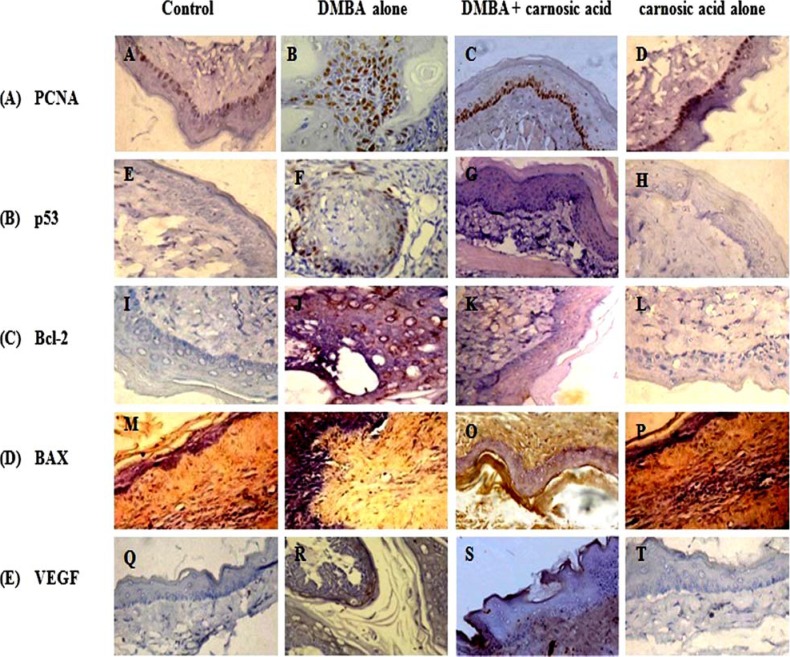
The immunoexpression pattern of cell proliferative (PCNA), apoptotic (p53, Bcl-2 and Bax) and angiogenic (VEGF) markers and the score of positively stained cells in control and experimental hamsters in each group are depicted in figure 1 (A–E) and Table 3 respectively. Over expression of all these markers except Bax was noticed in hamsters treated with DMBA alone. Oral administration of carnosic acid at a dose of 10mg/kg bw to hamsters treated with DMBA significantly restored the expression of above markers. Hamsters treated with carnosic acid alone revealed expression similar to that of control hamsters.
Figure 1.
(A–E). Immunoexpression pattern of PCNA, p53, Bcl-2, Bax and VEGF proteins observed in the buccal mucosa of control and experimental hamsters in each group (40X). (A) PCNA: A and D - Control and carnosic acid alone (expression not detectable), B - DMBA alone (over expressed), C - DMBA + carnosic acid (down regulated). (B) P53: E and H - Control and carnosic acid alone (expression not detectable); F - DMBA alone (over expression); G DMBA + carnosic acid (down regulated). (C) Bcl-2: I and L - Control and carnosic acid alone (expression not detectable); J - DMBA alone (over expression); K - DMBA + carnosic acid (down regulated). (D) Bax: M and P -Control and carnosic acid alone (nuclear expression positive), N - DMBA alone (nuclear expression negative), O DMBA + carnosic acid (nuclear and cytoplasmic expression positive). (E) VEGF: Q and T - Control and carnosic acid alone (expression not detectable), R - DMBA alone (overexpressed), S - DMBA + carnosic acid (down regulated).
Table 3.
The score of positively stained cells of PCNA, p53, Bcl-2, Bax and VEGF in control and experimental hamsters in each group
| Groups / Markers | PCNA | P53 | Bcl2 | Bax | VEGF | |||||||||||||||
| 0 | 1+ | 2+ | 3+ | 0 | 1+ | 2+ | 3+ | 0 | 1+ | 2+ | 3+ | 0 | 1+ | 2+ | 3+ | 0 | 1+ | 2+ | 3+ | |
| Control | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 8 | 2 | 0 | 0 | 1 | 4 | 3 | 2 | 10 | 0 | 0 | 0 |
| DMBA | 0 | 1 | 2 | 7 | 0 | 1 | 3 | 6 | 0 | 1 | 2 | 7 | 7 | 2 | 1 | 0 | 0 | 2 | 3 | 5 |
| DMBA + carnosic acid | 0 | 6 | 3 | 1 | 1 | 5 | 3 | 1 | 3 | 5 | 2 | 0 | 3 | 3 | 4 | 0 | 7 | 2 | 1 | 0 |
| Carnosic acid alone | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 7 | 3 | 0 | 0 | 2 | 3 | 3 | 2 | 10 | 0 | 0 | 0 |
Values are given as number of hamsters (n = 10). The percentage positive cells were scored as: 3+ = strong staining, more than 50% of cells were stained, 2+ = moderate staining, between 20 and 50% of cells were stained 1+ = week staining, between 1 and 20% of cells were stained, 0 = negative, less than 1% of cell staining.
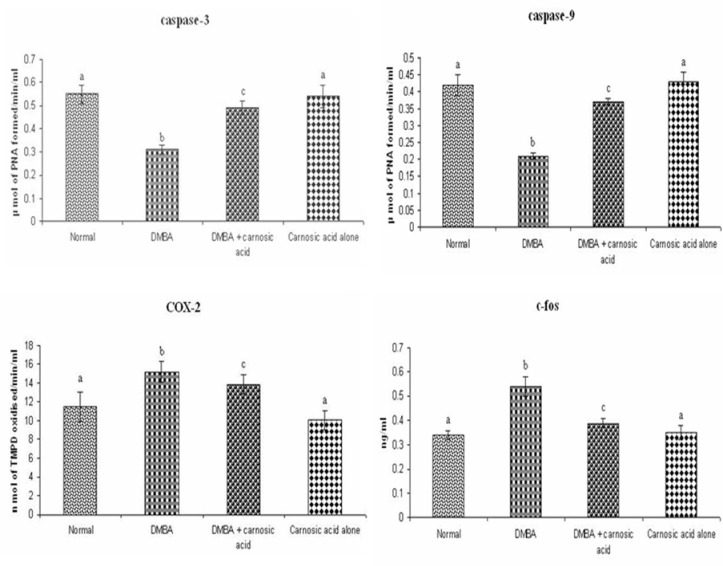
Buccal mucosa caspase-3 and 9, COX-2 and c-fos status of control and experimental hamsters in each group is shown in figure 2. The activities of caspase-3 and -9 were significantly decreased whereas COX-2 and c-fos were increased in hamsters treated with DMBA alone. Oral administration of carnosic acid to hamsters treated with DMBA brought back the status of above markers to near normal range. No significant difference was noticed in the status of above markers in control hamsters and hamsters treated with carnosic acid alone.
Figure 2.
Buccal mucosa Caspase-3 and -9, COX-2 and c-fos activity in control and experimental hamsters in each group. Values are expressed as mean ± SD for 10 hamsters in each group. Values that do not share a common superscript letter between groups differ significantly at p < 0.05. (Analysis of variance followed by DMRT).
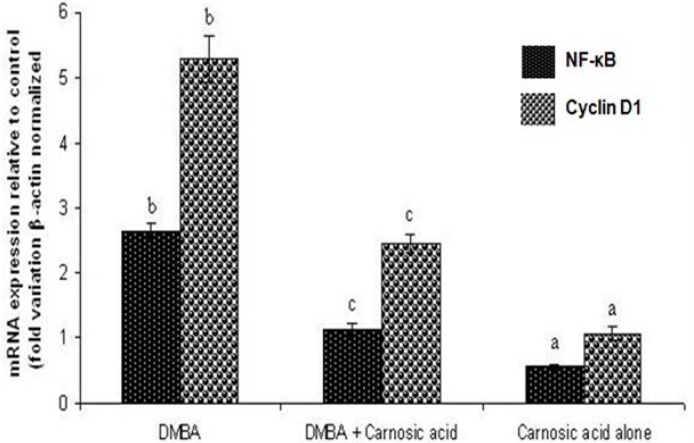
The NFκB and Cyclin D1 mRNA expression pattern of control and experimental hamsters in each group is depicted in figure 3. The expression of NFκB and Cyclin D1 were significantly higher in hamsters treated with DMBA alone as compared to control hamsters. Oral administration of carnosic acid to hamsters treated with DMBA suppressed the expression of Cyclin D1 and NFκB. Similar expression pattern of Cyclin D1 and NFκB was observed in control hamsters and hamsters treated with carnosic acid alone.
Figure 3.
Fold increase in the mRNA expression pattern for NF-κB and Cyclin D1 in hamsters treated with DMBA alone, DMBA + carnosic acid and carnosic acid alone. Values are expressed as mean ± SD for 10 hamsters in each group. Values that do not share a common superscript letter in the same column differ significantly at p < 0.05. (Analysis of variance followed by DMRT).
Discussion
Carnosic acid, a phenolic diterpene, exhibited antitumor properties in both in vivo and in vitro conditions (Manoharan et al., 2010b; Tsai et al., 2011). Manoharan et al. (2010b) reported that carnosic acid exhibited potent chemopreventive efficacy during DMBA-induced oral carcinogenesis. They concluded that the chemopreventive potential of carnosic acid is probably due to its antioxidant potential and modulating effect on phase I and II detoxification enzymes during DMBA-induced oral carcinogenesis. To further confirm the chemopreventive potential of carnosic acid, the present study focuses the modulating effect of carnosic acid on apoptotic, cell proliferative, inflammatory and angiogenic markers during DMBA-induced oral carcinogenesis. Deregulation in the above mentioned markers could result in neoplastic transformation.
In the present study, we noticed up-regulation of cell proliferation markers (PCNA, cyclin D1 and c-fos) during DMBA-induced hamster buccal pouch carcinogenesis. PCNA, a nuclear non-histone antigen, play an important role in both DNA synthesis and DNA repair (Mallick et al., 2010). Immunohistochemical studies using anti-PCNA antibody is commonly employed to measure abnormal cell proliferation during carcinogenesis. Over expression of PCNA has been shown in several cancers including oral cancers (Cheng et al., 2007; Harish Kumar et al., 2010). Cyclin D1, one of the most important cell cycle regulator protein, plays pivotal role in the control of restriction point of G1 phase. Deregulation in cyclin D1 expression has been associated with the progression and recurrence of tumours. It has been reported that p53 mutation preceded cyclin D1 amplification in carcinogenesis (Fu et al., 2004). Amplification and over expression of cyclin D1 resulted in the suppression of NFkB expression (Elayat et al., 2011). Over expression and amplification of cyclin D1 was reported in several human cancers including oral cancer (Shenoy, 2007; Holley et al., 2001). C-fos plays pivotal role in the regulation of cell growth and differentiation. Over expression of c-fos was reported in several cancers including cervical cancer, lung cancer, pancreatic carcinomas, osteosarcomas, hepatocellular carcinoma and colorectal adenoma (Pacheco et al., 2002; Petrov et al., 1994). Over expression of c-fos was reported in 70% and 38% of the oral cancers and oral pre-cancerous lesions respectively (Vairaktaris et al., 2008; Zhang et al., 2011). Our results corroborate these observations.
In the present study, over expression of inflammatory markers (NFkB and COX-2) was noticed in the buccal mucosa of hamsters treated with DMBA alone. NFkB, an anti-apoptotic transcription factor and inflammatory marker, integrates multiple signals and regulates the expression of genes involved in the pathogenesis of cancer (Karin, 2006). Inactivation of NFkB inhibited abnormal cell proliferation, invasion, metastasis and angiogenesis. Over expression of NFkB has been reported in human and experimental oral carcinogenesis (Wang et al., 2011). Cyclooxygenase-2 (COX-2), a key regulatory enzyme in the synthesis of prostaglandins, was upregulated in precancerous and cancerous conditions. In recent years, researchers thus utilize COX-2 as a novel target for the prevention of many cancers including oral cancer (McCormick et al., 2010). Deregulation of COX-2 expression could lead to abnormal production of VEGF thereby contributing to angiogenesis in tumour tissues (Turini and Dubois, 2002). Immunohistochemical analysis revealed over expression of COX-2 in oesophagus, stomach, breast, pancreas, lung, colon, skin, urinary bladder and prostate cancers (Mendes et al., 2009). Increased levels of COX-2 were shown in premalignant lesions of oral cavity (Mallery et al., 2008). A positive association between mutant p53 and over expression of COX-2 has been shown in oral carcinogenesis (Nishimura et al., 2004).
In the present study, up-regulation of p53 and bcl-2 and down-regulation of Bax was noticed in the buccal mucosa of hamsters treated with DMBA alone. Under normal conditions, p53 expression results in cell cycle arrest and apoptosis. Abnormalities in the p53 tumour suppressor gene are the most common molecular changes associated with the development of several cancers including oral cancer (Panjamurthy et al., 2009). p53 stimulates the up-regulation of Bax and down regulation of Bcl-2 to remove the unwanted cells from the host (Merchant et al.,1996). Anti-apoptotic and pro-apoptotic proteins comprises the Bcl-2 family and these proteins share sequence homology within conserved regions known as Bcl-2 homology (BH) domains. Immunohistochemical studies on normal epithelium showed Bcl-2 was restricted to the lower epithelial cell layers and Bax was diffused throughout the epithelium. Over expression of Bcl-2 indirectly inhibit the process of apoptosis and thus play a role in the onset of tumouregenesis (Kanekawa et al., 1999). Bcl-2 could favour tumour progression through extended cell survival (Chandra Mohan et al., 2006). Bax, a 21kD protein of 192 amino acids, play crucial role in the process of apoptosis. Over expression of Bcl-2 and down regulation of Bax has been reported in several cancers including oral cancer (Baltaziak et al., 2006; De Sousa et al., 2009; Balakrishnan et al., 2010).
A central component of the apoptotic machinery is the family of cysteine protease called caspases. Caspases are expressed as inactive precursors that are activated by proteolytic processing (Ho et al., 2009). In the present study, the activation of caspase 3 and 9 were decreased in the buccal mucosa of hamsters treated with DMBA alone. It has been reported that decreased caspase 3 expression was associated with vascular invasion, lymph node metastasis and advanced tumour stages in oral carcinogenesis (Ding et al., 2010). Reduced expression of caspase -3 and 9 has been reported in oral carcinogenesis (Letchoumy et al., 2007).
Angiogenesis, sprouting of new blood vessels from the pre-exiting vasculature, has been implicated not only under physiological conditions but also in various pathological conditions including cancer, diabetic retinopathy and rheumatoid arthristis. The purpose of angiogenesis in tumours is to sequester nutrients from the host for tumour cell survival and tumour growth (Carmeliet and Jain, 2000). Extensive studies pointed out that angiogenesis have been associated with tumour progression and aggressiveness in a number of malignancies, including oral cancer (Uehara et al., 2004; Shivamallappa et al., 2011). In the present study, we noticed over expression of VEGF in the buccal mucosa of hamsters treated with DMBA alone. Over expression of VEGF has been demonstrated in varying degrees of oral dysplasia and carcinoma (O-charoenrat et al., 2001). Deregulation of VEGF resulted in the progression of oral cancer, due to up-regulation of microvessel density. The VEGF serum concentration was significantly higher in patients with oral squamous cell carcinoma as compared to normal subjects (Seki et al., 2011).
Extensive studies investigated the modulating effect of carnosic acid on the expression pattern of molecular markers that are related to carcinogenesis. Visanji et al. (2006) reported that carnosic acid caused cell cycle arrest at G2/M phase in human colonic adenocarcinoma cell line. Mengoni et al. (2011) reported that carnosic acid suppressed the expression of COX-2 in the inflammatory skin. Tsai et al. (2011) reported that carnosic acid induced apoptosis by down regulating Bcl-2 expression and activating caspases -3 and 9 in human neuroblastoma IMR-32 cells. Kar et al. (2012) reported that carnosic acid induced apoptosis by activation of serine/threonine protein phosphatise 2A through modulation of Akt/IKK/NF-Kb pathway in human prostate carcinoma PC-3 cells. Carnosic acid induced apoptosis in prostate cancer DU 145 cells by activating caspase-3 and increasing cytochrome-c releases and Bax: Bcl-2 ratio. Barni et al. (2012) reported that carnosic acid inhibited the proliferation and migration capacity of human colorectal cancer cells by down regulating the expression of COX-2. Lopez-Jimenez et al. (2011) reported the anti-angiogenic properties of carnosic acid and suggested that the anti-angiogenic potential of carnosic acid could be due to its apoptotic potential. Pesakhov et al. (2010) demonstrated that combinations of the dietary plant polyphenols, curcumin and carnosic acid, at non-cytotoxic concentrations of each agent, produced a synergistic antiproliferative effect and a massive apoptotic cell death in HL-60 and KG-1a human AML cells.
In the present study, oral administration of carnosic acid at a dose of 10mg/kg bw restored the expression pattern of markers of cell proliferation, inflammation, apoptosis and angiogenesis in hamsters treated with DMBA. The results of the present study suggests that carnosic acid modulated the expression cell proliferative (PCNA, cyclin D1 and c-fos), apoptotic (p53, Bcl-2, caspase -3 and 9), inflammatory (NFκB and COX-2) and angiogenic (VEGF) in favour of inhibiting the abnormal cell proliferation occurring in DMBA induced hamster buccal pouch carcinogenesis.
Conclusion
The present study demonstrates the modulating effect of carnosic acid on the expression pattern of cell proliferative (PCNA, cyclin D1 and c-fos), apoptotic (p53, Bcl-2, caspase -3 and 9), inflammatory (NFκB and COX-2) and angiogenic (VEGF) during DMBA-induced oral carcinogenesis. Carnosic acid might have inhibited the tumour formation by exerting anti-cell-proliferative, anti-inflammatory, anti-angiogenic and apoptotic potential during DMBA-induced hamster buccal pouch carcinogenesis.
Acknowledgements
Financial assistance from Indian Council of Medical Research (ICMR), New Delhi, is gratefully acknowledged.
Abbreviations
- COX-2
cyclooxygenase-2
- DMBA
7,12-dimethylbenz(a)anthracene
- NFκB
Nuclear factor kappa B
- PCNA
Proliferating cell nuclear antigen
- VEGF
Vascular endothelial growth factor
References
- 1.Agra IM, Carvalho AL, Pinto CA, Martins EP, Filho JG, Soares FA, Kowalski LP. Biological markers and prognostic in recurrent oral cancer after salvage surgery. Arch Otolaryngol Head Neck Surg. 2008;134:743–749. doi: 10.1001/archotol.134.7.743. [DOI] [PubMed] [Google Scholar]
- 2.Balakrishnan S, Manoharan S, Alias LM, Nirmal MR. Effect of curcumin and ferulic acid on modulation of expression pattern of p53 and bcl-2 proteins in 7,12-dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesis. Indian J Biochem Biophys. 2010;47:7–12. [PubMed] [Google Scholar]
- 3.Balasenthil S, Saroja M, Ramachandran CR, Nagini S. Of humans and hamsters: comparative analysis of lipid peroxidation, glutathione, and glutathione-dependent enzymes during oral carcinogenesis. Brit J Oral Max Surg. 2000;38:267–270. doi: 10.1054/bjom.1999.0445. [DOI] [PubMed] [Google Scholar]
- 4.Baltaziak M, Duraj E, Koda M, Wincewicz A, Musiatowicz M, Kanczuga-Koda L, Szymanska M, Lesniewicz T, Musiatowicz B. Expression of Bcl-xL, Bax, and p53 in primary tumors and lymph node metastases in oral squamous cell carcinoma. Ann N Y Acad Sci. 2006;1090:18–25. doi: 10.1196/annals.1378.002. [DOI] [PubMed] [Google Scholar]
- 5.Barni MV, Carlini MJ, Cafferata EG, Puricelli L, Moreno S. Carnosic acid inhibits the proliferation and migration capacity of human colorectal cancer cells. Oncol rep. 2012;27:1041–1048. doi: 10.3892/or.2012.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 7.ChandraMohan KV, Devaraj H, Prathiba D, Hara Y, Nagini S. Antiproliferative and apoptosis inducing effect of lactoferrin and black tea polyphenol combination on hamster buccal pouch carcinogenesis. Biochim Biophys Acta. 2006;1760:1536–1544. doi: 10.1016/j.bbagen.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Cheng HC, Chien H, Liao CH, Yang YY, Huang SY. Carotenoids suppress proliferating cell nuclear antigen and cyclin D1 expression in oral carcinogenic models. J Nutr Biochem. 2007;18:667–675. doi: 10.1016/j.jnutbio.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 9.De Sousa FA, Paradella TC, Carvalho YR, Rosa LE. Comparative analysis of the expression of proliferating cell nuclear antigen, p53, bax, and bcl-2 in oral lichen planus and oral squamous cell carcinoma. Ann Diagn Pathol. 2009;13:308–312. doi: 10.1016/j.anndiagpath.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Ding YP, Li SX, Wu HR, Zhang XY, Tang XF, Sun Z. Expression of survivin, caspase-3 in oral precancerous lesions and oral squamous-cell carcinoma. Zhonghua Kou Qiang Yi Xue Za Zhi. 2010;45:85–88. [PubMed] [Google Scholar]
- 11.Elayat G, Selim AG, Gorman P, Tomlinson I, Wells CA. Cyclin D-1 protein over-expression is not associated with gene amplification in benign and atypical apocrine lesions of the breast. Pathol Res Pract. 2011;207:75–78. doi: 10.1016/j.prp.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Fu M, Wang C, Li Z, Sakamaki T, Pestell RG. Cyclin D1, normal and abnormal functions. Endocrinol. 2004;145:5439–5447. doi: 10.1210/en.2004-0959. [DOI] [PubMed] [Google Scholar]
- 13.Garcıa-Montesinos-Perea B, Val-Bernal JF, Saiz-Bustillo R. Epidermoid carcinoma of the lip: an immunohistochemical study. Med Oral Pathol Oral Cir Bucal. 2005;10:454–461. [PubMed] [Google Scholar]
- 14.Garg R, Ingle A, Maru G. Dietary turmeric modulates DMBA-induced p21ras, MAP kinases and AP-1/NF-kappaB pathway to alter cellular responses during hamster buccal pouch carcinogenesis. Toxicol Appl Pharmacol. 2008;232:428–439. doi: 10.1016/j.taap.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Harish Kumar G, Vidya Priyadarsini R, Vinothini G, Vidjaya Letchoumy P, Nagini S. The neem limonoids azadirachtin and nimbolide inhibit cell proliferation and induce apoptosis in an animal model of oral oncogenesis. Invest New Drugs. 2010;28:392–401. doi: 10.1007/s10637-009-9263-3. [DOI] [PubMed] [Google Scholar]
- 16.Ho YT, Lu CC, Yang JS, Chiang JH, Li TC, Ip SW, Hsia TC, Liao CL, Lin JG, Wood WG, Chung JG. Berberine induced apoptosis via promoting the expression of caspase-8, -9 and -3, apoptosis-inducing factor and endonuclease G in SCC-4 human tongue squamous carcinoma cancer cells. Anticancer Res. 2009;29:4063–4070. [PubMed] [Google Scholar]
- 17.Holley S, Parkes G, Bockmuhle U. Cyclin D1 polymorphism and expression in patients with squamous cell carcinoma of the head and neck. Am J Pathol. 2001;159:1917–1924. doi: 10.1016/S0002-9440(10)63038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanekawa A, Tsuji T, Oga A, Sasaki K, Shinozaki F. Chromosome 17 abnormalities in squamous cell carcinoma of the oral cavity, and its relationship with p53 and Bcl-2 expression. Anticancer Res. 1999;19:81–86. [PubMed] [Google Scholar]
- 19.Kar S, Palit S, Ball WB, Das PK. Carnosic acid modulates Akt/IKK/NF-κB signalling by PP2A and induces intrinsic and extrinsic pathway mediated apoptosis in human prostate carcinoma PC-3 cells. Apoptosis. 2012 doi: 10.1007/s10495-012-0715-4. [DOI] [PubMed] [Google Scholar]
- 20.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 21.Letchoumy PV, Mohan KV, Prathiba D, Hara Y, Nagini S. Comparative evaluation of antiproliferative, antiangiogenic and apoptosis inducing potential of black tea polyphenols in the hamster buccal pouch carcinogenesis model. J Carcinog. 2007;6:19. doi: 10.1186/1477-3163-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330:1340–1344. doi: 10.1126/science.1193494. [DOI] [PubMed] [Google Scholar]
- 23.Lindstrom AK, Stendahl U, Tot T, Lidström BM, Hellberg D. Predicting the outcome of squamous cell carcinoma of the uterine cervix using combinations of individual tumor marker expressions. Anticancer Res. 2007;27:1609–1615. [PubMed] [Google Scholar]
- 24.Lopez-Jimenez A, Garcia-Caballero M, Medina MA, Quesada AR. Anti-angiogenic properties of carnosol and carnosic acid, two major dietary compounds from rosemary. Eur J Nutr. 2011 doi: 10.1007/s00394-011-0289-x. [DOI] [PubMed] [Google Scholar]
- 25.Mallery SR, Zwick JC, Pei P, Tong M, Larsen PE, Shumway BS, Lu B, Fields HW, Mumper RJ, Stoner GD. Topical application of a bioadhesive black raspberry gel modulates gene expression and reduces cyclooxygenase 2 protein in human premalignant oral lesions. Cancer Res. 2008;68:4945–4957. doi: 10.1158/0008-5472.CAN-08-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mallick S, Agarwal J, Kannan S, Pawar S, Kane S, Teni T. PCNA and anti-apoptotic Mcl-1 proteins predict disease-free survival in oral cancer patients treated with definitive radiotherapy. Oral Oncol. 2010;46:688–693. doi: 10.1016/j.oraloncology.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Manoharan S, Balakrishnan S, Vinothkumar V, Silvan S. Anti-clastogenic potential of carnosic acid against 7,12-dimethylbenz(a)anthracene (DMBA)-induced clastogenesis. Pharmacol Rep. 2010b;62:1170–1177. doi: 10.1016/s1734-1140(10)70379-0. [DOI] [PubMed] [Google Scholar]
- 28.Manoharan S, Vasanthaselvan M, Silvan S, Baskaran N, Kumar Singh A, Vinoth Kumar V. Carnosic acid, a potent chemopreventive agent against oral carcinogenesis. Chem Biol Interact. 2010a;188:616–622. doi: 10.1016/j.cbi.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Manoharan S, Vasanthaselvan M, Silvan S, Muralinaidu R, Baskaran N. Carnosic acid protects cell surface glycoconjugates abnormalities during 7,12-dimethylbenz(a)anthracene (DMBA)-induced oral carcinogenesis. Int J Res Pharm Sci. 2011;2:237–243. [Google Scholar]
- 30.McCormick DL, Phillips JM, Horn TL, Johnson WD, Steele VE, Lubet RA. Over expression of cyclooxygenase-2 in rat oral cancers and prevention of oral carcinogenesis in rats by selective and nonselective COX inhibitors. Cancer Prev Res. 2010;3:73–81. doi: 10.1158/1940-6207.CAPR-09-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendes RA, Carvalho JF, Waal I. An overview on the expression of cyclooxygenase-2 in tumors of the head and neck. Oral Oncol. 2009;45:124–128. doi: 10.1016/j.oraloncology.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 32.Mengoni ES, Vichera G, Rigano LA, Rodriguez-puebla ML, Galliano SR, Cafferata EE, Pivetta OH, Moreno S, Vojnov AA. Suppression of COX-2, IL-1β and TNF-α expression and leukocyte infiltration in inflamed skin by bioactive compounds from Rosmarinus officinalis L. Fitoterapia. 2011;82:414–421. doi: 10.1016/j.fitote.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 33.Merchant AK, Loney TL, Maybaum J. Expression of wild-type p53 stimulates an increase in both Bax and Bcl-xL protein content in HT29 cells. Oncogene. 1996;13:2631–2637. [PubMed] [Google Scholar]
- 34.Nakagawa K, Yamamura K, Maeda S, Ichihashi M. bcl-2 expression in epidermal keratinocytic diseases. Cancer. 1994;74:1720–1724. doi: 10.1002/1097-0142(19940915)74:6<1720::aid-cncr2820740613>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 35.Neville BW, Day TA. Oral cancer and precancerous lesions. CA Cancer J Clin. 2002;52:195–215. doi: 10.3322/canjclin.52.4.195. [DOI] [PubMed] [Google Scholar]
- 36.Nishimura N, Urade M, Hashitani S, Noguchi K, Manno Y, Takaoka K, Sakurai K. Increased expression of cyclooxygenase (COX)-2 in DMBA-induced hamster cheek pouch carcinogenesis and chemopreventive effect of a selective COX-2 inhibitor celecoxib. J Oral Pathol Med. 2004;33:614–621. doi: 10.1111/j.1600-0714.2004.00254.x. [DOI] [PubMed] [Google Scholar]
- 37.O-charoenrat P, Rhys-Evans P, Eccles SA. Expression of vascular endothelial growth factor family members in head and neck squamous cell carcinoma correlates with lymph node metastasis. Cancer. 2001;92:556–568. doi: 10.1002/1097-0142(20010801)92:3<556::aid-cncr1355>3.0.co;2-q. 2001. [DOI] [PubMed] [Google Scholar]
- 38.Offord EA, Gautier JC, Avanti O, Scaletta C, Runge F, Kramer K, Applegate LA. Photoprotective potential of lycopene, β-carotene, vitamin E, vitamin C and carnosic acid in UVA-irradiated human skin fibroblasts. Free Radic Biol Med. 2002;32:1293–1303. doi: 10.1016/s0891-5849(02)00831-6. [DOI] [PubMed] [Google Scholar]
- 39.Pacheco MM, Kowalski LP, Nishimoto IN, Brentani MM. Differential expression of c-jun and c-fos mRNAs in squamous cell carcinoma of the head and neck: associations with uPA, gelatinase B, and matrilysin mRNAs. Head Neck. 2002;24:24–32. doi: 10.1002/hed.10009. [DOI] [PubMed] [Google Scholar]
- 40.Panjamurthy K, Manoharan S, Nirmal MR, Vellaichamy L. Protective role of Withaferin-A on immunoexpression of p53 and bcl-2 in 7,12-dimethylbenz(a)anthracene-induced experimental oral carcinogenesis. Invest New Drugs. 2009;27:447–452. doi: 10.1007/s10637-008-9199-z. [DOI] [PubMed] [Google Scholar]
- 41.Pesakhov s, Khanin M, Studzinski GP, Danilenko M. Distinct combinatorial effects of the plant polyphenols curcumin, carnosic acid and silibinin on proliferation and apoptosis in acute myloid leukemia cells. Nut Cancer. 2010;62:811–824. doi: 10.1080/01635581003693082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petrov SV, Mazurenko NN, Sukhova NM, Moroz IP, Katsenel'son VM, Raikhlin NT, Kiselev FL. Cell oncogene expression in normal, metaplastic, dysplastic epithelium and squamous cell carcinoma of the uterine cervix. Arkh Patol. 1994;56:22–31. [PubMed] [Google Scholar]
- 43.Saba NF, Goodman M, Ward K, Flowers C, Ramalingam S, Owonikoko T, Chen A, Grist W, Wadsworth T, Beitler JJ, Khuri FR, Shin DM. Gender and ethnic disparities in incidence and survival of squamous cell carcinoma of the oral tongue, base of tongue, and tonsils, a surveillance, epidemiology and end results program-based analysis. Oncol. 2011;81:12–20. doi: 10.1159/000330807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz JL. Biomarkers and molecular epidemiology and chemoprevention of oral carcinogenesis. Crit Rev Oral Biol Med. 2000;11:92–122. doi: 10.1177/10454411000110010501. [DOI] [PubMed] [Google Scholar]
- 45.Seki S, Fujiwara M, Matsuura M, Fujita S, Ikeda H, Asahina I, Ikeda T. Prediction of outcome of patients with oral squamous cell carcinoma using vascular invasion and the strongly positive expression of vascular endothelial growth factors. Oral Oncol. 2011;47:588–593. doi: 10.1016/j.oraloncology.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 46.Shenoy AM. Cyclin D1 over expression as a prognostic factor in patients with tobacco-related intraoral squamous cell carcinoma. Indian J Med Res. 2011;133:364–365. [PMC free article] [PubMed] [Google Scholar]
- 47.Shivamallappa SM, Venkatraman NT, Shreedhar B, Mohanty L, Shenoy S. Role of angiogenesis in oral squamous cell carcinoma development and metastasis: an immunohistochemical study. Int J Oral Sci. 2011;3:216–224. doi: 10.4248/IJOS11077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsai CW, Lin CY, Lin HH, Chen JH. Carnosic acid, a rosemary phenolic compound, induces apoptosis through reactive oxygen species-mediated p38 activation in human neuroblastoma IMR-32 cells. Neuro chem Res. 2011;36:2442–2451. doi: 10.1007/s11064-011-0573-4. [DOI] [PubMed] [Google Scholar]
- 49.Turini ME, Dubois RN. Cyclooxygenase-2, a therapeutic target. Ann Mev Med. 2002;53:35–57. doi: 10.1146/annurev.med.53.082901.103952. [DOI] [PubMed] [Google Scholar]
- 50.Uehara M, Sano K, Ikeda H, Sekine J, Irie A, Yokota T. Expression of vascular endothelial growth factor and prognosis of oral squamous cell carcinoma. Oral Oncol. 2004;40:321–325. doi: 10.1016/j.oraloncology.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 51.Vairaktaris E, Papakosta V, Derka S, Vassiliou S, Nkenke E, Spyridonidou S, Vylliotis A, Lazaris A, Kokkori A, Moulavassili P, Loukeri S, Perrea D, Donta I, Yapijakis C, Patsouris E. H-ras and c-fos exhibit similar expression patterns during most stages of oral oncogenesis. In Vivo. 2008;22:621–628. [PubMed] [Google Scholar]
- 52.Visanji JM, Thompson DG, Padfield PJ. Induction of G2/M phase cell cycle arrest by carnosol and carnosic acid is associated with alteration of cyclin A and cyclin B1 levels. Cancer Lett. 2006;237:130–136. doi: 10.1016/j.canlet.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 53.Wang LJ, Zhou X, Wang W, Tang F, Qi CL, Yang X, Wu S, Lin YQ, Wang JT, Geng JG. Andrographolide Inhibits Oral Squamous Cell Carcinogenesis through NF-{kappa}B Inactivation. J Dent Res. 2011;90:1246–1252. doi: 10.1177/0022034511418341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang L, Ye DX, Pan HY, Wei KJ, Wang LZ, Wang XD, Shen GF, Zhang ZY. Yes-associate protein promotes cell proliferation by activating Fos Related Activator-1 in oral squamous cell carcinoma. Oral Oncol. 2011;47:693–697. doi: 10.1016/j.oraloncology.2011.06.003. [DOI] [PubMed] [Google Scholar]