Abstract
A simple and accurate high-performance liquid chromatographic method was applied to the quantitative analysis of seven components of the traditional herbal prescription Jaeumganghwa-tang (JGT), including 5-hydroxymethyl-2-furaldehyde, albiflorin, paeoniflorin, liquiritin, ferulic acid, nodakenin, and glycyrrhizin. All seven compounds were separated in less than 40 min on a Gemini C18 column at 40°C by gradient elution using 1.0% (v/v) aqueous acetic acid and acetonitrile containing 1.0% (v/v) acetic acid as mobile phase. The flow rate was 1.0 mL/min and the detector was a photodiode array (PDA) set at 230 nm, 254 nm, 280 nm, and 330 nm. The calibration curves showed good linearity (r2 > 0.9998) in different concentration ranges. The recovery of each component was in the range of 91.47–102.62%, with relative standard deviations (RSDs, %) less than 4.5%. The RSDs (%) for intra- and interday precision were 0.06–2.85% and 0.06–2.83%, respectively. The concentrations of the seven components in JGT were in the range 0.74–5.48 mg/g.
Keywords: Herbal prescription, HPLC-PDA, Jaeumganghwa-tang, Zi-yin-jiang-huo-tang, Jiin-koka-to, Simultaneous determination
Introduction
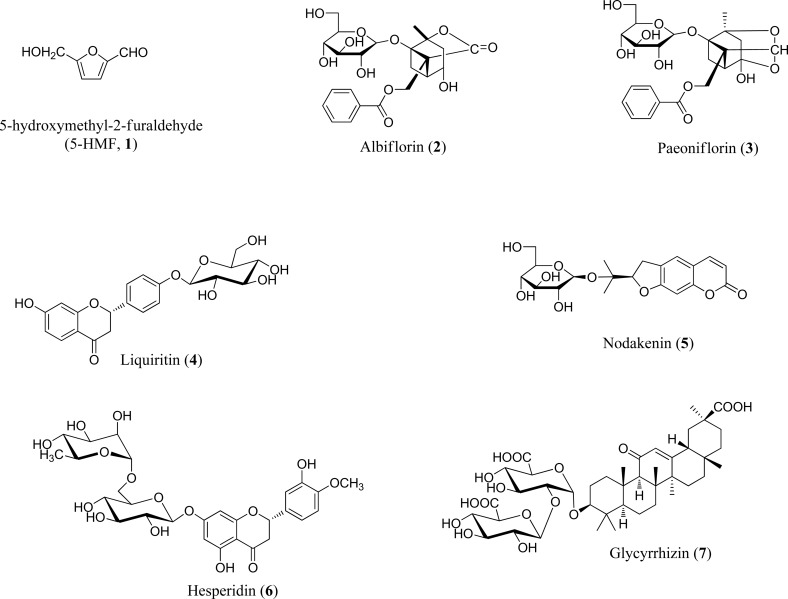
Many herbal formulae have been used to prevent and treat various diseases. Such herbal medicines have few side effects and exhibit multiple activities (Zhang et al., 2004; Jiang, 2005; Liu et al., 2008). Jaeumganghwa-tang (JGT, Zi-yin-jiang-huo-tang in Chinese, Jiin-koka-to in Japanese) is an herbal prescription that consists of 13 herbal medicines: Paeoniae radix, Angelicae gigantis Radix, Rehmanniae Radix Preparata, Asparagi tuber, Atractylodis Rhizoma Alba, Liriopis Tuber, Rehmanniae Radix Crudus, Citri Unshii Pericarpium, Anemarrhenae Rhizoma, Phellodendri Cortex, Glycyrrhizae Radix et Rhizoma, Zingiberis Rhizoma Crudus, and Zizyphi fructus. JGT has been used to treat symptoms such as sweating during sleep that ceases on awakening, fever that is more marked in the afternoon, cough accompanied by excessive phlegm, expectoration of blood or blood-stained sputum, and debilitating disease for thousands of years in Eastern countries (Heo, 2007>). It has been shown to have anti-inflammatory properties and to alter the immune response (Kim et al., 2004; Jung et al., 2010). However, no simultaneous analysis of JGT components has been reported. Therefore, we conducted simultaneous determination of major components for quality control of JGT. High-performance liquid chromatography (HPLC) coupled with photodiode array (PDA) detection is a convenient, widely used, and powerful approach for the rapid separation and identification of multiple components in herbal extracts and plants important in traditional Chinese medicine (Zhang et al., 2004; Park et al., 2009). The present study was performed as a quantitative determination and method validation of seven components of JGT, comprising 5-hydroxymethyl-2-furaldehyde (5-HMF, 1), albiflorin (2), paeoniflorin (3), liquiritin (4), nodakenin (5), hesperidin (6), and glycyrrhizin (7) (Figure 1), using the HPLC-PDA method.
Figure 1.
Chemical structures of seven components of Jaeumganghwa-tang.
Materials and Methods
Chromatographic system
The HPLC system consisted of a Shimadzu LC-20A HPLC system (Shimadzu Co., Kyoto, Japan) with a solvent delivery unit, on-line degasser, column oven, autosampler, and PDA detector. The data processor used LCsolution software (Version 1.24, Shimadzu, Kyoto, Japan). The column used was a Gemini C18 analytical column (250×4.6 mm; particle size 5 µm; Phenomenex, Torrance, CA, USA). The mobile phases were solvent A (1.0% v/v aqueous acetic acid) and solvent B (acetonitrile with 1.0% v/v acetic acid). The gradient conditions are shown in Table 1. Column temperature was maintained at 40°C. Analysis was performed at a flow rate of 1.0 mL/min and monitored at 230 nm (for albiflorin and paeoniflorin), 254 nm (for glycyrrhizin), 280 nm (for 5-HMF, liquiritin, and hesperidin), and 330 nm (for nodakenin). The injection volume was 10 µL.
Table 1.
Solvent gradient conditions for HPLC analysis.
1.0% (v/v) aqueous acetic acid.
1.0% (v/v) acetic acid in acetonitrile.
Materials
JGT samples consisting of 13 herbal medicines, Paeoniae Radix, Angelicae Gigantis Radix, Rehmanniae Radix Preparata, Asparagi Tuber, Atractylodis Rhizoma Alba, Liriopis Tuber, Rehmanniae Radix Crudus, Citri Unshius Pericarpium, Anemarrhenae Rhizoma, Phellodendri Cortex, Glycyrrhizae Radix et Rhizoma, Zingiberis Rhizoma Crudus, and Zizyphi Fructus, were purchased from Omniherb (Yeongcheon, Korea) and HMAX (Jecheon, Korea). The origin of the samples was confirmed taxonomically by Prof. Je Hyun Lee and Young Bae Seo, Dongguk University, Gyeongju, Korea and Daejeon University, Daejeon, Korea, respectively. A voucher specimen (2008-KE01-1 through KE01-13) has been deposited at the Basic Herbal Medicine Research Group, Korea Institute of Oriental Medicine.
Reagents
The reference components 5-HMF and hesperidin were purchased from Sigma-Aldrich (St Louis, MO, USA) and Chengdu Biopurify Phytochemicals Ltd (Chengdu, China), respectively. Albiflorin, paeoniflorin, and glycyrrhizin were obtained from Wako (Osaka, Japan). Liquiritin and nodakenin were purchased from NPC BioTechnology Inc. (Daejeon, Korea). The purity of all reference standards was >98.0%. HPLC-grade methanol, acetonitrile, and water were obtained from J.T. Baker (Phillipsburg, NJ, USA). Glacial acetic acid was of analytical reagent grade and procured from Junsei (Tokyo, Japan).
Preparation of standard solutions
Standard stock solutions of 5-HMF, albiflorin, paeoniflorin, liquiritin, nodakenin, hesperidin, and glycyrrhizin (all at 1,000 µg/mL) were prepared in methanol and stored below 4°C. Working standard solutions were prepared by serial dilution of stock solutions with methanol.
Preparation of sample solutions
A decoction of JGT was prepared in our laboratory (Table 2, 20.01 kg; 43.125 g × 464) from a mixture of chopped crude herbs, and extracted in distilled water (10 times the sample amount) at 100°C for 2 h. The solution was evaporated to dryness and freeze-dried (4.163 kg). The yield of JGT extract was 20.8%. Lyophilized JGT extract (200 mg) was dissolved in distilled water (20 mL) and mixed. The solution was filtered through a SmartPor GHP syringe filter (0.2 µm pore size; Woongki Science, Seoul, Korea).
Table 2.
Crude components of Jaeumganghwa-tang.
| Scientific name | Latin name | Amount (g) |
Company of purchase |
Source |
| Paeonia lactiflora | Paeoniae Radix | 4.875 | Omniherb | Hwasun, Korea |
| Angelica gigas | Angelicae Gigantis Radix | 4.5 | Omniherb | Pyeongchang, Korea |
| Rehmannia glutinosa | Rehmanniae Radix Preparata | 3.75 | Omniherb | Jangheung, Korea |
| Asparagus cochinchinensis | Asparagi Tuber | 3.75 | HMAX | China |
| Atractylodes japonica | Atractylodis Rhizoma Alba | 3.75 | Omniherb | China |
| Liriope platyphylla | Liriopis Tuber | 3.75 | Omniherb | Yeongcheon, Korea |
| Rehmannia glutinosa | Rehmanniae Radix Crudus | 3.00 | Omniherb | Jangheung, Korea |
| Citrus unshiu | Citri Unshius Pericarpium | 2.625 | Omniherb | Jeju, Korea |
| Anemarrhena asphodeloides | Anemarrhenae Rhizoma | 1.875 | Omniherb | China |
| Phellodendron amurense | Phellodendri Cortex | 1.875 | Omniherb | China |
| Glycyrrhiza uralensis | Glycyrrhizae Radix et Rhizoma | 1.875 | HMAX | China |
| Zingiber officinale | Zingiberis Rhizoma Crudus | 3.75 | Omniherb | Yeongcheon, Korea |
| Zizyphus jujube | Zizyphi Fructus | 3.75 | Omniherb | Yeongcheon, Korea |
| Total amount | 43.125 | |||
Linearity, limits of detection (LOD) and quantification (LOQ)
All calibration curves were obtained by assessment of peak areas from standard solutions in the following concentration ranges: 5-HMF and albiflorin, 0.94–30.00 µg/mL; paeoniflorin, 1.56–100.00 µg/mL; liquiritin and nodakenin, 0.39–50.00 µg/mL; hesperidin, 0.78–100.00 µg/mL; and glycyrrhizin, 1.56–50.00 µg/mL. The LOD and LOQ data obtained under the chromatographic conditions used in the present study were determined using signal-to-noise (S/N) ratios of 3 and 10, respectively.
Recovery
Recovery tests were performed by adding known amounts (low, medium, and high) of reference standards to JGT samples before extraction. An average recovery was calculated using the formula: Recovery (%) = (Amountdetermined − Amountoriginal)/Amountspiked × 100.
Precision and accuracy
Reproducibility was assessed by analysis of five independently prepared standard solutions. The relative standard deviation (RSD) of analyte peak areas and peak retention times for each standard were calculated. Intra- and inter-day precision values were determined using a standard addition method to prepare spiked samples, employing both standards and controls.
Results and Discussion
Optimization of chromatographic conditions
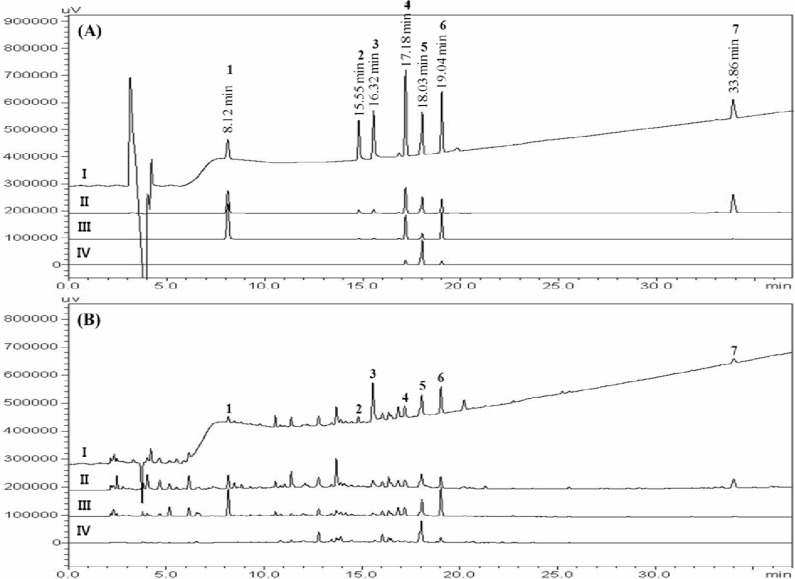
We obtained good separation chromatograms using mobile phases consisting of (A) 1.0% (v/v) aqueous acetic acid and (B) acetonitrile with 1.0% (v/v) acetic acid, with a gradient flow of 0–40 min, 5–70% B; 40–45 min, 70–100% B; 45–50 min, 100% B; 50–55 min, 100–5% B; and 55–70 min, 5% B. Quantitation was achieved by PDA detection in the region 190–400 nm, based on retention time and UV spectra compared with those of the standard. Using optimized chromatography conditions, all analytes eluted before 35 min, showed a resolution better than 1.75, and afforded good specificity upon sample analysis. Representative HPLC chromatograms of standards and the extract are shown inFigure 2.
Figure 2.
HPLC chromatographs of a standard mixture (A) of seven components and Jaeumganghwa-tang sample (B) with detection at 230 nm (I), 254 nm (II), 280 nm (III), and 330 nm (IV). The peaks represent 5-HMF (1), albiflorin (2), paeoniflorin (3), liquiritin (4), nodakenin (5), hesperidin (6), and glycyrrhizin (7).
Linearity, range, LOD, and LOQ
Calibration curves were obtained using standard solutions containing 0.94–30.00 µg/mL of 5-HMF and albiflorin; 1.56–100.00 µg/mL of paoniflorin; 0.39–50.00 µg/mL of liquiritin and nodakenin; 0.78–100.00 µg/mL of hesperidinorin; and 1.56–50.00 µg/mL of glycyrrhizinn, as marker components. The linearity of the peak area (y) versus concentration (x, µg/mL) curve for each component was used to calculate the content of each main component in JGT. The correlation coefficients (r2) of the calibration curves for seven constituents were >0.9998. Line equations and correlation coefficients (r2) of calibration curves are summarized in Table 3. The LODs and LOQs were in the ranges 0.021–0.275 µg/mL and 0.069–0.918 µg/mL, respectively. These data are shown in Table 3.
Table 3.
Linearities, correlation coefficients, LODs, and LOQs for marker compounds (n = 3).
| Compound | Linear range (µg/mL) |
Regression equationa | Correlation coefficient (r2) |
LODb (µg/mL) |
LOQc (µg/mL) |
| 5-HMF (1) | 0.94–30.00 | Y = 80,959.89x − 10,045.13 | 0.9999 | 0.021 | 0.069 |
| Albiflorin (2) | 0.94–30.00 | Y = 8,070.68x − 1,989.45 | 0.9999 | 0.235 | 0.783 |
| Paeoniflorin (3) | 1.56–100.00 | Y = 11,588.34x − 10,855.84 | 0.9998 | 0.255 | 0.867 |
| Liquiritin (4) | 0.39–50.00 | Y = 16,346.25x − 2,116.57 | 0.9999 | 0.069 | 0.229 |
| Nodakenin (5) | 0.39–50.00 | Y = 20,991.73x − 2,426.98 | 0.9999 | 0.048 | 0.160 |
| Hesperidin (6) | 0.78–100.00 | Y = 18,380.79x − 4,931.69 | 0.9999 | 0.060 | 0.200 |
| Glycyrrhizin (7) | 1.56–50.00 | Y = 8,236.28x − 1,681.31 | 0.9999 | 0.275 | 0.918 |
Y: peak area (mAU) of components; x: concentration (µg/mL) of components.
LOD = 3 × signal-to-noise (S/N) ratio.
LOQ = 10 × signal-to-noise (S/N) ratio.
Recovery
A recovery test was performed by addition of known amounts of 5-HMF, albiflorin, paeoniflorin, liquiritin, nodakenin, hesperidin, and glycyrrhizin to the extract. Standard compounds, at each of three different levels (low, medium, and high), were mixed with sample powder, and extracted. The recovery of each reference standard was in the range 91.47–102.62%, and the RSD range was 0.19–4.24% (Table 4).
Table 4.
Recovery levels of the seven marker compounds (n = 5).
| Compound | Original mean level (µg/mL) |
Spiked level (µg/mL) |
Detected mean level (µg/mL) |
Recovery mean (%) |
RSD (%) |
| 2.00 | 9.55 | 102.62 | 0.96 | ||
| 5-HMF (1) | 7.50 | 5.00 | 12.64 | 98.86 | 4.19 |
| 10.00 | 17.00 | 94.97 | 0.38 | ||
| 2.00 | 12.87 | 98.96 | 2.28 | ||
| Albiflorin (2) | 10.89 | 5.00 | 15.89 | 99.81 | 1.67 |
| 10.00 | 20.41 | 95.14 | 2.60 | ||
| 10.00 | 56.26 | 99.35 | 2.31 | ||
| Paeoniflorin (3) | 46.33 | 25.00 | 70.70 | 97.48 | 1.83 |
| 50.00 | 94.47 | 96.28 | 2.04 | ||
| 2.00 | 16.62 | 100.53 | 2.36 | ||
| Liquiritin (4) | 14.60 | 5.00 | 19.64 | 100.63 | 1.12 |
| 10.00 | 24.14 | 95.32 | 0.36 | ||
| 4.00 | 27.78 | 96.66 | 2.59 | ||
| Nodakenin (5) | 23.92 | 10.00 | 33.29 | 93.74 | 1.65 |
| 20.00 | 43.36 | 97.21 | 2.73 | ||
| 6.00 | 39.04 | 102.53 | 1.08 | ||
| Hesperidin (6) | 32.88 | 15.00 | 46.60 | 91.47 | 0.26 |
| 30.00 | 61.27 | 94.62 | 0.18 | ||
| 4.00 | 20.51 | 100.77 | 1.78 | ||
| Glycyrrhizin (7) | 16.48 | 10.00 | 25.96 | 94.86 | 0.24 |
| 20.00 | 35.16 | 93.41 | 0.53 | ||
Accuracy and precision
Reproducibility or intra-assay precision was assessed by repeatedly measuring retention times and peak areas for three independently prepared samples of analyte. Reproducibility for all analytes was less than RSD 1.0% for peak responses and less than RSD 0.1% for retention times (data not shown). Thus, the HPLC assay showed good repeatability under optimized conditions. To test the accuracy and precision of our analytical method, intra-and inter-day variations in measurement of seven major constituents were determined, and are summarized in Table 5. The intra-day accuracy was in the range 97.32–106.66%, and inter-day accuracy was 96.77–107.43%.
Table 5.
Precision and accuracy of analytical results (n = 5).
| Compound | Fortified conc. (µg/mL) |
Intra-day | Inter-day | ||||
| Observed conc. (µg/mL) |
Precision (%) |
Accuracy (%) |
Observed conc. (µg/mL) |
Precision (%) |
Accuracy (%) |
||
| 2.00 | 2.01 | 1.13 | 100.44 | 2.02 | 1.10 | 100.82 | |
| 5-HMF (1) | 5.00 | 5.25 | 0.38 | 104.93 | 5.27 | 0.98 | 105.35 |
| 10.00 | 9.88 | 0.11 | 98.75 | 9.86 | 0.23 | 98.63 | |
| 2.00 | 2.04 | 2.77 | 102.11 | 2.00 | 2.03 | 99.94 | |
| Albiflorin (2) | 5.00 | 5.15 | 1.78 | 102.96 | 5.17 | 2.22 | 103.32 |
| 10.00 | 9.92 | 0.46 | 99.18 | 9.92 | 0.51 | 99.17 | |
| 10.00 | 10.10 | 1.04 | 100.97 | 10.14 | 1.22 | 101.42 | |
| Paeoniflorin (3) | 25.00 | 25.20 | 0.74 | 100.79 | 25.17 | 1.77 | 100.66 |
| 50.00 | 49.88 | 0.15 | 99.76 | 49.89 | 0.44 | 99.78 | |
| 2.00 | 1.97 | 1.54 | 98.75 | 2.01 | 2.04 | 100.56 | |
| Liquiritin (4) | 5.00 | 5.23 | 0.93 | 104.69 | 5.18 | 0.82 | 103.64 |
| 10.00 | 9.89 | 0.20 | 98.88 | 9.91 | 0.14 | 99.07 | |
| 4.00 | 4.19 | 2.02 | 104.81 | 4.06 | 1.87 | 101.58 | |
| Nodakenin (5) | 10.00 | 10.41 | 2.85 | 104.13 | 9.75 | 2.83 | 97.46 |
| 20.00 | 19.90 | 0.81 | 99.49 | 20.11 | 0.64 | 100.57 | |
| 6.00 | 6.40 | 1.07 | 106.66 | 6.45 | 0.71 | 107.43 | |
| Hesperidin (6) | 15.00 | 14.60 | 0.28 | 97.32 | 14.52 | 0.30 | 96.77 |
| 30.00 | 30.12 | 0.06 | 100.40 | 30.15 | 0.06 | 100.51 | |
| 4.00 | 4.18 | 1.38 | 104.53 | 4.17 | 1.04 | 104.37 | |
| Glycyrrhizin (7) | 10.00 | 10.07 | 1.32 | 100.73 | 10.05 | 0.40 | 100.46 |
| 20.00 | 19.93 | 0.28 | 99.64 | 19.94 | 0.07 | 99.71 | |
Sample analysis
This newly established analytical method was applied to simultaneous determination of seven components in JGT. Figure 2 shows chromatograms of reference components and a water extract of JGT, with detection of eluents at 230 nm, 254 nm, 280 nm, and 330 nm. The contents of seven constituents, 5-HMF, albiflorin, paeoniflorin, liquiritin, nodakenin, hesperidin, and glycyrrhizin were 0.74 mg/g, 1.09–1.11 mg/g, 5.44–5.48 mg/g, 1.38–1.43 mg/g, 2.37–2.46 mg/g, 3.24–3.29 mg, and 1.67–1.68 mg/g, respectively. The analytical results for each component identified are summarized in Table 6.
Table 6.
Contents of seven marker compounds in Jaeumganghwa-tang (n = 3).
| Batch (#) | Content (mg/g) | |||||||||||
| 5-HMF (1) | Albiflorin (2) | Paeoniflorin (3) | Liquiritin (4) | |||||||||
| Mean | SD | RSD (%) | Mean | SD | RSD (%) Mean | SD | RSD (%) | Mean | SD | RSD (%) | ||
| 1 | 0.74 | 0.002 | 0.258 | 1.11 | 0.005 | 0.418 | 5.44 | 0.022 | 0.408 | 1.38 | 0.011 | 0.799 |
| 2 | 0.74 | 0.003 | 0.424 | 1.10 | 0.002 | 0.162 | 5.48 | 0.020 | 0.372 | 1.43 | 0.010 | 0.727 |
| 3 | 0.74 | 0.003 | 0.361 | 1.09 | 0.002 | 0.218 | 5.46 | 0.034 | 0.617 | 1.43 | 0.004 | 0.285 |
| Batch (#) | Content (mg/g) | ||||||||
| Nodakenin (5) | Hesperidin (6) | Glycyrrhizin (7) | |||||||
| Mean | SD | RSD (%) | Mean | SD | RSD (%) | Mean | SD | RSD (%) | |
| 1 | 2.39 | 0.019 | 0.802 | 3.24 | 0.027 | 0.845 | 1.68 | 0.005 | 0.309 |
| 2 | 2.46 | 0.009 | 0.366 | 3.29 | 0.031 | 0.947 | 1.67 | 0.006 | 0.345 |
| 3 | 2.34 | 0.015 | 0.642 | 3.27 | 0.033 | 1.018 | 1.67 | 0.005 | 0.326 |
In conclusion, we developed a simple and accurate HPLC method for simultaneous separation and determination of seven components, in order to evaluate the quality of JGT. This study is the first report of the simultaneous analysis of seven constituents in JGT using HPLC-PDA detection. In the present work, simultaneous determination of seven marker compounds in JGT was validated with respect to linearity, precision, and accuracy. The method will be helpful to improve the quality control and analysis of JGT.
Acknowledgments
This research was supported by a grant (no. K12030) from the Korea Institute of Oriental Medicine.
References
- 1.Hur J. Donguibogam. 8th ed. Namsandang, Seoul: 2007. p. 424. [Google Scholar]
- 2.Jiang WY. Therapeutic wisdom in traditional Chinese medicine: a perspective from modern science. Trends Pharmacol Sci. 2005;26:558–563. doi: 10.1016/j.tips.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Jung D, Ha H, Lee HY, Lee JA, Lee JK, Huang DS, Shin HK. Stimulation of the immune response by Yin-Tonifying formula. J Korean Oriental Med. 2010;31:112–123. [Google Scholar]
- 4.Kim YK, Kim HJ, Kim WS, Park HJ, Moon G, Kim DW, Won JH. Inhibitory effect of Jaeumganhwa-tang on allergic inflammatory reaction. Korean J Orient Inter Med. 2004;25:174–182. [Google Scholar]
- 5.Liu S, Yi LZ, Liang YZ. Traditional Chinese medicine and separation science. J Sep Sci. 2008;31:2113–2137. doi: 10.1002/jssc.200800134. [DOI] [PubMed] [Google Scholar]
- 6.Park AY, Park SY, Lee J, Jung M, Kim J, Kang SS, Youm JR, Han SB. Simultaneous determination of five coumarins in Angelicae dahuricae Radix by HPLC/UV and LC-ESI-MS/MS. Biomed Chromatogr. 2009;23:1034–1043. doi: 10.1002/bmc.1219. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H, Shen P, Cheng Y. Identification and determination of the major constituents in traditional Chinese medicine Si-Wu-Tang by HPLC coupled with DAD and ESI-MS. J Pharm Biomed Anal. 2004;34:705–713. doi: 10.1016/s0731-7085(03)00650-2. [DOI] [PubMed] [Google Scholar]