Abstract
As part of our search for natural antioxidants, this work presents an evaluation of antioxidant activities of methanolic extract of Oxalis corniculata and its sub-fractions in hexane, chloroform, ethyl acetate, n-butanol and water. The total phenolic contents in terms of µg of gallic acid equivalents per mg of dried mass were approximately 21.0, 28.2, 34.5, 162.0, 70.0, and 49.2 in methanolic, hexane, chloroform, ethyl acetate, n-butanolic and aqueous fractions respectively, while the flavonoid contents in these solvents were 362.4, 214.1, 317.1, 177.1, 98.8 and 53.5 respectively in terms of µg of rutin per mg of dried mass. In DPPH assay, the ethyl acetate fraction showed the highest free radical scavenging activity, 24.0% with 1 mg/mL concentration. The second strongest fraction was chloroform (21.5%). The EC50 and TEC50 values of the methanolic extract were 3.63 mg/mL and 23 min respectively. The FRAP values in terms of µg of ascorbic acid equivalents per mg of dried mass for these solvents were 288.0, 1705.3, 437.1, 72.0, 28.0, and 44.0 respectively while total antioxidant activity measured by phosphomolybdate assay in terms of µg of ascorbic acid equivalents per mg of dried mass were 50.0, 117.0, 78.6, 57.8, 3.4 and 8.3 respectively. All the samples showed remarkable ability to inhibit lipid peroxidation exhibiting much better and sustainable peroxidation inhibitory activity than the standard butylated hydroxyanisole.
Keywords: Oxalis corniculata, antioxidant, free radical, phenolics, flavonoids
Introduction
During the metabolic process in human body a number of free radicals are formed which can cause harmful effects leading to various diseases, such as, cardiovascular ailments, inflammation, cancer, neural disorders, Alzheimer disease, arteriosclerosis, allergies, and aging (Tiganis, 2011; Oktay and Gulcin, 2003; Niki et al., 1994; Cook and Samman, 1996; Sohal, 2002; Choi et al., 2002; Finkel and Holbook, 2000). To control these reactive free radicals, a number of synthetic antioxidants are available but they have harmful side effects (Rechner et al., 2002). There is, thus, a dire need of natural antioxidants which are not only safer but also cheaper and more easily available to a common person (Madsen, 1995; Lim and Murtiyaja, 2007). Plant kingdom that possesses a virtually unending reservoir of therapeutic and nutraceutical agents must, therefore, be explored for chemical principles having antioxidant properties (Mishra et al., 2007).
Oxalis corniculata (Oxalidaceae) is a cosmopolitan herb and abundantly found in agricultural farms, gardens and lawns, etc. (Lourteig, 2000; Mabberley, 2008). In Pakistan, the plant is distributed through out the country (Nasir, 1971). It is a small perennial herb with leaves having sour taste, and locally known as khatcorla or khati buti in Hindko (Shah and Khan, 2006b). In folklore, the plant is used to cure a number of diseases such as indigestion and stomach disorders, liver and bladder inflammation and jaundice (Qureshi et al., 2008; Hussain et al. 2008; Abbasi et al., 2010). Fresh leaves are also applied as antiscorbutic (Shah and Khan, 2006b), aperient, diaphoretic, diuretic, antidiabetic, enthelminthic (Shah and Khan, 2006a). The crushed fresh leaves are used to stop bleeding from wounds (Hebbar, et al., 2004).
Oxalis corniculata has also been reviewed (Badwaik et al., 2011; Kathiriya et al., 2010) highlighting its diverse ethnomedicinal applications and research studies carried out on the plant so far. The plant has been reported to contain flavonoids, tannins, phytosterol, phenolics, glycosides, fatty acids, volatile oil, vitamin C and oxalates (Badwaik et al., 2011). Sakat et al. (2009) evaluated phenolics and flavonoids contents of the defatted methanolic extract of Oxalis corniculata from Mumbai, India, and analyzed the antioxidant properties of the extract using DPPH and nitric oxide free radical scavenging assays. Reddy et al. (2010) also investigated the antioxidant properties of the methanolic extract of the plant from Talakona (India) using DPPH assay and reducing power method. More recently, Alam et al. (2011) conducted a more detailed evaluation of the antioxidant activities of the plant from Khulna, Bangladesh. The study was conducted on crude methanolic extract and its sub-fractions in dichloromethane, ethyl acetate, and n-butanol to evaluate phenolic and flavonoid contents, total antioxidant activity, DPPH free radical scavenging, nitric oxide radical scavenging, and reducing power activity. In these studies, the crude methanolic extract was obtained through Soxhlet extraction.
In view of its extensive ethnomedicinal application by the local people, we investigated the antioxidant properties of the Pakistani variety of Oxalis corniculata. In the study, a different methodology was used to obtain the crude methanolic extract and its fractions in various solvents.
Materials and Methods
Chemicals
Ascorbic acid and DPPH [1,1-diphenyl-2-picrylhydrazyl] radical were purchased from MP biomedicals, Inc. (France). Rutin, Linoleic acid and Folin-Ciocalteu reagent were purchased from Sigma Aldrich. Butylated hydroxyanisole (BHA) and Tween-20 were obtained from Merck (Darmstadt, Germany). Gallic acid was purchased from Scharlau-Switzerland and 2,4,6-(2-tripyridyl)-S-triazine (TPTZ) was obtained from Alfa-Aesar, (Germany). All other solvents and chemicals used were of analytical grade.
Plant material and Preparation of plant extract and fractions
The aerial parts of the herb, Oxalis corniculata, were collected from the hilly area near Abbottabad, Hazara (Pakistan) in June 2010 and identified by the taxonomist. The plant was cleaned and air-dried in the shade for ten days and then ground to obtain a fine powder. The powdered material (100 g) was soaked in 500 mL of methanol and kept at room temperature for ten days. The extract was filtered and the solvent was evaporated from the filtrate under reduced pressure with rotary evaporator to obtain crude methanolic (MeOH) extract. The MeOH extract (1.90 g) was suspended in double distilled water and extracted successively with hexane, chloroform (CHCl3), ethyl acetate (EtOAc) and n-butanol (n-BuOH). In this way, six samples were obtained, namely, MeOH extract, and hexane, CHCl3, EtOAc, n-BuOH and aqueous (H2O) fractions. They were dried under reduced pressure, weighed and kept in refrigerator until further use.
Total phenolic content
The total phenolic content in the crude methanolic extract and its sub-fractions of O. corniculata was determined according to a well-cited protocol (Singleton and Rossi, 1965). Briefly, 1.5 mg of an extract was dissolved in 5 mL of methanol from which 40 µL was taken and dissolved in 3.16 mL of distilled water. To this 200 µL of Folin-Ciocalteu reagent was mixed and, after an interval of 8 min, 20% of 600 µL sodium carbonate solution was added. The mixture was incubated at 40 °C for 30 min and absorbance was measured at 765 nm on UV/Visible spectrophotometer. The standard calibration curve was prepared with gallic acid standard solution (50 to 500 mg/L) following the same procedure. The gallic acid equivalent (GAE) was determined from the following equation of the standard curve and the results were expressed as µg of GAE per mg of dried sample.
GAE = [Absorbance (765 nm) / 0.0009567] + 0.02091, R2 0.9995.
Total flavonoid content
The total flavonoid contents in the samples were determined following the method reported by Park et al. (2008). In each experiment, 1.5 mg of methanolic extract or a fraction was dissolved in 5 mL of methanol, from which 300 µL were transferred into 3.4 mL of 30% aqueous methanol. To this mixture, 150 µL each of 0.5M NaNO2 and 0.3 M AlCl3.6H2O were added and mixed thoroughly. After the interval of 5 min, 1 mL of 1 M NaOH was also added. The absorbance was observed immediately at 506 nm against a blank. The standard curve was plotted using rutin as a standard following the same method. The results were expressed as µg of Rutin per mg of dried mass as obtained from the following equation.
Rutin Equivalent (RE) = Absorbance (506 nm) / 0.0002428 − 0.008141, R2 0.9987.
Free radical scavenging activity by DPPH assay
The free radical scavenging activities of the samples by DPPH method were determined according to the method reported by Brand-Williams et al. (1995). In this method, 2.4 mg of DPPH free radical were dissolved in 100 mL of methanol to prepare its stock solution, which was kept at 20 °C until required. The working solution of DPPH was obtained by diluting its stock solution with methanol till the absorbance was noted to be 0.980 ± 0.02 at 517 nm. Then, 3 mL of the working solution was mixed with 100 µL of a sample (1 mg/mL). After incubating the mixture in the dark for 30 min, absorbance was measured at 517 nm. The scavenging activity was calculated by using the formula:
% inhibition= [(Absorbance of blank - Absorbance of sample)/Absorbance of blank] × 100
The blank contained all reagents except the test sample. In order to establish the relationship between concentration of methanolic extract and its free radical scavenging activity, solutions of extract with different concentrations were prepared and their activities were studied. The EC50 value was also determined. To determine the variation in free radical scavenging activity of the extract with time, %DPPH remaining was also found out using the following formula:
Where [DPPH]T=0 is the concentration of DPPH radical before reaction with antioxidant sample and [DPPH]T=t is the concentration of DPPH radical after reaction with antioxidant sample at any time t. From the data, TEC50 was also calculated which is the time required for 50% inhibition of DPPH by the extract.
Reducing power by FRAP assay
The ferric reducing antioxidant power (FRAP) assay was carried out according to the protocol reported by Benzie and Strain (1999). The FRAP reagent is prepared as follows: 300 mM sodium acetate buffer (pH 3.6, 25 mL), 10 mM TPTZ solution (2.5 mL) in 40 mM HCl solution (20 mL) and 20 mM FeCl3.6H2O solution (2.5 mL) were mixed thoroughly and kept at 37 °C throughout the experiment. After initial incubation for 10 min, the absorbance of the reagent was noted at 593 nm. For a test, 3 mL of FRAP reagent was added to 100 µL of sample (1 mg/mL) and absorbance was noted at 593 nm after 5 min. The blank was prepared by dissolving 100 µL of methanol in 3 mL of FRAP reagent. Ascorbic acid was used as a standard antioxidant and the same procedure was employed to determine its FRAP values for different concentrations and a calibration curve was plotted. The results were expressed in terms of µg of ascorbic acid equivalents (AAE) per mg of the dried mass, which was determined from the following equation;
Ascorbic Acid Equivalent = [(Absorbance at 593 nm/0.002) − 0.004], R2 1
Total antioxidant activity by phosphomolybdate assay
To carry out phosphomolybdate assay, the procedure followed by Umamaheswari and Chatterjee (2008) was used. The phosphomolybdate reagent was prepared by mixing equal volumes (100 mL) of 0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate. Test samples were prepared by dissolving 1 mg of plant methanolic extract or any of its sub-fraction in 1 mL of methanol. Then, 0.1 mL of the sample was dissolved in 1 mL of reagent solution in a test tube which was capped with silver foil and incubated in water bath at 95 °C for 90 min. After cooling the sample to room temperature, the absorbance was observed at 765 nm against a blank. Ascorbic acid was used as a standard antioxidant. Various concentrations of ascorbic acid (50 to 500 mg/L) were prepared and the same protocol was carried out in order to plot a standard curve. The results were expressed as µg of ascorbic acid equivalent (AAE) per mg of the dried weight of the sample as determined from the equation of the standard calibration curve.
AAE = [(Absorbance (765 nm) / 0.0025) + 0.0593], R2 0.997
Lipid peroxidation inhibition
The lipid peroxidation assay was carried out following the protocol given by Mitsuda et al. (1966). To prepare an emulsion of linoleic acid, 155 µL of the acid were mixed with 175 µg of Tween-20, and the mixture was put into 50 mL of potassium phosphate buffer (pH 7). Meanwhile, 5 mg of a sample was dissolved in 1 mL of methanol. An aliquot of 100 µL was dissolved in 2.4 mL of potassium phosphate buffer (0.04 M, pH 7) and the mixture was put in 2.5 mL of linoleic acid emulsion. The mixture was incubated at 37 °C. The 100 µL of aliquot from the incubated solution was regularly taken at 24 h intervals and allowed to react with 100 µL each of 20 mM FeCl2 and 30% ammonium thiocyanate. The absorbance was noted at 500 nm after every 24 h interval for 4 days. A 5 mL solution consisting of equal quantities of linoleic acid emulsion and potassium phosphate buffer was taken as a blank. Butylated hydroxyanisole (BHA) was used as a standard and same procedure was followed to measure its ability to inhibit peroxidation in linoleic acid.
Statistical analysis
All experiments were carried out in triplicate and mean values were calculated. One way analysis of variance (ANOVA) was applied and the results were correlated.
Results and Discussion
Total phenolic content
The methanolic extract and fractions in various solvents of Oxalis corniculata were subjected to evaluation of total phenolic content and the results are shown in Table 1. In this study on the Pakistani variety of the plant, ethyl acetate (EtOAc) fraction showed the highest phenolic content (162.03 µg of Gallic Acid Equivalent pr mg of the dried mass, GAE), while the crude methanolic (MeOH) extract had the lowest value (20.91 GAE). The MeOH value was close to that reported earlier by Sakat et al. (2010) for the Indian plant. The phenolic content in various solvents decreases in the order of EtOAc > n-BuOH > H2O, the trend also exhibited by the Bangladeshi variety (Alam et al., 2011). However, the crude methanolic extract of Bangladeshi plant showed a higher value of total phenolics than both the Pakistani and Indian. The reason may lie in the methods of extraction as well as climatic conditions.
Table 1.
Percent yield, total flavonoid and total phenolic contents of Oxalis corniculata
| Extract/Fraction | Percent yield* | Total flavonoids in µg of **RE/mg of dried mass |
Total phenolics in µg of +GAE /mg of dried mass |
| MeOH | --- | 362.43 ± 9.26 | 20.91 ± 4.26 |
| Hexane | 29.73 | 214.14 ± 3.31 | 28.23 ± 2.08 |
| CHCl3 | 22.12 | 317.13 ± 7.50 | 34.50 ± 2.62 |
| EtOAc | 16.24 | 177.09 ± 5.89 | 162.03 ± 9.98 |
| n-BuOH | 14.51 | 98.82 ± 2.76 | 70.05 ± 7.30 |
| H2O | 15.40 | 53.52 ± 1.32 | 49.15 ± 4.45 |
The amount of crude methanolic extract was 1.90 g;
Rutin equivalents;
Gallic acid equivalents; Each value listed in the table is expressed as mean ± SD (n=3).
Total flavonoid content
Total flavonoid content of the methanolic extract and fractions in various solvents was determined in terms of µg of Rutin Equivalent per mg of the dried material (RE) and the results are shown in Table 1. In our study, the total flavonoid content decreases in the following order: MeOH > CHCl3 > Hexane > EtOAc > n-BuOH > H2O. The overall trend was the same as reported by Alam et al. (2011), however, MeOH extract in our study exhibited a much higher value.
In EtOAc, n-BuOH and H2O fractions, the phenolic and flavonoid contents showed the same trend; EtOAc being the highest and H2O the lowest. The CHCl3 fraction, however, showed much higher value of flavonoids (317.13 RE) than phenolics (34.50 GAE)
Free radical scavenging activity by DPPH assay
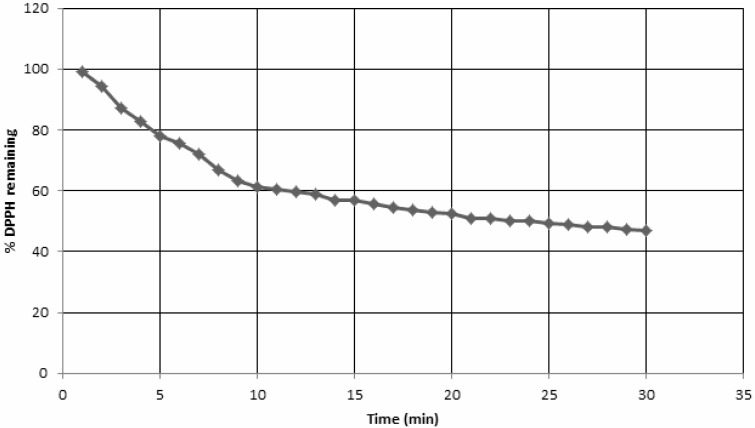
Free radical scavenging activities of the samples were determined using well-known DPPH assay and the results are displayed in Table 2. The DPPH is a stable free radical (DPPH•) that accepts an electron or a hydrogen radical to form diamagnetic molecule (Oktay et al., 2005; Nakayama, 1994). During the process, the purple colour of the radical turns pale yellow. The DPPH radical shows maximum absorbance at 517 nm. In the present study, the EtOAc fraction exhibited the highest capability to scavenge free radicals. This is in agreement with the data reported by Alam et al. (2011). While the CHCl3 fraction showed good antioxidant potential, the residual H2O fraction was completely inactive. The high free radical scavenging activity of EtOAc and CHCl3 fractions may be related to their high phenolic contents. This is in consonance with findings of other workers as well (Ahmed et al., 2010; Rahman et al., 2008). The hexane fraction proved to be a very weak free radical scavenger, which can be explained on the basis of its very low phenolic content (Table 1). The %DPPHremaining decreases with time, first rapidly but then slowly. The TEC50 value was 23 min which is the time required for 50% decrease in the concentration of DPPH radical. The effect of the change in concentration of the methanolic extract on DPPH radical was also determined, and there was an almost direct relationship between the concentration of the extract and its ability to scavenge radicals. The EC50 value was 3.63 mg/mL which is the concentration of the extract required to give 50% inhibition of free radical activity of DPPH (Baig et al., 2012).
Table 2.
Free radical scavenging by DPPH assay, reducing power by FRAP assay, total antioxidant activity by phosphomolybdate assay
| Extract / Fraction | %Free radical scavenging by DPPH assay* |
Reducing power by FRAP assay µg of **AAE/ mg of driend mass |
Total antioxidant activity by phosphomolybdate assay µg of **AAE/mg of dried mass |
| MeOH | 3.67 | 287.98 ± 5.26 | 49.80 ± 2.91 |
| Hexane | 1.68 | 1705.26 ± 9.57 | 117.00 ± 7.26 |
| CHCl3 | 21.45 | 437.13 ± 3.24 | 78.60 ± 2.20 |
| EtOAc | 24.03 | 71.98 ± 3.33 | 57.80 ± 8.10 |
| n-BuOH | 7.54 | 27.96 ± 0.98 | 3.40 ± 0.60 |
| H2O | 0.00 | 43.85 ± 1.27 | 8.30 ± 0.72 |
The amount of crude methanolic extract was 1.90 g;
Rutin equivalents;
Gallic acid equivalents; Each value listed in the table is expressed as mean ± SD (n=3).
Reducing power by FRAP assay
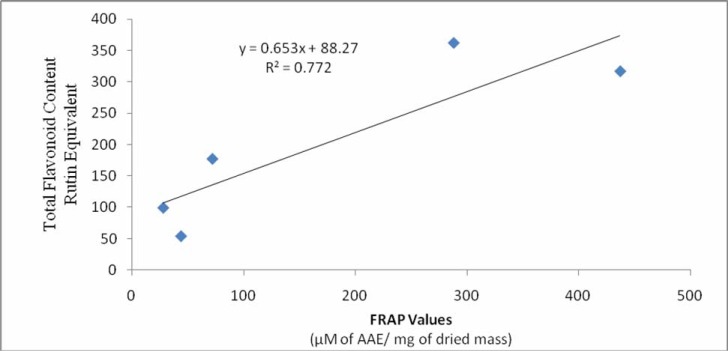
In the Ferric Reducing Antioxidant Power (FRAP) assay, ferric ions are reduced to ferrous ions in the presence of an antioxidant (or, a reducing agent) and a coloured ferrous tripyridyltriazine complex is formed at pH 3.6 which is monitored spectrophotometrically (λmax 593 nm) (Huang et al., 2005). The results of the FRAP assay in terms of Ascorbic Acid Equivalent (AAE) are shown in Table 2. The hexane fraction showed extraordinarily high reducing power (1705 AAE). The ability of the samples to reduce iron(III) to iron(II) decreases in the order of hexane > CHCl3 > MeOH > EtOAc >H2O > n-BuOH. The FRAP values of various samples show parallel behavior with the flavonoid content (Figure 3), explaining the possible role of flavonoids as potential antioxidants.
Figure 3.
The significant correlation was observed between the total flavonoid content and FRAP values expressed as rutin equivalent and ascorbic acid equivalent respectively.
Total antioxidant activity by phosphomolybdate assay
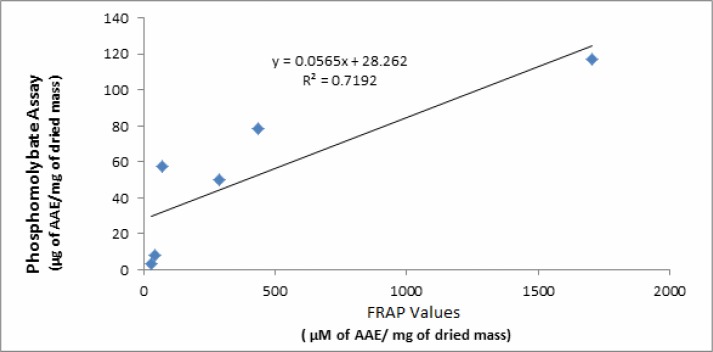
This assay is based on the reduction of molybdenum (VI) to molybdenum(V) which takes place in the presence of a reducing agent (antioxidant). The product is a green phosphomolybdate(V) complex whose formation is monitored with a spectrophotometer. The assay is often used to estimate total antioxidant activity of a sample and the results are expressed in terms of Ascorbic Acid Equivalents (AAE). The total antioxidant activity of various samples decreases in the following order: hexane > CHCl3 > EtOAc > MeOH >H2O > n-BuOH. There is a good correlation between the FRAP values and total antioxidant activity measured by phosphomolybdate assay (Figure 1). Here EtOAc fraction showed a higher value than n-BuOH fraction which is in agreement with the findings of Alam et al. (2011). In our study, the MeOH fraction showed much less antioxidant activity than the findings of Alam et al. (2011). The reason possibly lies in the method of extraction. Soxhlet extraction used by Alam et al. may have converted flavonoids into simpler phenolics as we had a much greater content of flavonoids than phenolics while the order in findings of Alam et al. (2011) is exactly the opposite.
Figure 1.
The significant correlation was observed between the antioxidant activities as determined by phosphomolybdate assay and FRAP assay.
Lipid peroxidation inhibition
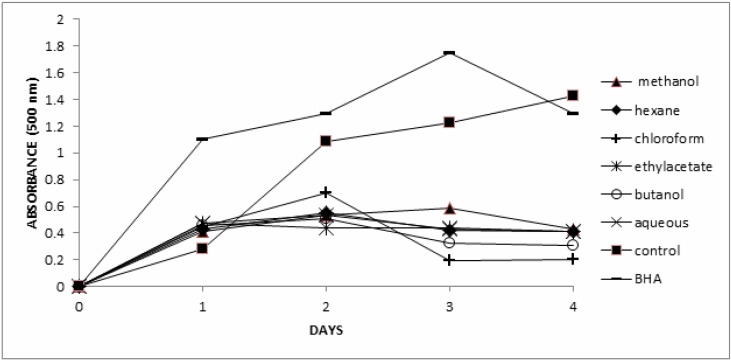
This assay measures the ability of a sample to inhibit peroxidation that may occur in lipids due to free radicals. Peroxidation of linoleic acid produces peroxyl free radicals, which are used to oxidize ferrous ions into ferric ions. The ferric ions so produced form coloured complex, ferric thiocyanate, with thiocyanate ions present in the medium. The formation of the complex is detected spectrophotometrically at 500 nm. Antioxidants will slow down the peroxidation in lipids resulting in low production of peroxides. As a result, there will be less oxidation of ferrous into ferric and therefore less amount of the complex will be formed. Thus, the stronger the antioxidant, the less is the formation of ferric thiocyanate and lower will be the absorbance. In our study, antioxidant activity of the plant samples was plotted as a function of time and compared with a standard antioxidant, BHA (butylated hydroxyanisole) and the results are displayed inFigure 4. The methanolic extract and the fractions in various solvents all showed remarkable ability to inhibit peroxidation in linoleic acid. The plant samples proved to be better antioxidants than BHA. Moreover, the samples remained almost equally effective through the whole duration of the experiment and at the end of the fourth day there was a slight increase in the activity. Oxalis corniculata, thus, contains active chemical substances having sustainable antioxidant activities. The CHCl3 fraction showed an interesting behavior; its activity first decreases reaching its lowest value after 48 hours, and then starts to increase ultimately acquiring a steady state after day 3.
Figure 4.
Lipid peroxidation assay. The change in absorbance of ferric thiocyanate in the present of various samples of Oxalis corniculata or BHA over 4 days. *Each value is the mean of triplicate determinations. Lower absorbance means a stronger antioxidant.
Conclusion
Oxalis corniculata is a common weed distributed throughout Pakistan. In ethnomedicine, it is used as cheap and easily accessible remedy to cure a number of diseases. The plant has good quantities of phenolics and flavonoids, the compounds known for their antioxidant properties. The methanolic extract and its sub-fractions in various solvents, in general, showed good antioxidant activities. Ethyl acetate fraction was found to be most active. Although the plant has already been investigated for its phytochemicals, there is a need for more extensive studies. Assay-guided isolation of natural products from the plant may result into the discovery of new and cheaper therapeutic agents.
Figure 2.
Effect of the methanolic extract of Oxalis corniculata on DPPH with time. %DPPH remaining decreases with time grdually reaching almost a steady state after 15 min.
Table 3.
Change of % inhibition of DPPH free radical with changing concentration of methanolic extract of Oxalis corniculata
| S.No | Concentration (mg/mL) | % Inhibition |
| 1 | 0.2 | 01.79 |
| 2 | 0.4 | 05.84 |
| 3 | 0.5 | 08.31 |
| 4 | 0.6 | 12.35 |
| 5 | 0.7 | 15.06 |
| 6 | 0.8 | 17.42 |
| 7 | 0.9 | 21.66 |
| 8 | 01 | 25.90 |
| 9 | 02 | 31.78 |
| 10 | 03 | 40.60 |
| 11 | 04 | 53.45 |
| 12 | 05 | 62.09 |
| 1 | 07 | 74.71 |
| 14. | 10 | 85.57 |
| 15 | 15 | 98.64 |
| EC50 (mg/mL) 3.63 | ||
References
- 1.Abbasi A M, Khan M A, Ahmed M, Zafar M. Herbal medicines used to cure various ailments by the inhabitants of Abbottabad district, North West Frontier Province, Pakistan. Indian Journal of Traditional Knowledge. 2010;9:175–183. [Google Scholar]
- 2.Ahmed D, Arshad MA, Ikram M, Asghar MN. Antioxidant and Free Radical Scavenging Potential of Otostegia limbata. Asian Journal of Chemistry. 2010;22(6):4524–4532. (2010) [Google Scholar]
- 3.Alam M B, Hossain M S, Chowdhury N S, Mazumder M E H, Haque M E, Islam A. In vitro and in vivo Antioxidant and Toxicity Evaluation of Different Fractions of Oxalis corniculata Linn. J Pharmacol Toxicol. 2011;6:337–348. [Google Scholar]
- 4.Badwaik H, Singh M K, Thakur D, Giri T K, Tripathi D K. The botany, chemistry, pharmacological and therapeutic application of Oxalis corniculata Linn.- A Review. International J of Phytomedicine. 2011;3:01–08. [Google Scholar]
- 5.Baig H, Ahmed D, Saman Zara S, Aujla MI, Asghar MN. In vitro Evaluation of Antioxidant Properties of Different Solvent Extracts of Rumex acetosella Leaves. Oriental Journal of Chemistry. 2012;27(4):1509–1516. [Google Scholar]
- 6.Benzie I F F, Strain JJ. The Ferric Reducing Ability of Plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP Assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 7.Brand-Williams W, Cuvelier M E, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss & Technol/Food Science and Technology. 1995;28:25–30. [Google Scholar]
- 8.Choi H R, Choi JS, Han Y N, Bae SJ, Chung H Y. Peroxynitrite scavenging activity of herb extracts. Phytotherapy Res. 2002;16:364–367. doi: 10.1002/ptr.904. [DOI] [PubMed] [Google Scholar]
- 9.Cook NC, Samman S. Flavonoids- chemistry, metabolism, cardioprotective effects, and dietary sources. J Nutr Biochem. 1996;7:66–76. [Google Scholar]
- 10.Finkel T, Holbook N J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 11.Hebbar SS, Harsha VH, Shripati V, Hedge G R. Ethnomedicine of Dharwad district of Karnataka India. J Ethnopharmacol. 2004;94:261–266. doi: 10.1016/j.jep.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 12.Huang D, Ou B, Prior R L. The chemistry behind antioxidant assays. J Agric Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 13.Hussain K, Shahazad A, Hussnain S Z. An Ethnobotanical Survey of Important Wild Medicinal Plants of Hattar District Haripur, Pakistan. Ethnobotanical Leaflets. 2008;12:29–35. [Google Scholar]
- 14.Kathiriya A, Das K, Kumar E P, Mathai K B. Evaluation of antitumor and antioxidant activity of oxalis corniculata linn. Against ehrlich ascites carcinoma on mice. Iranian journal of cancer prevention. 2010;4:157–165. [Google Scholar]
- 15.Kathiriya A K, Das K, Joshipura M, Mandal N. Oxalis corniculata Linn. -The Plant of Indian subtropics. Herbal Tech Industry. 2010:7–11. [Google Scholar]
- 16.Lim YY, Murtiyaja J. Antioxidant properties of Phyllanthus amarus extracts as affected by different drying methods. LWT -Food Science and Technology. 2007;40:1664–1669. [Google Scholar]
- 17.Lourteig A. Oxalis L., subgenera Monoxalis (Small) Lourt., Oxalis y Trifidus Lourt. Bradea. 2000;7:201–629. [Google Scholar]
- 18.Mabberley D J. The Plant-Book A portable dictionary of higher plants. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- 19.Madsen HL, Bertelsen G. Spices as antioxidants. Trends in Food Sci Technol. 1995;6:271–277. [Google Scholar]
- 20.Mishra J, Srivastava R K, Shukla S V, Raghav CS. Antioxidants in aromatic and medicinal plants. Science Tech Entreprenuer. 2007:9–10. [Google Scholar]
- 21.Mitsuda H, Yasumoto K, Iwami I. Antioxidative action of indole compounds during the autoxidation of linoleic acid. Eiyoto Shokuryo. 1966;19:210–214. [Google Scholar]
- 22.Nakayama T. Suppression of hydroperoxide-induced cytotoxicity by plyphenols. Cancer Res. 1994;54:1991s–1993s. [PubMed] [Google Scholar]
- 23.Nasir Y. In: Flora of Pakistan. Nasir Y, Ali SI, editors. 1971. No. 4 Oxalidaceae, Rawalpindi. [Google Scholar]
- 24.Niki E, Shimaski H, Mino M. Antioxidant-free radical and biological defense. Vol. 3. Tokyo: Gakkai Syuppn Center; 1994. [Google Scholar]
- 25.Oktay M, Gulcin I, Kufrevioglu OI. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. Lebensmittel-Wissenchaft und Technologie. 2003;36:263–271. [Google Scholar]
- 26.Park YS, Jung S T, Kang SG, Heo BK, Arancibia-Avila P, Toledo F, et al. Antioxidants and proteins in ethylene treated kiwifruits. Food Chemistry. 2008;107:640–648. [Google Scholar]
- 27.Qureshi S J, Khan M A, Ahmad M. A survey of useful medicinal plants of Abbottabad in northern Pakistan. Trakia Journal of Sciences. 2008;6:39–51. [Google Scholar]
- 28.Rahman A, Rahman M M, Sheikh M M I, Rahman M M, Shadli S M, Alam M F. Free Radical scavenging activity and phenolic content of Cassia sophera L. Afr J Biotechnol. 2008;7:1591–1593. [Google Scholar]
- 29.Rechner AR, Kuhnle G, Brember P, Hubbard G P, Moore K P, Rive-Evans CA. The metabolic fate of dietary polyphenols in humans. Free Radic Biol Med. 2002;33:220–235. doi: 10.1016/s0891-5849(02)00877-8. [DOI] [PubMed] [Google Scholar]
- 30.Reddy K Y, Kumar A S, Lakshmi S M, Angothu S. Antioxidasnt properties of methanolic extract of Oxalis corniculata. International Journal of Phytopharmacology. 2010;1:43–46. [Google Scholar]
- 31.Sakat SS, Juvekar A R, Gambhire M N. In vitro antioxidant and anti-infammatory activity of methanol extract of oxalis corniculata Linn. Int J Pharm and Pharmaceut Sci. 2010;2:146–155. [Google Scholar]
- 32.Shah G M, Khan M A. Check List of Medicinal Plants of Siran Valley Mansehra-Pakistan. Ethnobotanical Leaflets. 2006a;10:63–71. [Google Scholar]
- 33.Shah G M, Khan M A. Common Medicinal Folk Recipes of Siran Valley, Mansehra, Pakistan. Ethnobotanical Leaflets. 2006b;10:49–62. [Google Scholar]
- 34.Singleton V L, Rossi J A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- 35.Sohal S R. Role of oxidative stress and protein oxidation in the aging process. Free Radical Bio Med. 2002;33:37–44. doi: 10.1016/s0891-5849(02)00856-0. [DOI] [PubMed] [Google Scholar]
- 36.Tiganis T. Reactive oxygen species and insulin resistance: the good, the bad and the ugly. Trends in Pharmacological Sciences. 2011;32(2):82–89. doi: 10.1016/j.tips.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Umamaheswari M, Chatterjee T K. In vitro antioxidant activities of the fractions of Coccinia grandis L. leaf extract. African J Trad Compl Altern Medicines. 2008;5:61–73. [PMC free article] [PubMed] [Google Scholar]