Abstract
Eosinophilic gastroenteritis (EGE) is a rare disorder characterized by eosinophilic infiltration of the bowel wall with various gastrointestinal manifestations. Till date only 280 cases have been described in the literature. A high index of suspicion, by excluding other causes of peripheral eosinophilia, is a pre requisite for accurate diagnosis. EGE is an uncommon gastrointestinal disease affecting both children and adults. It was first described by Kaijser in 1937. Presentation may vary depending on location as well as depth and extent of bowel wall involvement and usually runs a chronic relapsing course. This condition can respond to low dose steroid therapy, thereby preventing grave complications like ascites and intestinal obstruction that might need surgical intervention. The natural history of EGE has not been well documented. Eosinophilic gastroenteritis is a chronic, waxing and waning condition. Mild and sporadic symptoms can be managed with reassurance and observation, whereas disabling gastrointestinal (GI) symptom flare-ups can often be controlled with oral corticosteroids. When the disease manifests in infancy and specific food sensitization can be identified, the likelihood of disease remission by late childhood is high. GI obstruction is the most common complication. Fatal outcomes are rare.
Keywords: Eosinophilic gastroenteritis, Unusual type, Review of literature
Core tip: Eosinophilic gastroenteritis is a rare disorder characterised by eosinophilic infiltration of the bowel wall and various gastrointestinal manifestations. Diagnosis requires a high index of suspicion and exclusion of various disorders that are associated with peripheral eosinophilia. Corticosteroids are the mainstay of therapy with a 90% response rate.
INTRODUCTION
Eosinophilic gastroenteritis is a rare disorder that can present with various gastrointestinal manifestations depending on the specific site and specific layer of the gastrointestinal tract involved. Majority of the cases involve stomach and proximal small bowel. The diagnostic criteria include demonstration of eosinophilic infiltration of bowel wall, lack of evidence of extra intestinal disease and exclusion of other causes of peripheral eosinophilia[1-4].
Eosinophilic gastroenteritis is characterized by the presence of abnormal gastrointestinal (GI) symptoms, most often abdominal pain, eosinophilic infiltration in one or more areas of the GI tract, defined as 50 or more eosinophils per high-power field, the absence of an identified cause of eosinophilia and the exclusion of eosinophilic involvement in organs other than the GI tract.
It can be classified into mucosal, muscular and serosal types based on the depth of involvement[5,6]. The stomach is the organ most commonly affected, followed by small intestine and colon[7,8]. The anatomical locations of eosinophilic infiltrates and the depth of GI involvement determine clinical symptoms. The therapeutic role of steroids and antihelminthic drugs in the treatment of eosinophilic gastroenteritis is not established. In a few cases, steroids have produced symptomatic improvement in controlling malabsorption syndrome[1,9].
EPIDEMIOLOGY
Eosinophilic gastroenteritis occurs over a wide age range from infancy through the seventh decade, but most commonly between third to fifth decades of life[10,11]. A slight male preponderance has been reported[12].
Although cases have been reported worldwide, the exact incidence of eosinophilic gastroenteritis is unclear. After first described by Kaijser[10], a little less than 300 cases have been reported in the literature. Kim et al[2] reported 31 new cases of eosinophilic gastroenteritis in Seoul, Korea, between January 1970 and July 2003.
Venkataraman et al[5] reported 7 cases of eosinophilic gastroenteritis over a 10-year period in India[5]. Chen et al[3] reported 15 patients including 2 children, with eosinophilic gastroenteritis in 2003. In eosinophilic enteritis the morbidity is mainly due to combination of chronic nonspecific GI symptoms which include abdominal pain, nausea, vomiting, diarrhea, weight loss, and abdominal distension and more serious complications like intestinal obstruction and perforation[13,14].
PATHOPHYSIOLOGY
Eosinophilic gastroenteritis can involve any part of gastrointestinal tract from esophagus down to the rectum. The stomach and duodenum are the most common sites of involvement[1,13-17]. The etiology and pathogenesis is not well understood. There is evidence to suggest that a hypersensitivity reaction may play a role. The clinical presentations of eosinophilic gastroenteritis vary according to the site and depth of eosinophilic intestinal infiltration. The presence of peripheral eosinophilia, abundant eosinophils in the gastrointestinal tract and dramatic response to steroids provide some support that the disease is mediated by a hypersensitivity reaction[1,18]. Moreover, a study at Mayo clinic showed that 50% of patients with eosinophilic gastroenteritis give history of allergy such as asthma, rhinitis, drug allergy and eczema[1]. Peripheral blood eosinophilia and elevated serum immunoglobulin E (IgE) are usual but not universal. The damage to the gastrointestinal tract wall is caused by eosinophilic infiltration and degranulation[19]. Eosinophils are normally present in gastrointestinal mucosa as a part of host defense mechanism, though the finding in deeper tissue is almost always pathologic[20]. In eosinophilic gastroenteritis (EGE) cytokines interleukin (IL)-3, IL-5 and granulocyte macrophage colony stimulating factor may be responsible for the recruitment and activation of eosinophils and hence the pathogenesis. They have been observed immunohistochemically in diseased intestinal wall[21]. In addition eotaxin has been shown to have an integral role in regulating the homing of eosinophils into the lamina propria of stomach and small intestine[22]. Indeed, many patients have history of food allergy and other atopic conditions like eczema, asthma etc. In this allergic subtype of disease, it is thought that food allergens cross the intestinal mucosa and trigger an inflammatory response that includes mast cell degranulation and recruitment of eosinophils[23,24].
CLINICAL PRESENTATIONS
The clinical presentations of eosinophilic gastroenteritis vary according to the site and depth of inflammatory involvement of different layers of the intestinal wall. Approximately 80% have symptoms for several years[25]. Occasionally, the disease may manifest itself as an acute abdomen or bowel obstruction[13,14]. Children and adolescents can present with growth retardation, failure to thrive, delayed puberty or amenorrhea. Adults have abdominal pain, diarrhea or dysphagia. Mucosal disease is the commonest variety that presents with features of protein losing enteropathy, bleeding or malabsorption. Failure to thrive and anaemia may also be present. Lower gastrointestinal bleeding may imply colonic involvement[1,26,27]. Involvement of muscle layer may cause bowel wall thickening and intestinal obstruction. Cramping and abdominal pain associated with nausea and vomiting occurs frequently. It can also present as an obstructing caecal mass or intussusception. The subserosal form, which is least common but can cause more morbidity, usually presents as eosinophilic ascites, which is usually an exudate, with abundant peripheral eosinophilia. Serosal and visceral peritoneal inflammation leads to leakage of fluids but has a more favourable response to corticosteroids. In literature features like cholangitis, pancreatitis[28], eosinophilic splenitis, acute appendicitis and giant refractory duodenal ulcer are also mentioned.
DIAGNOSTIC EVALUATION
Four criteria are required for the diagnosis of eosinophilic gastroenteritis namely-presence of gastrointestinal symptoms, eosinophilic infiltration of gastrointestinal tract, exclusion of parasitic disease and absence of other systemic involvement. The presence of peripheral eosinophilia is not a universal phenomenon[1,29].
A thorough evaluation of the patient is necessary, starting with laboratory evaluation.
After a detailed history and physical examination, a complete blood count plays an important role. Peripheral blood eosinophilia is found in 20%-80% of cases. Average count is 2000 eosinophils (eos)/μL in patients with mucosal layer involvement, 1000 eos/μL in patients with muscle layer involvement, and 8000 eos/μL in patients with serosal involvement. Iron-deficiency anemia may be evident on mean corpuscular volume. Serum albumin may be low, especially in patients with mucosal involvement.
Fecal protein loss can be assessed by measuring alpha1-antitrypsin in a 24-h feces collection. It is used to identify the inability to digest and absorb proteins in the GI tract. The normal value is 0-54 mg/dL. Patients with eosinophilic gastroenteritis have elevated alpha1-antitrypsin in their feces. Protein loss can also result in low levels of total immunoglobulins, but serum IgE could be elevated, which then strongly supports the diagnosis of eosinophilic gastroenteritis in conjunction with other findings. The erythrocyte sedimentation rate can be elevated in few cases.
Stool examination should be performed to rule out parasitic infestation. Mild-to-moderate steatorrhea is present in approximately 30% of patients. This can be measured by qualitative and quantitative stool tests. Skin prick tests help to identify sensitization to specific ingestant and/or inhalant allergens.
Computed tomography (CT) scan may show nodular and irregular thickening of the folds in the distal stomach and proximal small bowel, but these findings can also be present in other conditions like Crohn’s disease and lymphoma. On ultrasonography ascitic fluid is usually detected in patients with serosal involvement.
Radiographic changes are variable, nonspecific, and/or absent in at least 40% of patients. Gastric folds can be enlarged, with or without nodular filling defects. In extensive disease strictures, ulceration or polypoid lesions may occur and valvulae conniventes may be thickened and flattened. In eosinophilic gastroenteritis involving the muscle layer, localized involvement of the antrum and pylorus may occur, causing narrowing of the distal antrum and gastric retention. The small intestine may be dilated, with an increase in the thickness of the mucosal folds. Prominent mucosal folds may be observed in the colon. Other tests like exploratory laparotomy may be indicated in patients with serosal eosinophilic gastroenteritis.
The endoscopic appearance is nonspecific. It includes erythematous, friable, nodular, and occasional ulcerative changes[3] (Figure 1). Sometimes diffuse inflammation results in complete loss of villi, involvement of multiple layers, submucosal oedema and fibrosis[30,31]. When performing endoscopy, it is necessary to obtain at least 6 biopsy specimens from normal and abnormal areas of the bowel to exclude the possibility of sampling error. In patients with esophageal or colonic symptoms, additional biopsy specimens may be obtained from the relevant sites to aid the diagnosis.
Figure 1.
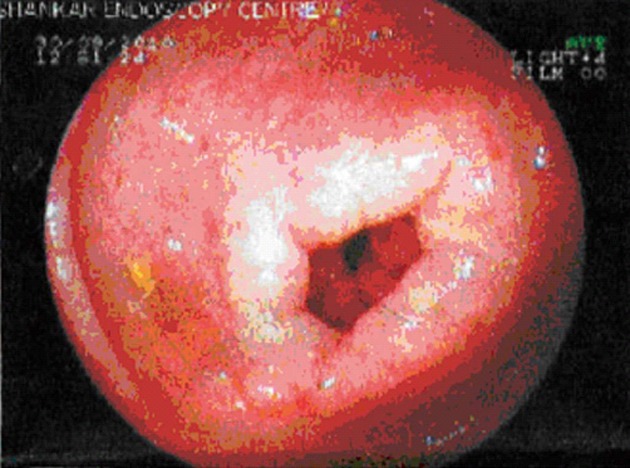
Endoscopy showing small superficial ulcers in stomach.
Patients with serosal disease present with ascites. Abdominal paracentesis demonstrates a sterile fluid with a high eosinophil count. Pleural effusion also may be present.
The diagnosis can be confirmed on histopathological examination of gastric and duodenal biopsies. The gross appearance of eosinophilic gastroenteritis upon endoscopy shows erythematous, friable, nodular, and often ulcerated mucosa. Microscopy demonstrates increased numbers of eosinophils (often > 50 eos per high-power field) in the lamina propria. Large numbers of eosinophils are often present in the muscularis and serosa (Figure 2). Localized eosinophilic infiltrates may cause crypt hyperplasia, epithelial cell necrosis, and villous atrophy. Diffuse enteritis with complete loss of villi, submucosal edema, infiltration of the GI wall, and fibrosis may be apparent. Mast cell infiltrates and hyperplastic mesenteric lymph nodes infiltrated with eosinophils may be present[1,27,31,32]. Infiltration is often patchy, can be missed and laparoscopic full thickness biopsy may be required.
Figure 2.

Large numbers of eosinophils are often present in the muscularis and serosa. A, B: Showing dense eosinophilic infiltrates in the lamina propria and mucosa (× 10); C: Showing dense eosinophilic infiltrates in the lamina propria and mucosa (× 40).
Histologic analysis of the small intestine reveals increased deposition of extracellular major basic proteins and eosinophilic cationic proteins.
Radio isotope scan using technetium (99mTc) exametazime-labeled leukocyte single-photon emission CT may be useful in assessing the extent of disease and response to treatment but has little value in diagnosis, as the scan does not help differentiating EGE from other causes of inflammation[33,34].
When eosinophilic gastroenteritis is observed in association with eosinophilic infiltration of other organ systems, the diagnosis of idiopathic hypereosinophilic syndrome should be considered[35].
Differential diagnosis
The main differential diagnoses are: (1) eosinophilic esophagitis; (2) eosinophilic ascites; (3) coeliac disease; (4) protein losing enteropathy from intolerance to cow milk protein; (5) infantile formula protein intolerance; and (6) idiopathic hypereosinophilic syndrome.
A diagnosis of idiopathic hypereosinophilic syndrome can be ruled out when there is absence of eosinophilic infiltration in all other organs except the bowel[35].
In celiac disease, biopsy of small bowel shows blunting of villi, crypt hyperplasia, and predominantly lymphocyte infiltration of crypts. Coeliac disease is caused by a reaction to gliadin, a prolamin (glutenprotein) found in wheat, and similar proteins found in other grains[36].
In eosinophilic esophagitis only the eosophagus is involved and not the whole bowel. A minimum of 15 eosinophils per high power field is required to make the diagnosis. Typically, eosinophils can be found in superficial clusters near the surface of the epithelium. An expansion of the basal layer is also seen in response to the inflammatory damage to the epithelium. At the time of endoscopy, ridges or furrows may be seen in the esophageal mucosa. Presence of white exudates in esophagus is also suggestive of the diagnosis[37,38].
Treatment
The role of steroids and antihelminthic drugs is not well established. However, in a few cases, steroids have been reported to produce symptomatic improvement in controlling diarrhea and protein losing enteropathy[9].
Corticosteroids are the mainstay of therapy with a 90% response rate in some studies (Figure 3). Appropriate duration of steroid treatment is unknown and relapse often necessitates long term treatment. Various steroid sparing agents, e.g., sodium cromoglycate (a stabilizer of mast cell membranes), ketotifen (an antihistamine), and montelukast (a selective, competitive leukotriene receptor antagonist) have been proposed, centering around an allergic hypothesis, with mixed results[24,39,40].
Figure 3.

Post treatment (low dose steroid) biopsy showing resolution of disease.
Corticosteroids
Fluticasone inhaled (Flovent): Decreases recruitment of inflammatory cells including eosinophils and decreases the release of eotaxins and other inflammatory mediators. Dosage required is higher than that used in asthma.
Prednisolone (AK-Pred, Delta-Cortef): Decreases inflammation by suppressing migration of polymorphonuclear leukocytes and reducing capillary permeability. Equivalent dosages of prednisone or methylprednisolone may be used.
Budesonide (Pulmicort Respule) oral viscous suspension: Decreases inflammation, reduces capillary permeability[6].
MAST CELL STABILIZERS
Cromolyn (Intal, Gastrocrom): Inhibits release of histamine, leukotrienes, and other mediators from sensitized mast cells. It also inhibits the influx of neutrophils, as well as the formation of the active form of NADPH oxidase, which in turn prevents tissue damage caused by oxygen radicals.
Leukotriene receptor antagonists
Prevent or reverse some of the pathologic features associated with the inflammatory process mediated by leukotrienes C4, D4 and E4. Successful treatment of eosinophilic gastroenteritis has been reported in few cases, mainly with Montelukast (Singulair) which is a potent and selective antagonist of leukotriene D4 at the cysteinyl leukotriene receptor, CysLT1[41].
Role of surgical care
Surgery is avoided, except when it is necessary to relieve persistent pyloric or small bowel obstruction. Most patients respond to conservative measures and oral glucocorticosteroids. Recurrence is possible, even after surgical excision.
Prognosis
The natural history of EGE has not been well documented. Eosinophilic gastroenteritis is a chronic, waxing and waning condition. Mild and sporadic symptoms can be managed with reassurance and observation, whereas disabling GI symptom flare-ups can often be controlled with oral corticosteroids. When the disease manifests in infancy and specific food sensitization can be identified, the likelihood of disease remission by late childhood is high. GI obstruction is the most common complication. Fatal outcomes are rare.
Preventive and diet therapy
The strong association of eosinophilic gastroenteritis with food allergies has prompted the use of restrictive or elemental diets. Initially, a trial elimination diet that excludes milk, eggs, wheat and/or gluten, soy, and beef may be helpful. Skin testing can identify food hypersensitivity. If a prohibitive number of food reactions are found, an amino-acid-based diet or elemental diet may be considered. Educate patients to avoid foods that they cannot tolerate and to seek medical care when needed.
Footnotes
P- Reviewer Krishnan T S- Editor Wen LL L- Editor A E- Editor Li JY
References
- 1.Ingle SB, Patle YG, Murdeshwar HG, Pujari GP. A case of early eosinophilic gastroenteritis with dramatic response to steroids. J Crohns Colitis. 2011;5:71–72. doi: 10.1016/j.crohns.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Kim NI, Jo YJ, Song MH, Kim SH, Kim TH, Park YS, Eom WY, Kim SW. Clinical features of eosinophilic gastroenteritis. Korean J Gastroenterol. 2004;44:217–223. [PubMed] [Google Scholar]
- 3.Chen MJ, Chu CH, Lin SC, Shih SC, Wang TE. Eosinophilic gastroenteritis: clinical experience with 15 patients. World J Gastroenterol. 2003;9:2813–2816. doi: 10.3748/wjg.v9.i12.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu YQ, Lo CY. A case of eosinophilic gastroenteritis. Hong Kong Med J. 1998;4:226–228. [PubMed] [Google Scholar]
- 5.Venkataraman S, Ramakrishna BS, Mathan M, Chacko A, Chandy G, Kurian G, Mathan VI. Eosinophilic gastroenteritis--an Indian experience. Indian J Gastroenterol. 1998;17:148–149. [PubMed] [Google Scholar]
- 6.Aceves SS, Bastian JF, Newbury RO, Dohil R. Oral viscous budesonide: a potential new therapy for eosinophilic esophagitis in children. Am J Gastroenterol. 2007;102:2271–2279; quiz 2280. doi: 10.1111/j.1572-0241.2007.01379.x. [DOI] [PubMed] [Google Scholar]
- 7.Chehade M, Magid MS, Mofidi S, Nowak-Wegrzyn A, Sampson HA, Sicherer SH. Allergic eosinophilic gastroenteritis with protein-losing enteropathy: intestinal pathology, clinical course, and long-term follow-up. J Pediatr Gastroenterol Nutr. 2006;42:516–521. doi: 10.1097/01.mpg.0000221903.61157.4e. [DOI] [PubMed] [Google Scholar]
- 8.De Angelis P, Morino G, Pane A, Torroni F, Francalanci P, Sabbi T, Foschia F, Caldaro T, di Abriola GF, Dall’Oglio L. Eosinophilic esophagitis: management and pharmacotherapy. Expert Opin Pharmacother. 2008;9:731–740. doi: 10.1517/14656566.9.5.731. [DOI] [PubMed] [Google Scholar]
- 9.Sharma S, Singh M, Naik S, Kumar S, Varshney S. Case report of eosinophilic gastroenteritis. Bmbay Hospital Journal. 2004:46. Available from: http://www.bhj.org.in/journal/2004_4603_july/july_2004/htm/case_reports_eosonophilic.htm. [Google Scholar]
- 10.Kaijser R. Zur Kenntnis der allergischen Affektionen des Verdauugskanals vom Standpunkt des Chirurgen aus. Arch Klin Chir. 1937;188:36–64. Available from: http://ci.nii.ac.jp/naid/10010523250/ [Google Scholar]
- 11.Klein NC, Hargrove RL, Sleisenger MH, Jeffries GH. Eosinophilic gastroenteritis. Medicine (Baltimore. ) 1970;49:299–319. doi: 10.1097/00005792-197007000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Guandalini S. Essential pediatric gastroenterology, hepatology and nutrition. New York: McGraw Hill; 2004. p. 210. [Google Scholar]
- 13.Shweiki E, West JC, Klena JW, Kelley SE, Colley AT, Bross RJ, Tyler WB. Eosinophilic gastroenteritis presenting as an obstructing cecal mass--a case report and review of the literature. Am J Gastroenterol. 1999;94:3644–3645. doi: 10.1111/j.1572-0241.1999.01625.x. [DOI] [PubMed] [Google Scholar]
- 14.Tran D, Salloum L, Tshibaka C, Moser R. Eosinophilic gastroenteritis mimicking acute appendicitis. Am Surg. 2000;66:990–992. [PubMed] [Google Scholar]
- 15.Schulze K, Mitros FA. Eosinophilic gastroenteritis involving the ileocecal area. Dis Colon Rectum. 1979;22:47–50. doi: 10.1007/BF02586758. [DOI] [PubMed] [Google Scholar]
- 16.Chisholm JC, Martin HI. Eosinophilic gastroenteritis with rectal involvement: case report and a review of literature. J Natl Med Assoc. 1981;73:749–753. [PMC free article] [PubMed] [Google Scholar]
- 17.Moore D, Lichtman S, Lentz J, Stringer D, Sherman P. Eosinophilic gastroenteritis presenting in an adolescent with isolated colonic involvement. Gut. 1986;27:1219–1222. doi: 10.1136/gut.27.10.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobbins JW, Sheahan DG, Behar J. Eosinophilic gastroenteritis with esophageal involvement. Gastroenterology. 1977;72:1312–1316. [PubMed] [Google Scholar]
- 19.Talley NJ, Shorter RG, Phillips SF, Zinsmeister AR. Eosinophilic gastroenteritis: a clinicopathological study of patients with disease of the mucosa, muscle layer, and subserosal tissues. Gut. 1990;31:54–58. doi: 10.1136/gut.31.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan AC, Kruimel JW, Naber TH. Eosinophilic gastroenteritis treated with non-enteric-coated budesonide tablets. Eur J Gastroenterol Hepatol. 2001;13:425–427. doi: 10.1097/00042737-200104000-00021. [DOI] [PubMed] [Google Scholar]
- 21.Blackshaw AJ, Levison DA. Eosinophilic infiltrates of the gastrointestinal tract. J Clin Pathol. 1986;39:1–7. doi: 10.1136/jcp.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desreumaux P, Bloget F, Seguy D, Capron M, Cortot A, Colombel JF, Janin A. Interleukin 3, granulocyte-macrophage colony-stimulating factor, and interleukin 5 in eosinophilic gastroenteritis. Gastroenterology. 1996;110:768–774. doi: 10.1053/gast.1996.v110.pm8608886. [DOI] [PubMed] [Google Scholar]
- 23.Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest. 2001;107:83–90. doi: 10.1172/JCI10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pérez-Millán A, Martín-Lorente JL, López-Morante A, Yuguero L, Sáez-Royuela F. Subserosal eosinophilic gastroenteritis treated efficaciously with sodium cromoglycate. Dig Dis Sci. 1997;42:342–344. doi: 10.1023/a:1018818003002. [DOI] [PubMed] [Google Scholar]
- 25.Christopher V, Thompson MH, Hughes S. Eosinophilic gastroenteritis mimicking pancreatic cancer. Postgrad Med J. 2002;78:498–499. doi: 10.1136/pmj.78.922.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baig MA, Qadir A, Rasheed J. A review of eosinophilic gastroenteritis. J Natl Med Assoc. 2006;98:1616–1619. [PMC free article] [PubMed] [Google Scholar]
- 27.Lee CM, Changchien CS, Chen PC, Lin DY, Sheen IS, Wang CS, Tai DI, Sheen-Chen SM, Chen WJ, Wu CS. Eosinophilic gastroenteritis: 10 years experience. Am J Gastroenterol. 1993;88:70–74. [PubMed] [Google Scholar]
- 28.Lyngbaek S, Adamsen S, Aru A, Bergenfeldt M. Recurrent acute pancreatitis due to eosinophilic gastroenteritis. Case report and literature review. JOP. 2006;7:211–217. [PubMed] [Google Scholar]
- 29.Kamal MF, Shaker K, Jaser N, Leimoon BA. Eosinophilic gastroenteritis with no peripheral eosinophilia. Ann Chir Gynaecol. 1985;74:98–100. [PubMed] [Google Scholar]
- 30.Johnstone JM, Morson BC. Eosinophilic gastroenteritis. Histopathology. 1978;2:335–348. doi: 10.1111/j.1365-2559.1978.tb01726.x. [DOI] [PubMed] [Google Scholar]
- 31.Katz AJ, Goldman H, Grand RJ. Gastric mucosal biopsy in eosinophilic (allergic) gastroenteritis. Gastroenterology. 1977;73:705–709. [PubMed] [Google Scholar]
- 32.Talley N. Eosinophilic Gastroenteritis. In: Feldman M, Scharschmidt BF, Sleisenger M, Zorab R, edtidors , et al., editors. Sleisenger and Fordtran‘s Gastrointestinal and Liver Disease: Pathophysiology/Diagnosis/Management. 6th ed. Philadelphia: WB Saunders; 1998. pp. 1679–1686. [Google Scholar]
- 33.Lee KJ, Hahm KB, Kim YS, Kim JH, Cho SW, Jie H, Park CH, Yim H. The usefulness of Tc-99m HMPAO labeled WBC SPECT in eosinophilic gastroenteritis. Clin Nucl Med. 1997;22:536–541. doi: 10.1097/00003072-199708000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Imai E, Kaminaga T, Kawasugi K, Yokokawa T, Furui S. The usefulness of 99mTc-hexamethylpropyleneamineoxime white blood cell scintigraphy in a patient with eosinophilic gastroenteritis. Ann Nucl Med. 2003;17:601–603. doi: 10.1007/BF03006675. [DOI] [PubMed] [Google Scholar]
- 35.Matsushita M, Hajiro K, Morita Y, Takakuwa H, Suzaki T. Eosinophilic gastroenteritis involving the entire digestive tract. Am J Gastroenterol. 1995;90:1868–1870. [PubMed] [Google Scholar]
- 36.Di Sabatino A, Corazza GR. Coeliac disease. Lancet. 2009;373:1480–1493. doi: 10.1016/S0140-6736(09)60254-3. [DOI] [PubMed] [Google Scholar]
- 37.Zimmerman SL, Levine MS, Rubesin SE, Mitre MC, Furth EE, Laufer I, Katzka DA. Idiopathic eosinophilic esophagitis in adults: the ringed esophagus. Radiology. 2005;236:159–165. doi: 10.1148/radiol.2361041100. [DOI] [PubMed] [Google Scholar]
- 38.Samadi F, Levine MS, Rubesin SE, Katzka DA, Laufer I. Feline esophagus and gastroesophageal reflux. AJR Am J Roentgenol. 2010;194:972–976. doi: 10.2214/AJR.09.3352. [DOI] [PubMed] [Google Scholar]
- 39.Barbie DA, Mangi AA, Lauwers GY. Eosinophilic gastroenteritis associated with systemic lupus erythematosus. J Clin Gastroenterol. 2004;38:883–886. doi: 10.1097/00004836-200411000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Moots RJ, Prouse P, Gumpel JM. Near fatal eosinophilic gastroenteritis responding to oral sodium chromoglycate. Gut. 1988;29:1282–1285. doi: 10.1136/gut.29.9.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neustrom MR, Friesen C. Treatment of eosinophilic gastroenteritis with montelukast. J Allergy Clin Immunol. 1999;104:506. doi: 10.1016/s0091-6749(99)70404-5. [DOI] [PubMed] [Google Scholar]


