Abstract
AIM: To study effect of diterpenoid C extracted from radix curcumae on Helicobacter pylori (H. pylori)-infected inflammation, intestinal metaplasia, and nuclear factor kappa B (NF-κB) signaling pathway in vitro.
METHODS: We used I-type H. pylori to infect human gastric epithelial gastric epithelium cell line (GES-1) cell lines, and then H. pylori-infected GES-1 cells were treated with radix curcumae (RC)-derived diterpenoid C of different concentrations (5, 10, 20 μg/mL) and amoxicillin. The expression of p65, IκB kinase (IKK) α and IKKγ proteins was detected with Western blotting, and the expression of interleukin (IL)-8, IL-6 and IL-4 was determined with enzyme-linked immunosorbent assay method. Data were analyzed using SPSS software ver18.0. For comparisons between groups of more than two unpaired values, one-way analysis of variance (ANOVA) was used. If an ANOVA F value was significant, post hoc comparisons were performed between groups. If results were not normally distributed, the Mann-Whitney U test was used to compare two groups of unpaired values, whereas for comparisons between groups of more than two unpaired values, the Kruskal-Wallis H test was used. Statistical significance was established at P < 0.05.
RESULTS: The MTT assay results revealed the inhibited rate of GES-1, and indicated that the IC5 of RC-derived diterpenoid C and amoxicillin all were 5 μg/mL for gastric GES-1 cells. The expression of IL-8 was significantly increased, especially at 12 h time point; and the expression of IL-4 was decreased in H. pylori-infected GES-1 cells. After H. pylori-infected GES-1 cells were treated with RC-derived diterpenoid C of different concentrations and amoxicillin, the expression of IL-8 was decreased at 12, 24, 48, 72 h points (P < 0.01), especially in high-concentration diterpenoid C (20 μg/mL) group; and the expression of IL-4 was increased, especially in moderate and high-concentration diterpenoid C (10 and 20 μg/mL) groups. RC-derived diterpenoid C had the inhibitory effects on H. pylori-induced p65 translocation from cytoplasm into cell nucleus, H. pylori-stimulant IkBα degradation, the phosphorylation of p65 and IkBα, and the expression of IKKα and IKKβ proteins.
CONCLUSION: RC-derived diterpenoid C can block NF-κB signal pathway, effectively reducing the secretion of H. pylori-induced proinflammatory cytokine and increasing the secretion of anti-inflammatory cytokine.
Keywords: Radix curcumae-derived diterpenoid C, Helicobacter pylori, Nuclear factor-κB, Inflammatory cytokine
Core tip: Radix curcumae (RC), a common Chinese crude drug, has a wide range of pharmacological activity including hypolipidemic effect, hepatoprotective effect, anti-tumor, anti-radiation and anti-anaphylaxis. RC-derived diterpenoid C is recently obtained from RC ether extract by us, and its chemical properties and constitution are different from curcumin and β-elemene. Our results showed that RC-derived diterpenoid C can block nuclear factor kappa B signal pathway, effectively reducing the secretion of Helicobacter pylori-induced proinflammatory cytokine and increasing the secretion of anti-inflammatory cytokine. RC-derived diterpenoid C may become an effective drug for treatment of chronic gastritis.
INTRODUCTION
Gastric carcinogenesis is usually believed to undergo the process including Helicobacter pylori (H. pylori) infection, chronic gastritis, atrophy, intestinal metaplasia, atypical hyperplasia abd gastric cancer[1]. H. pylori infection can bring to inflammation continuing through activating nuclear factor kappa B (NF-κB) signal pathway[2]. As H. pylori drug resistance becomes strong, it is difficult to eradicate H. pylori. How early to block the progression of chronic gastritis and to reduce gastric carcinogenesis is a main problem for us[3]. At present, there are no effective drugs for treatment of chronic gastritis. Our previous review has indicated that the total effective rate and pathological improvement (atrophy and intestinal metaplasia) are better in Chinese medicine group than in Western medicine group in the treatment of chronic gastritis[4]. But the mechanism of Chinese medicine is still unclear.
Radix curcumae (RC), a common Chinese crude drug, has a wide range of pharmacological activity including hypolipidemic effect, hepatoprotective effect, anti-tumor, anti-radiation and anti-anaphylaxis. RC-derived diterpenoid C is recently obtained from RC ether extract by us, and its chemical properties and constitution are different from curcumin and β-elemene. Our previous experiments have shown that RC-derived diterpenoid C has better anti-tumor activity and RC-derived diterpenoid C of high concentration can induce apoptosis[5,6]. Inflammation is strongly associated with tumor and the activation of some signal pathways occur in both inflammation and tumor[7,8], so we investigated the role of RC-derived diterpenoid C in anti-inflammation. Since biological properties are similar in gastric epithelium cell line (GES-1) cells and normal gastric epithelial cells, GES-1 cells were used in this study. The purpose of this study was to observe the effects of RC-derived diterpenoid C on inflammation, intestinal metaplasia and the expression of NF-κB signal pathway-related proteins in H. pylori-treated GES-1 cells.
MATERIALS AND METHODS
Materials
H. pylori strain, (CagA+, VacA+) NCTCl 1637 consistent with international standards, was purchased from China Disease Control and Prevention Center (Beijing, China). Human gastric epithelial GES-1 cells were purchased from the Institute of Cancer Research, Peiking University. RC-derived diterpenoid C (molecular weight: 380; molecular formula: C22H36O5) was provided by the College of Pharmacy, Zhejiang University (Hangzhou, China). Amoxicillin (molecular weight: 365.4) dispersible tablets with the batch number 63-110604 were from Xiansheng (Nanjing, China). Enzyme-linked immunosorbent assay (ELISA) kits was purchased from Nanjing KeyGey Biotech Co., Ltd. Primary antibodies were used. Horseradish peroxidasecoupled secondary antibodies were bought from Promega (Promega). The protein bands were detected employing electrochemi-luminescence chemiluminescence (Thermo Scientific).
Preparation of RC-derived diterpenoid C
Extraction of RC-derived diterpenoid C: RC-dried rhizome (10 kg) was used in extraction with 80 L of 95% ethanol, which was repeated four times to obtain 247 g of crude extract. After dispersion with 500 mL of water, the crude extract was respectively extracted with 500 mL of petroleum ether, dichloromethane and n-butanol to obtain 95.1 g of methylene bichloride. The methylene bichloride underwent silice gel column chromatography with petroleum ether/acetic ether (100:0, 100:10, 100:20, 100:30, 100:40, 100:50, 100:60, 100:70, 100:80 and 100:90), respectively, to obtain fractions A-J. The fraction E underwent chromatograph with acetonitrile/water (7:3) for 0-10, 10-20, 20-30, 30-40, 40-50, 50-60, 60-70 and 70-80 min, respectively, to obtain subfractions E1-E8. The subfraction E8 underwent RP-HPLC with acetonitrile/water (45:55) as eluant to obtain diterpenoid C (5.0 mg, tR: 43.7 min). Its molecular structure was shown in Figure 1.
Figure 1.
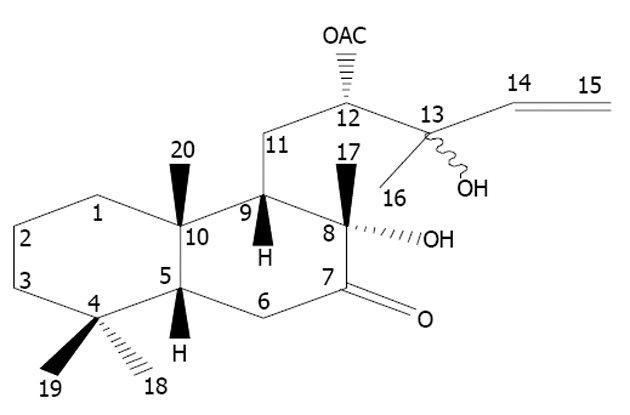
The molecular structure of radix curcumae-derived diterpenoid C. Originated from Huang et al[9], with permission.
Preparation of diterpenoid C of different concentrations: RC-derived diterpenoid C was made into 10 mg/mL of stock solution with dimethyl sulfoxide (DMSO), and then stored at -20 °C. The stock solution was diluted with fetal calf serum-free Dulbecco’s Modification of Eagle’s Medium (DMEM) containing high glucose for use in the experiment. DMSO concentration was controlled at 0.1% (volume percentage).
Cell culture
The tube containing frozen cells was placed in 37 °C water bath with constant shaking, and the frozen cells were melted within one minute. The tube was sterilized with 75% alcohol, and then quickly placed on a sterile bench for operation. After the tube was opened, cells were placed in high glucose-DMEM containing 10% fetal calf serum for incubation at 37 °C in an atmosphere of 5% CO2. Next day, the medium was changed. When cells reached 80% confluence, cells were digested with 0.25% trypsin for passage. One passage was performed every 2-3 d and the cells after passage 3 were used in this experiment.
Preparation of viable H. pylori suspensions
NCTCI 1637 was incubated in Bushi-modified selective plating medium containing 10% yolk, 10% fetal calf serum, soluble amylum, vancomycin, trimethoprim, amphotericin and polymyxin B at 37 °C in an atmosphere of 85% nitrogen, 5% oxygen and 10% CO2 for 3 d for future use. H. pylori was placed in 0.01 mol/L of PBS followed by quantitation with 752 type-spectrophotometer, and then diluted to 3.2 × 104-2.0 × 107 CFU/mL with RPMI1640 containing 2% fetal calf serum. The assays of Gram’s stain, urease, katalase and oxidase were performed to confirm the presence of H. pylori before application.
Cell infection and intervention
Gastric epithelial GES-1 cells were cultured in an incubator containing antibiotics-free RPMI1640 with 10% fetal calf serum. Gastric epithelial GES-1 cells in logarithmic phase were digested with 0.25% trypsin for counting, and then were seeded in 96-well plate at 5 × 104/mL-1 × 105/mL. When cells reached 80% confluence, H. pylori-negative control group without H. pylori was set. After adherence of viable H. pylori suspensions, H. pylori/GES-1 cells (200:1) were incubated at 37 °C in an atmosphere of 5% CO2 for 2 h, and then RC-derived diterpenoid C of different concentrations were added to incubate for 12, 24, 48 and 72 h, respectively, followed by observation on cell morphology under an electron microscopy. Three wells were set for each group. There were 3 RC-derived diterpenoid C groups with different concentrations, negative control group with 100 μL of RPMI1640 containing GES-1 cells, model group with H. pylori and positive control group with amoxicillin.
Inhibitory effects of RC-derived diterpenoid C and amoxicillin on GES-1 cell proliferation (MTT assay)
After GES-1 cells were incubated for 24 h, RC-derived diterpenoid C and amoxicillin (0, 5, 10, 20, 40, 80 ng/mL) were added for 24 h-culture. Three wells were set for each group. MTT (20 μL, 5 mg/mL) was added in each well for 3 h-incubation, and then the supernatant was taken followed by addition of 150 μL of DMSO. At the same time, the blank control group without RC-derived diterpenoid C and amoxicillin was set. Absorbance values were measured with a microplate reader (490 nm) for calculating inhibition rates. The inhibitory concentration 5% (IC5) was adopted in the following experiments, and inhibitory rate (IR) was calculated as follows: IR = (A of control group - A of experimental group/A of control group) × 100%.
Cell morphology
The status of cell growth was observed under an optical microscope after GES-1 cells were incubated for 12, 24, 48 and 72 h, respectively.
Levels of IL-8 and IL-4 in cell supernatant determined with ELISA
We detect the level of IL-8 and IL-4 with ELISA methods according to the manufacturer’s instructions.
Effects of RC-derived diterpenoid C on NF-κB signal pathways in H. pylori-induced GES-1 cell inflammation (Western blotting)
The effects of RC-derived diterpenoid C on the nuclear localization of NF-κB p65 were analyzed with Western blotting. Cells were divided into blank control group, model (H. pylori) group in which cells were treated for 60 min, and RC-derived diterpenoid C (20 μg/mL) + H. pylori group in which cells were first treated with RC-derived diterpenoid C for 2 h, and then infected with H. pylori. After nuclear proteins and cytoplasmic proteins were extracted, p65 protein in them was respectively determined. The effects of RC-derived diterpenoid C on the key proteins of NF-κB signal pathways were analyzed.
Statistical analysis
The experiments were repeated three times independently. Data were presented as the mean ± SD. Data were analyzed using SPSS software ver18.0. If the results were distributed normally, the two independent samples t test was used for comparison. For comparisons between groups of more than two unpaired values, one-way analysis of variance (ANOVA) was used. If an ANOVA F value was significant, post hoc comparisons were performed between groups. If results were not normally distributed, the Mann-Whitney U test was used to compare two groups of unpaired values, whereas for comparisons between groups of more than two unpaired values, the Kruskal-Wallis H test was used. Statistical significance was established at P < 0.05.
RESULTS
Effects of RC-derived diterpenoid C and amoxicillin on GES-1 cell proliferation
As shown in Table 1 and Figure 1, RC-derived diterpenoid C and amoxicillin inhibited human gastric GES-1 cell proliferation in time and dose-dependent manners, namely that with the increase in drug concentration and the extension in drug action time, the inhibition rate was increased. The maximum un-cytotoxic concentration (IC5) was 5 μg/mL. We adopted 5, 10, 20 μg/mL of RC-derived diterpenoid C as low, moderate and high-concentration diterpenoid C groups, and 5 μg/mL of moxicillin as drug-intervention group in the following experiments. The highest inhibition rate was 79.527% ± 6.879% obtained by 80 μg/mL of diterpenoid C with 72 h action time.
Table 1.
Inhibition rates of radix curcumae-derived diterpenoid C on human gastric gastric epithelium cell line cell proliferation (n = 3)
| Drug level (μg/mL) |
Action time |
||
| 24 h | 48 h | 72 h | |
| Radix curcumae-derived diterpenoid C | |||
| 0 (negative control) | - | - | - |
| 5 | 4.320% ± 0.056% | 5.695% ± 0.657% | 9.043% ± 0.121% |
| 10 | 8.409% ± 0.879% | 11.734% ± 0.547% | 20.512% ± 1.098% |
| 20 | 10.537% ± 1.098% | 19.96% ± 2.093% | 29.841% ± 2.345% |
| 40 | 13.273% ± 0.897% | 28.473% ± 5.093% | 45.723% ± 5.876% |
| 80 | 15.805% ± 0.975% | 65.056% ± 6.098% | 79.527% ± 6.879% |
| Amoxicillin | |||
| 0 (negative control) | - | - | - |
| 5 | 6.671% ± 0.987% | 7.935% ± 0.567% | 10.769% ± 1.087% |
| 10 | 8.325% ± 0.765% | 14.769% ± 0.897% | 19.130% ± 1.098% |
| 20 | 9.731% ± 0.345% | 18.530% ± 1.876% | 29.154% ± 1.543% |
| 40 | 12.929% ± 1.098% | 25.691% ± 1.786% | 31.832% ± 1.346% |
| 80 | 14.953% ± 1.876% | 38.427% ± 2.765% | 43.790% ± 2.983% |
Effects of RC-derived diterpenoid C on human gastric GES-1 cell morphology
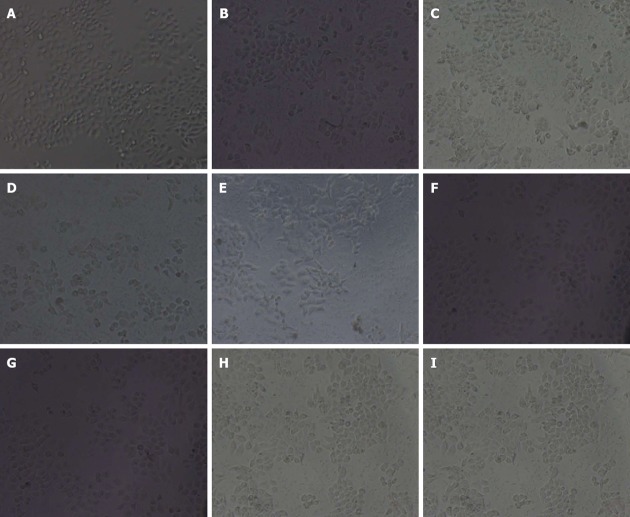
In bland group, GES-1 cells were polygon-shaped or spindle-shape with pseudopodia and island-like growth. Cells gradually were adherent. With prolonged incubation time, the number and density of cells were increased with a few floating cells (Figure 2A). In the GES-1 cells treated with H. pylori for 12 (Figure 2B), 24 (Figure 2C), 48 (Figure 2D) and 72 h (Figure 2E), cells became round; adherent cells were decreased and floating cells were increased; fragments occurred around cells; cell junction was reduced; the boundaries between cell nucleus and cytoplasm were obscure, and nucleus-cytoplasm fusion was seen. In the GES-1 cells treated with RC-derived diterpenoid C (5, 10, 20 μg/mL), adherent cells increased and cell morphology gradually recovered at 24 h (Figure 2F-I, respectively). Amoxicillin had no marked effects on cell morphology.
Figure 2.
Gastric epithelium cell line cell morphology (× 200). In bland group, gastric epithelium cell line (GES-1) cells were polygon-shaped or spindle-shape with pseudopodia and island-like growth. Cells gradually were adherent. With prolonged incubation time, the number and density of cells were increased with a few floating cells (A). In the GES-1 cells treated with Helicobacter pylori for 12 (B), 24 (C), 48 (D) and 72 (E), cells became round; adherent cells were decreased and floating cells were increased; fragments occurred around cells; cell junction was reduced; the boundaries between cell nucleus and cytoplasm were obscure, and nucleus-cytoplasm fusion was seen. In the GES-1 cells treated with radix curcumae-derived diterpenoid C (5, 10, 20 μg/mL), adherent cells increased and cell morphology gradually recovered at 24 h (F-I, respectively). Amoxicillin had no marked effects on cell morphology.
Effects of RC-derived diterpenoid C on H. pylori-induced human gastric GES-1 cell inflammation
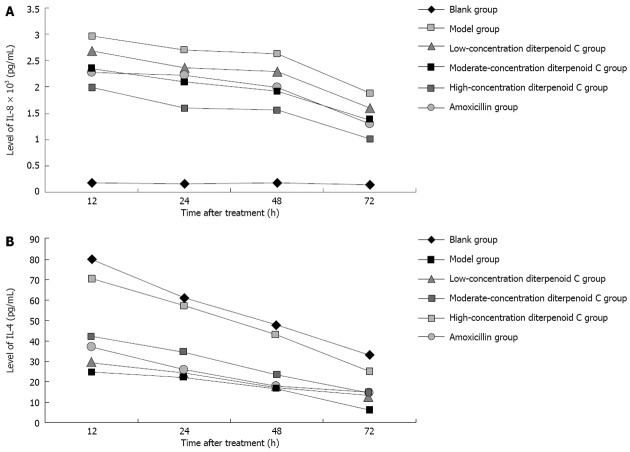
Effects of RC-derived diterpenoid C on the secretion of IL-8: As shown in Figure 3A, after human gastric GES-1 cells were infected with H. pylori, IL-8 in the supernatant was significantly increased, especially at 12 h time point. With prolonged time, IL-8 level was gradually decreased. There were statistical differences in IL-8 levels at 12, 24, 48 and 72 h time points (all P = 0.000). After human gastric GES-1 cells were treated with diterpenoid C of different concentrations and amoxicillin, compared with model group, IL-8 level at each time point was significantly decreased with statistical significance.
Figure 3.
Effects of radix curcumae-derived diterpenoid C on Helicobacter pylori-induced human gastric epithelium cell line cell inflammation. A: The changes in the level of interleukin (IL)-8 in cell supernatant; B: The changes in the level of IL-4 in cell supernatant.
Effects of RC-derived diterpenoid C on the secretion of IL-4: As shown in Figure 3B, after human gastric GES-1 cells were infected with H. pylori, IL-4 in the supernatant was significantly decreased with statistical differences compared with that at each time point of blank control group. After human gastric GES-1 cells were treated with diterpenoid C of low concentration, IL-4 level at each time point was increased, but P values at 12, 24, 48 and 72 h time points were 0.472, 0.550, 0.446 and 0.067, respectively, without statistical differences. After human gastric GES-1 cells were treated with diterpenoid C of moderate and high concentrations, IL-4 level at each time points was increased with statistical differences. After human gastric GES-1 cells were treated with amoxicillin, IL-4 level at each time point was increased, but their P values at 12, 24, 48 and 72 h time points were 0.092, 0.245, 0.446 and 0.053, respectively, without statistical differences. The results above suggest that the diterpenoid C of moderate and high concentrations can promote GES-1 cells to secrete IL-4, while amoxicillin has no the similar effect.
Effects of RC-derived diterpenoid C on NF-κB signal pathway activated by H. pylori in human gastric GES-1 cells
Nucleic localization of NF-κB p65: Our results indicated that 60 min after H. pylori infected human gastric GES-1 cells, p65 expression was increased in cell nucleus, but decreased in cytoplasm, suggesting that H. pylori can allow p65 translocation from cytoplasm to cell nucleus. In blank control group, there was a lot of p65 expression in cytoplasm. In high-concentration group of RC-derived diterpenoid C, p65 translocation was reduced, demonstrating that RC-derived diterpenoid C can inhibit p65 translocation from cytoplasm into cell nucleus induced by H. pylori (Figure 4).
Figure 4.
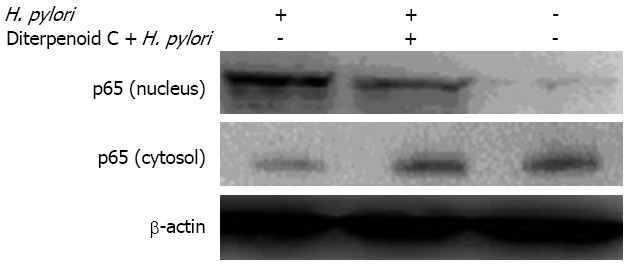
Effects of radix curcumae-derived diterpenoid C on nucleic localization of nuclear factor kappa B p65. H. pylori: Helicobacter pylori.
Effects of RC-derived diterpenoid C on IkBα degradation caused by H. pylori
After GES-1 cells were respectively treated with H. pylori for 0, 15, 30, 60 and 90 min, cytoplasm was isolated to be used for determination of IkBα degradation with Western blotting. Results indicated that IkBα began reducing at 15 min time point and was the lowest at 30 min time point; 60 min later, the decreased IkBα gradually recovered (Figure 5A and B). These results suggest that H. pylori can lead to IkBα degradation. Based on this, we observed the effects of RC-derived diterpenoid C on IkBα degradation caused by H. pylori, and foud that IkBα was basically unchanged. This suggests that RG-derived diterpenoid C can inhibit IkBα degradation caused by H. pylori (Figure 5C).
Figure 5.
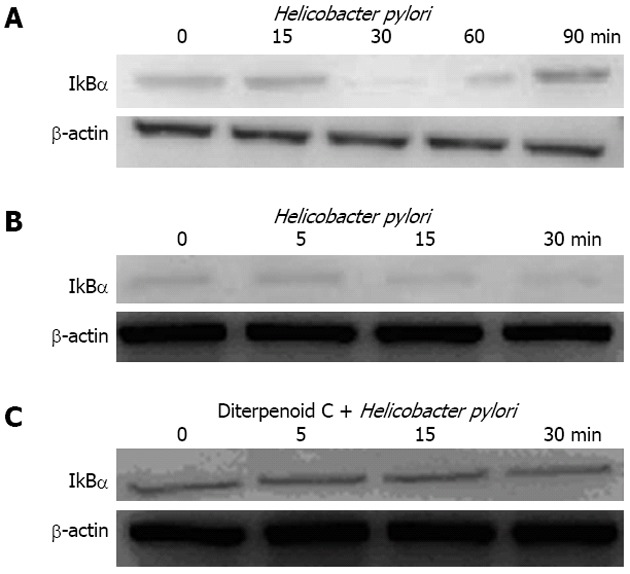
Effects of radix curcumae-derived diterpenoid C on IkBα degradation caused by Helicobacter pylori. A: After gastric epithelium cell line cells were respectively treated with Helicobacter pylori for 0, 15, 30, 60 and 90 min, cytoplasm was isolated to be used for determination of IkBα degradation with Western blotting; B: Helicobacter pylori for 0, 5, 15 and 30 min; C: Diterpenoid C + Helicobacter pylori for 0, 5, 15 and 30 min.
Expression of IkBα and p65 phosphorylated proteins, and IκB kinase α, IκB kinase β and p65 proteins
H. pylori rapidly induced phosphorylation of p65 and IkBα proteins. p65 phosphorylation was clearly seen at 5 min time point, and was the most strong between 15 and 30 min, and then gradually weakened. IkBα phosphorylation was seen at 5 min time point, and was the most strong at 15 min time point, and then gradually weakened. In a short time, the expression of p65, IκB kinase (IKK)α and IKKβ proteins was not markedly changed in H. pylori group. These results suggest that H. pylori is a good activator of NF-κB signal pathways. RC-derived diterpenoid C inhibited H. pylori-induced p65 and IkBα phosphorylation, decreased the expression of p65, IKKα and IKKβ proteins (Figure 6). These results indicated that RC-derived diterpenoid C decreased IkBα protein degradation through inhibiting phosphorylation of p65 and IkBα and the expression of IKKα and IKKβ proteins. RC-derived diterpenoid C may be an effective inhibitor of NF-κB.
Figure 6.
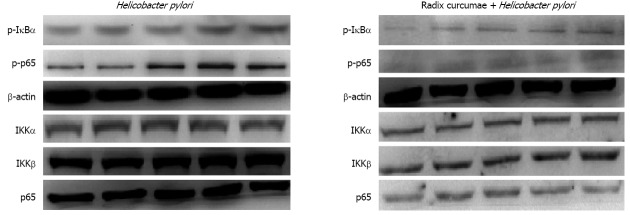
Effects of radix curcumae-derived diterpenoid C on the expression of nuclear factor kappa B proteins. p-IκBα: Phosphorylated IκBα; IKK: IκB kinase.
DISCUSSION
Recent studies indicate that H. pylori activates NF-κB through two pathways. One pathway is dependent on Cag pathogenicity island (CagPAI), but independent of CD14 and interleukin-1 receptor-associated kinase. Another pathway is dependent on CD14 and toll-like receptor 4, but independent of CagPAI. H. pylori chiefly activates NF-κB classics approach. So it is important to p53 moving nuclear and IkBα degradation in NF-κB classics approach. In addition, H. pylori infection induces IkB-β attenuation. In gastric cancer cells, the activities of IkB-α and IkB-β are increase, and the phosphorylation of serine residues of IkB-α and IkB-β induces the degradation of regulatory proteins of NF-κB, activating NF-κB. H. pylori infection may induce gastric mucosal inflammatory, and increase the release of PGE2, IL-8 and ROS[10-12], the possible mechanism of which may be related to NF-κB pathways[13].
NF-κB, an important nuclear factor, is involved in cell proliferation[14], immune response[15] and inflammation[16] through regulating the transcription of many genes[17]. In recent years, a great deal of attention has been paid to its role in inflammation and cancer[18,19]. Kim et al[20] believes that chronic inflammation is the seventh feature of tumor, chronic inflammation is strongly associated with tumor, and carcinogenesis is from the site of chronic inflammation. In some chronic inflammation-related tumors such as ulcerative colitis and colon cancer, chronic hepatitis and liver cancer, and chronic cervicitis and cervical cancer, NF-κB is found to be super-activated. NF-κB is an important molecule between chronic inflammation and tumor, and is regarded as a bridge between chronic inflammation and tumor.
Many studies have found that the curcumin, a main component of RC-ethanal extract, has highly effective anti-cancer activity with tumor cells[21-24], tumor-associated proteins[25,26], tumor-associated genes[27] and tumor-associated signal transduction pathways[28,29] as targets. It has been classified as the third-generation cancer-chemoprophylactic drug by United States National Cancer Institute. The elemene, a main component of RC-ether extract, can induce cancer apoptosis through down-regulating the expression of Bcl-2 and vascular endothelial growth factor, increasing the levels of cytochrome C and caspase-3 and blocking cell cycle progression[30-32]. Elemene emulsion with β-elemene as the main raw material has been widely used in the treatment of solid tumors, malignant hydrothorax and ascites, and metastasis tumor of brain[33,34]. However, the bioavailability of curcumin is lower, and elemene can produce vein injury, so their clinical application is limited. Therefore, due to this, we successfully obtained a new diterpenoid C from RC-ether extract, and its chemical constitution and properties are different from curcumin and elemene[35,36]. In this study, we explored the inhibitory effects of RC-derived diterpenoid C on H. pylori-induced GES-1 cell inflammation.
In this study, in the absence of stimulus, GES-1 cells secrete a tille cytokine. After GES-1 cells were treated with H. pylori, the levels of proinflammatory cytokins including IL-8 and IL-6 were significantly increased, and the the level of anti-inflammatory cytokine IL-4 was significantly decreased. RC-derived diterpenoid C was conducive to the balance between proinflammatory cytokines and anti-inflammatory cytokines. The possible mechanism is that RC-derived diterpenoid C has the cascaded inhibitory effects on the expression of IKKα and IKKβ, H. pylori-induced IkBα degradation, H. pylori-induced p65 translocation from cytoplasm into cell nucleus, the combination of p65 with inflammatory target genes and the release of inflammatory cytokins. Therefore, we infer that RC-derived diterpenoid C is an effective inhibitor of NF-κB.
In summary, RC-derived diterpenoid C, a newly effective anti-inflammatory factor, plays its role in H. pylori-infected GES-1 cells possibly through inhibiting NF-κB pathway. In view of the complexity of human life control and cell-signal transduction network, there may be more potential mechanisms about the anti-inflammatory effects of RC-derived diterpenoid C. Exploring RC-derived diterpenoid C to block the combination of NF-κB with its target gene with a reduction or elimination of cytokines has become a new idea to interrupt the progression of chronic gastritis into gastric cancer. This has important values in research and application.
COMMENTS
Background
Gastric carcinogenesis is usually believed to undergo the process including Helicobacter pylori (H. pylori) infection, chronic gastritis, atrophy, intestinal metaplasia, atypical hyperplasia abd gastric cancer. H. pylori infection can bring to inflammation continuing through activating nuclear factor kappa B (NF-κB) signal pathway. As H. pylori drug resistance becomes strong, it is difficult to eradicate H. pylori. How early to block the progression of chronic gastritis and to reduce gastric carcinogenesis is a main problem for them.
Research frontiers
At present, there are no effective drugs for treatment of chronic gastritis. Their previous experiments have shown that radix curcumae-derived diterpenoid C has better anti-tumor activity and radix curcumae (RC)-derived diterpenoid C of high concentration can induce apoptosis. Inflammation is strongly associated with tumor and the activation of some signal pathways occur in both inflammation and tumor, so the authors investigated the role of RC-derived diterpenoid C in anti-inflammation.
Innovations and breakthroughs
Since biological properties are similar in gastric epithelium cell line (GES-1) cells and normal gastric epithelial cells, GES-1 cells were used in this study. The purpose of this study was to observe the effects of RC-derived diterpenoid C on inflammation, intestinal metaplasia and the expression of NF-κB signal pathway-related proteins in H. pylori-treated GES-1 cells. However, prior study is rare.
Applications
The study demonstrated RC-derived diterpenoid C to block the combination of NF-κB with its target gene with a reduction or elimination of cytokines has become a new idea to interrupt the progression of chronic gastritis into gastric cancer. This has important values in research and application.
Terminology
RC, a common Chinese crude drug, has a wide range of pharmacological activity including hypolipidemic effect, hepatoprotective effect, anti-tumor, anti-radiation and anti-anaphylaxis. RC-derived diterpenoid C is recently obtained from RC ether extract by us, and its chemical properties and constitution are different from curcumin and β-elemene.
Peer review
This paper showed that RC-derived diterpenoid C can block NF-κB signal pathway, effectively reducing the secretion of H. pylori-induced proinflammatory cytokine and increasing the secretion of anti-inflammatory cytokine. RC-derived diterpenoid C may become an effective drug for treatment of chronic gastritis.
Footnotes
Supported by The Natural Science Foundation of Zhejiang Province of China, No. LY12H29002; and by grants of Scientific Research from Chinese Herbal Drug Administration, No. 2011ZB032
P- Reviewers Day AS, Iera E S- Editor Gou SX L- Editor A E- Editor Zhang DN
References
- 1.Li SR, Zhang L. Progress on the study of the relation between Helicobacter pylori and stomach cancer. Zhonghua Yixue Zazhi. 2004;84:1210–1211. [PubMed] [Google Scholar]
- 2.Maeda S, Omata M. Inflammation and cancer: role of nuclear factor-kappaB activation. Cancer Sci. 2008;99:836–842. doi: 10.1111/j.1349-7006.2008.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang X, Lv B, Zhang S, Fan YH, Meng LN. Itopride therapy for functional dyspepsia: a meta-analysis. World J Gastroenterol. 2012;18:7371–7377. doi: 10.3748/wjg.v18.i48.7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang X, Lv B. Treatment of chronic atrophic gastritis with Chinese medicines: a systematic review. Shijie Huaren Xiaohua Zazhi. 2010;18:1056–1062. [Google Scholar]
- 5.Jin HF, Lv B, Chen Z, Ma ZJ. Inhibitory effects of diterpenoid C extracted from radix curcumae on human gastric cancer SGC-7901 cells and the influence of protein expression of Bcl-2, Bax. Zhonghua Zhongyiyao Xuekan. 2011;11:2570–2573. [Google Scholar]
- 6.Shen Y, Lv B, Zhang S, Ma ZJ. Apoptosis of human colon adenocarcinoma cell line SW620 induced by diterpenoid C from radix curcumae and its related pathways. Zhonghua Yaolixue Tongbao. 2011;27:296–401. [Google Scholar]
- 7.Calzado MA, Bacher S, Schmitz ML. NF-kappaB inhibitors for the treatment of inflammatory diseases and cancer. Curr Med Chem. 2007;14:367–376. doi: 10.2174/092986707779941113. [DOI] [PubMed] [Google Scholar]
- 8.Inoue J, Gohda J, Akiyama T, Semba K. NF-kappaB activation in development and progression of cancer. Cancer Sci. 2007;98:268–274. doi: 10.1111/j.1349-7006.2007.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang W, Zhang P, Jin YC Shi Q, Cheng YY, Qu HB, Ma ZJ. Cytotoxic Diterpenes from the Root Tuber of Curcuma wenyujin. Helvetica Chimica Acta. 2008;91:944–950. [Google Scholar]
- 10.Zaidi SF, Ahmed K, Yamamoto T, Kondo T, Usmanghani K, Kadowaki M, Sugiyama T. Effect of resveratrol on Helicobacter pylori-induced interleukin-8 secretion, reactive oxygen species generation and morphological changes in human gastric epithelial cells. Biol Pharm Bull. 2009;32:1931–1935. doi: 10.1248/bpb.32.1931. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi H, Zhang YN, Israel DA, Peek RM, Kamioka M, Yanai H, Morimoto N, Sugiura T. Effect of Helicobacter pylori cdrA on interleukin-8 secretions and nuclear factor kappa B activation. World J Gastroenterol. 2012;18:425–434. doi: 10.3748/wjg.v18.i5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeshima E, Tomimori K, Teruya H, Ishikawa C, Senba M, D’Ambrosio D, Kinjo F, Mimuro H, Sasakawa C, Hirayama T, et al. Helicobacter pylori-induced interleukin-12 p40 expression. Infect Immun. 2009;77:1337–1348. doi: 10.1128/IAI.01456-08. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Mori N, Ishikawa C, Senba M. Induction of CD69 expression by cagPAI-positive Helicobacter pylori infection. World J Gastroenterol. 2011;17:3691–3699. doi: 10.3748/wjg.v17.i32.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo JL, Zheng SJ, Li YN, Jie W, Hao XB, Li TF, Xia LP, Mei WL, Huang FY, Kong YQ, et al. Toxicarioside A inhibits SGC-7901 proliferation, migration and invasion via NF-κB/bFGF signaling. World J Gastroenterol. 2012;18:1602–1609. doi: 10.3748/wjg.v18.i14.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giardino Torchia ML, Conze DB, Jankovic D, Ashwell JD. Balance between NF-κB p100 and p52 regulates T cell costimulation dependence. J Immunol. 2013;190:549–555. doi: 10.4049/jimmunol.1201697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakagawa H, Maeda S. Inflammation- and stress-related signaling pathways in hepatocarcinogenesis. World J Gastroenterol. 2012;18:4071–4081. doi: 10.3748/wjg.v18.i31.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colleran A, Collins PE, O’Carroll C, Ahmed A, Mao X, McManus B, Kiely PA, Burstein E, Carmody RJ. Deubiquitination of NF-κB by Ubiquitin-Specific Protease-7 promotes transcription. Proc Natl Acad Sci USA. 2013;110:618–623. doi: 10.1073/pnas.1208446110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiba T, Marusawa H, Seno H, Watanabe N. Mechanism for gastric cancer development by Helicobacter pylori infection. J Gastroenterol Hepatol. 2008;23:1175–1181. doi: 10.1111/j.1440-1746.2008.05472.x. [DOI] [PubMed] [Google Scholar]
- 19.Edwards MR, Bartlett NW, Clarke D, Birrell M, Belvisi M, Johnston SL. Targeting the NF-kappaB pathway in asthma and chronic obstructive pulmonary disease. Pharmacol Ther. 2009;121:1–13. doi: 10.1016/j.pharmthera.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen CF, Fang JY. Mitogen-activated protein kinase signal transduction pathways and gastric cancer. Zhonghua Xiaohua Zazhi. 2005;25:316–318. [Google Scholar]
- 22.Benlloch S, Payá A, Alenda C, Bessa X, Andreu M, Jover R, Castells A, Llor X, Aranda FI, Massutí B. Detection of BRAF V600E mutation in colorectal cancer: comparison of automatic sequencing and real-time chemistry methodology. J Mol Diagn. 2006;8:540–543. doi: 10.2353/jmoldx.2006.060070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thong-Ngam D, Choochuai S, Patumraj S, Chayanupatkul M, Klaikeaw N. Curcumin prevents indomethacin-induced gastropathy in rats. World J Gastroenterol. 2012;18:1479–1484. doi: 10.3748/wjg.v18.i13.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye F, Zhang GH, Guan BX, Xu XC. Suppression of esophageal cancer cell growth using curcumin, (-)-epigallocatechin-3-gallate and lovastatin. World J Gastroenterol. 2012;18:126–135. doi: 10.3748/wjg.v18.i2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun H, Ren J, Zhu Q, Kong FZ, Wu L, Pan BR. Effects of lysophosphatidic acid on human colon cancer cells and its mechanisms of action. World J Gastroenterol. 2009;15:4547–4555. doi: 10.3748/wjg.15.4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W, Wang X, Peng L, Deng Q, Liang Y, Qing H, Jiang B. CD24-dependent MAPK pathway activation is required for colorectal cancer cell proliferation. Cancer Sci. 2010;101:112–119. doi: 10.1111/j.1349-7006.2009.01370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qu JL, Qu XJ, Zhao MF, Teng YE, Zhang Y, Hou KZ, Jiang YH, Yang XH, Liu YP. Gastric cancer exosomes promote tumour cell proliferation through PI3K/Akt and MAPK/ERK activation. Dig Liver Dis. 2009;41:875–880. doi: 10.1016/j.dld.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Seto M, Ohta M, Asaoka Y, Ikenoue T, Tada M, Miyabayashi K, Mohri D, Tanaka Y, Ijichi H, Tateishi K, et al. Regulation of the hedgehog signaling by the mitogen-activated protein kinase cascade in gastric cancer. Mol Carcinog. 2009;48:703–712. doi: 10.1002/mc.20516. [DOI] [PubMed] [Google Scholar]
- 29.Keswani RN, Chumsangsri A, Mustafi R, Delgado J, Cohen EE, Bissonnette M. Sorafenib inhibits MAPK-mediated proliferation in a Barrett’s esophageal adenocarcinoma cell line. Dis Esophagus. 2008;21:514–521. doi: 10.1111/j.1442-2050.2007.00799.x. [DOI] [PubMed] [Google Scholar]
- 30.Chin R, Earnest-Silveira L, Koeberlein B, Franz S, Zentgraf H, Dong X, Gowans E, Bock CT, Torresi J. Modulation of MAPK pathways and cell cycle by replicating hepatitis B virus: factors contributing to hepatocarcinogenesis. J Hepatol. 2007;47:325–337. doi: 10.1016/j.jhep.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Sun DF, Lu R, Chen ZF, Chen YX, Fang JY. RAF may induce cell proliferation through hypermethylation of tumor suppressor gene promoter in gastric epithelial cells. Cancer Sci. 2009;100:117–125. doi: 10.1111/j.1349-7006.2008.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng H, Ravikumar TS, Yang WL. Bone morphogenetic protein-4 inhibits heat-induced apoptosis by modulating MAPK pathways in human colon cancer HCT116 cells. Cancer Lett. 2007;256:207–217. doi: 10.1016/j.canlet.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Looby E, Abdel-Latif MM, Athié-Morales V, Duggan S, Long A, Kelleher D. Deoxycholate induces COX-2 expression via Erk1/2-, p38-MAPK and AP-1-dependent mechanisms in esophageal cancer cells. BMC Cancer. 2009;9:190. doi: 10.1186/1471-2407-9-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caja L, Sancho P, Bertran E, Iglesias-Serret D, Gil J, Fabregat I. Overactivation of the MEK/ERK pathway in liver tumor cells confers resistance to TGF-{beta}-induced cell death through impairing up-regulation of the NADPH oxidase NOX4. Cancer Res. 2009;69:7595–7602. doi: 10.1158/0008-5472.CAN-09-1482. [DOI] [PubMed] [Google Scholar]
- 35.Chong H, Vikis HG, Guan KL. Mechanisms of regulating the Raf kinase family. Cell Signal. 2003;15:463–469. doi: 10.1016/s0898-6568(02)00139-0. [DOI] [PubMed] [Google Scholar]
- 36.Grynkiewicz G, Ślifirski P. Curcumin and curcuminoids in quest for medicinal status. Acta Biochim Pol. 2012;59:201–212. [PubMed] [Google Scholar]