Abstract
AIM: To determine the diagnostic accuracy of a new point-of-care assay detecting anti-deamidated gliadin peptides in celiac disease (CD) patients.
METHODS: One-hundred-and-twelve patients (age range: 1.8-79.2 years old) with clinical symptoms suggestive of CD and/or first-degree relatives (FDR) of CD patients (n = 66), and confirmed CD on a gluten-free diet (GFD) (n = 46), were prospectively enrolled in the study at Gastroenterology outpatient clinics for adult patients and from the Gastroenterology Consultation Ward at the Pediatric Department of the University Hospital of Geneva. Written informed consent was obtained from all subjects enrolled. The study received approval from the local ethics committee. The original CD diagnosis had been based on serum-positive IgA anti-tissue transglutaminase enzyme-linked immunosorbent assay (ELISA) (QuantaLite™, Inova Diagnostics, San Diego, CA, United States) and on biopsy results. Serum samples from all study participants were tested by the new CD lateral flow immunochromatographic assay (CD-LFIA) device, Simtomax® Blood Drop (Augurix SA, BioArk, Monthey, Switzerland) to detect immunoglobulin (Ig)A and IgG antibodies against deamidated gliadin peptides. The diagnostic performance was evaluated using receiver operating characteristic curves with 95%CIs. A cut-off of 2 on the Rann colorimetric scale was used to calculate the device’s sensitivity and specificity.
RESULTS: CD-LFIA was highly accurate in detecting untreated celiac patients. In the group of patients with CD symptoms and/or FDR, eight new cases of CD were detected by ELISA and biopsy. All of these new cases were also correctly identified by CD-LFIA. The test yielded four false positive and four false negative results. The false positive results were all within the groups with clinical symptoms suggestive of CD and/or FDR, whereas the false negative results were all within the GFD group. The test yeld a sensitivity of 78.9% (95%CI: 54.4-93.9) and specificity of 95.7% (95%CI: 89.4-98.8), and the area under the curve reached 0.893 (95%CI: 0.798-0.988). The Kappa coefficient, calculated according to the values obtained by two readers from the same device, was of 0.96 (SE: 0.06). When the GFD patients were excluded from the analysis, the area under the curve reached 0.989 (95%CI: 0.971-1.000) and the Kappa coefficient, calculated according to the values obtained by two readers from the same device, became 0.96 (SE: 0.07). Furthermore, using the Rann scale cut-off of 2 without the GFD patients, sensitivity was 100% and specificity was 93.1% (95%CI: 83.3-98.1).
CONCLUSION: The new CD-LFIA rapid screening test shows good diagnostic accuracy, sensitivity and specificity, and may rule out CD in patients with CD-related symptoms.
Keywords: Celiac disease, Deamidated gliadin, Total immunoglobulin A, Screening, Point-of-care assay
Core tip: The aim of the present study was to evaluate the clinical accuracy of a new point-of-care device that is based on deamidated gliadin peptides (DGP) for diagnosis of celiac disease (CD). One-hundred-and-twelve patients with clinical symptoms suggestive of CD and/or first-degree relatives of CD patients, and patients with confirmed CD on a gluten-free diet, were prospectively enrolled in the study. The actual CD diagnosis had been based on serum-positive immunoglobulin A anti-tissue transglutaminase results by enzyme-linked immunosorbent assay and on biopsy findings. Overall evaluation shows that the new DGP-based rapid point-of-care test is an excellent screening tool for high-risk populations.
INTRODUCTION
Celiac disease (CD) is a common T cell-mediated gluten-sensitive enteropathy. CD diagnosis remains challenging since only a minority of celiac patients presents with specific gastrointestinal symptoms and the majority of patients manifests atypical extra-intestinal symptoms that may lead to missed diagnosis or misdiagnosis[1].
The initial diagnosis of CD is made by serological testing and confirmed either by histopathologic examination of small-bowel biopsy or further blood tests, depending on the serum concentration of anti-tissue transglutaminase (tTG) autoantibodies and the patient’s age. Serology markers of CD have evolved over the years, as more specific antibodies have been identified. Currently, endomysial and anti-tTG autoantibodies are considered among the most reliable CD diagnostic markers[2,3]. Although both of these markers exhibit high sensitivity and specificity, their accuracy remains controversial in patients of a very young age or with a minor degree of mucosal damage; moreover, their accuracy for monitoring CD status in patients following a gluten-free diet (GFD) remains controversial[4,5]. Very recently, a new generation of assays based on the detection of antibodies against deamidated gliadin peptides (DGP) has demonstrated very high sensitivity, as well as a diagnostic accuracy that is at least equivalent to the established serological assays[6-9].
Given the high prevalence of the disease and likelihood of missed diagnosis, several simple immunoassays have been developed as a first step toward reducing the turnaround time for result delivery and initiating patient counseling and treatment[10]. Unfortunately, these new assays feature several drawbacks, including the reliance on serum samples, requirement for some basic laboratory equipment, their lack of sensitivity to identify celiac disease and to identify patients suffering from an immunoglobulin (Ig)A deficiency[11].
To overcome these issues, a multi-analytic lateral-flow immunochromato-graphic assay (LFIA) device, the Simtomax® Blood Drop system, has been developed that is based upon the detection of both IgA and IgG anti-DGP and total IgA. In this study, this new CD-LFIA test was evaluated in a ward setting to determine its accuracy, sensitivity, and specificity as compared to the established laboratory serology assay.
MATERIALS AND METHODS
Patients
Patients visiting the gastroenterology adult outpatient clinic and the gastroenterology consultation ward in the pediatric department of the University Hospital of Geneva from April 2008 to December 2009 were prospectively enrolled in this study. Criteria for study inclusion were clinical symptoms suggestive of CD and/or first-degree relatives (FDR) of CD-confirmed patients (n = 66), and CD-confirmed patients on a gluten-free diet (n = 46). Written informed consent was obtained from all subjects prior to study participation. The study was carried out with approval from the local ethics committee board (University Hospital of Geneva application 07-217).
Diagnostic methods
The diagnosis of CD was based on results of serologic enzyme-linked immunosorbent assay (ELISA) tests (described below) and small intestine mucosal biopsy examination.
The IgA and IgG anti-tTG QuantaLite™ ELISA tests from Inova Diagnostics (San Diego, CA, United States) were used to detect serum samples from all study participants. For both tests, concentrations > 30 U/mL were considered moderate to strongly positive for CD.
Total IgA was measured by the BNII nephelometer (Dade Behring Ltd., Milton Keynes, United Kingdom) according to the manufacturer’s protocol. Results were evaluated by referring to a standard curve and by using < 0.05 g/L as the cut-off point to identify IgA deficiency. For the study population, normal values ranged between 0.05 and 4.07 g/L, depending on the patient’s age.
Small-bowel biopsies were obtained from all patients who tested positive by serology tests. The mucosal biopsy sections were analyzed by an experienced histopathologist, who assessed the following pathologic features of CD: villus atrophy, crypt hyperplasia, increased intraepithelial lymphocytes, and chronic inflammation in the lamina propria. The diagnosis of CD was subsequently confirmed according to the modified Oberhuber-Marsh classification system[12].
CD-LFIA test
Serum samples were collected from all study participants, stored at -20 °C, and tested in duplicate on the Simtomax® Blood Drop system (Augurix SA, BioArk, Monthey, Switzerland). This CD-LFIA device was developed as an antigen direct sandwich assay capable of detecting both human IgA and IgG anti-DGP, as well as total IgA. A synthetic DGP conjugated to a carrier protein[7] was attached to the device’s nitrocellulose membrane at the test line A position for detection of IgA and IgG anti-DGP. Mouse anti-human IgA was attached at the test line B position for detection of total IgA. In the test, secondary gold-conjugated antibodies bind to the patient’s antibodies to form detectable complexes that are captured by the test in lines A and B. The control line, CT, is formed by the interaction of nitrocellulose-attached goat anti-mouse antibodies with the secondary gold-conjugated antibodies. All the lines are formed in 10-15 min. A CD-positive test result was indicated by detection of both the CT and A lines. IgA deficiency was indicated by absence of the B line. Figure 1 illustrates the device run with samples representative of the various diagnoses. Each sample was tested by two independent user-operators blinded to the subject’s histories and laboratory findings and each of whom performed the CD-LFIA interpretations twice on two independent devices.
Figure 1.
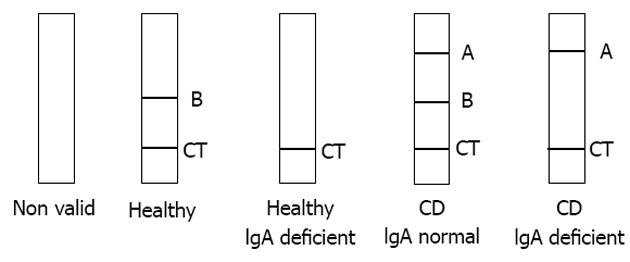
Celiac disease lateral-flow immunochromatographic assay visual result interpretation. CT: Control line; A: Position for detection of IgA and IgG anti-DGP; B: Position for detection of total IgA; CD: Celiac disease; IgA: Immunoglobulin A.
The CD-LFIA test lines were semi-quantitatively evaluated by using the Rann colorimetric scale (British Biocell International, Cardiff, United Kingdom). A series of five pink/red lines with a colloidal gold solution of decreasing optical density were sprayed on a card, and yielded line intensities ranging from 10 (maximum line intensity) to 2 (weakest visible line). Accordingly, the cut-off value for a positive result was set at 2. Spiked celiac serum equivalent to the ELISA QuantaLite™ cut-off value was used to set the visual limit of detection.
Statistical analysis
Statistical analyses were carried out by the STATA software (version 11; College Station, TX, United States). The StatXact-8 software (Cytel Inc., Cambridge, MA, United States) was used to calculate the 95%CIs. The diagnostic performance of the CD-LFIA test was evaluated by generating receiver operating characteristic (ROC) curves for each CD-LFIA device used and for each user-operator[13]. The areas under the ROC curves (AUCs) were provided with the corresponding 95%CIs. The “gold standard” diagnostic methods of laboratory ELISA and biopsy results were used for comparative analyses to evaluate the testing features of CD-LFIA. The cut-off of 2 Rann, which represented the delimitation between a “positive” and “negative” result (visible/invisible band) was used to calculate the CD-LFIA test’s sensitivity, specificity, and positive and negative likelihood ratios (LR+, LR-). Concordance between sample and device replicates was evaluated by calculating the Kappa coefficient and its SE.
RESULTS
Overall agreement between CD-LFIA and ELISA IgA-tTG laboratory test results
A total of 112 patients (71 females, 36 males; no sex information was available for five patients) with a mean age of 24.6 years old (median 13.9 years; range: 1.8-79.2 years) were analyzed.
Based on the laboratory values and biopsy results, a group of eight newly diagnosed celiac patients was found amongst the group of 66 patients composed of FDR and patients with clinical symptoms suggestive of CD. Thus, the CD prevalence in this study was 12.1%. All of the eight newly diagnosed CD patients were correctly identified by the CD-LFIA test (range of Rann values between 3-10). Among them, one did not undergo intestinal biopsy but had typical clinical presentation of CD and high positive titers of IgA-tTG (137 U/mL). The remaining seven had a positive intestinal biopsy (Marsh 3 and 4) and positive titers of IgA-tTG (119 -197 U/mL). Out of the 58 CD sero-negative patients, four were positive by the CD-LFIA test, however their Rann scores were just near cut-off: 2-3.
Of the 46 CD GFD patients, two patients showed selective IgA deficiency, and the CD-LFIA test detected this at 100%. Out of the 46 CD GFD patients, eleven of the CD GFD patients tested positive on the IgA-tTG ELISA, with three having high levels (116-170 U/mL) and the remaining eight having moderate levels (near the cut-off value; 30-55 U/mL). Among those 11 patients with positive IgA-tTG serology, four had negative results with the CD-LFIA test. These four patients had IgA-tTG ELISA levels near the cut-off values (36-55 U/mL for IgA-tTG for ELISA) and values of 0 Rann for CD-LFIA. The remaining 35 CD GFD patients were correctly identified as CD-negative by CD-LFIA. The overall agreement between the CD-LFIA test and the ELISA laboratory test results is shown in Figure 2.
Figure 2.
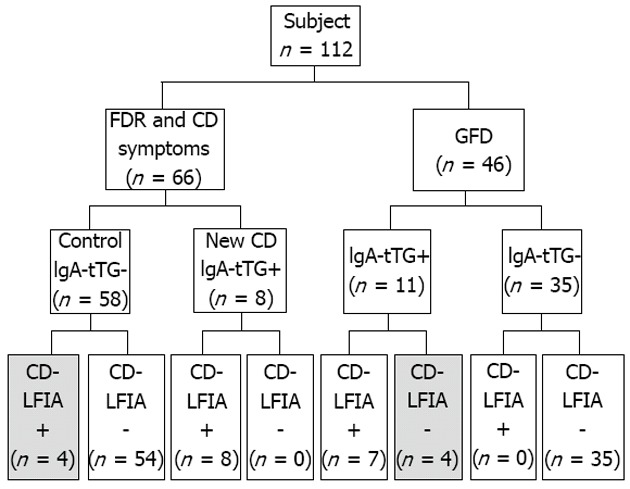
Histogram showing the immunoglobulin A-tissue transglutaminase enzyme-linked immunosorbent assay and celiac disease lateral-flow immunochromatographic assay test results. Text in gray indicates false-positive and false-negative results by celiac disease lateral-flow immunochromatographic assay (CD-LFIA). FDR: First-degree relatives; GFD: Gluten-free diet; Control: First-degree relatives and patients with celiac disease symptoms diagnosed as celiac disease (CD)-negative; IgA-tTG: Immunoglobulin A-tissue transglutaminase.
Thus, CD-LFIA tests showed four false-positive results, all in the FDR and CD symptoms group. All of the ELISA laboratory test results were below the cut-off value and the Rann scores were between 2 and 3, just near the cut-off value. There were also four false-negative results obtained by the CD-LFIA device, all of which were from the CD GFD group. The serological IgA-tTG level of these patients was near the cut-off values.
Evaluation of the diagnostic performance of CD-LFIA on a population including patients monitored for compliance with GFD
The AUCs for each CD-LFIA device used and for each user-operator were 0.869 (95%CI: 0.764-0.975) and 0.893 (95%CI: 0.798-0.988), indicating excellent diagnostic performance of the test (Figure 3).
Figure 3.
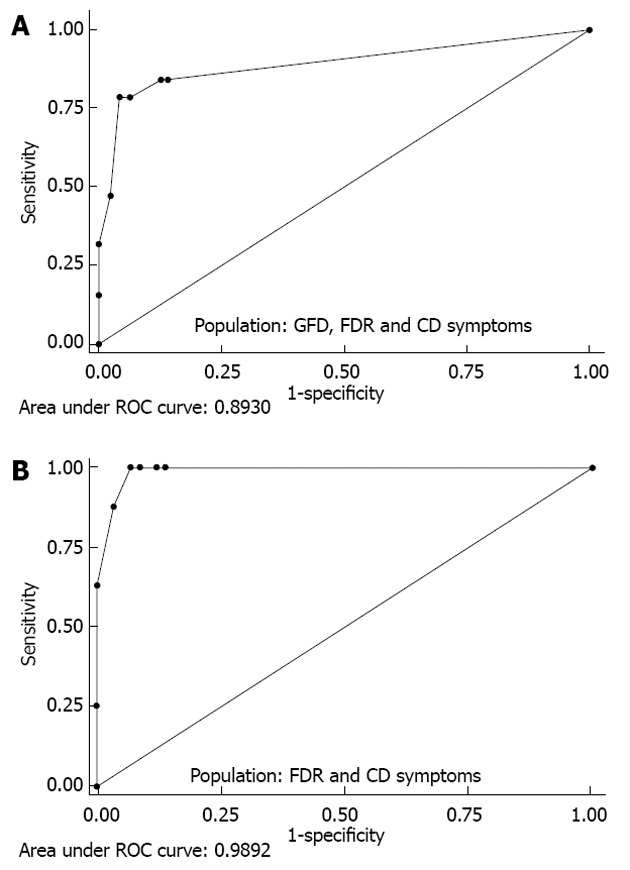
Diagnostic performance of the celiac disease lateral-flow immunochromatographic assay test determined by receiver operating characteristic curve analysis. A: GFD, FDR and CD symptoms; B: FDR and CD symptoms. FDR: First-degree relatives; CD: Celiac disease symptoms; GFD: Gluten-free diet; ROC: Receiver operating characteristic.
These results yield a sensitivity for the CD-LFIA device of 78.9% (95%CI: 54.4-93.9) and a specificity of 95.7% (95%CI: 89.4-98.8), as compared to the serological IgA-tTG levels detected by the ELISA laboratory tests. Considering the newly diagnosed CD patients (n = 8), the sensitivity was 100% (95%CI: 63.1-100) for both user-operators (Table 1).
Table 1.
Celiac disease lateral-flow immunochromatographic assay result compared to diagnosis of celiac disease
|
IgA-tTG ELISA |
Total | |||
| Positive | Negative | |||
| GFD, FDR and CD symptoms | ||||
| CD-LFIA | Positive | 15 | 4 | 19 |
| Negative | 4 | 89 | 93 | |
| 19 | 93 | 112 | ||
| FDR and CD symptoms | ||||
| CD-LFIA | Positive | 8 | 4 | 12 |
| Negative | 0 | 54 | 54 | |
| 8 | 58 | 66 | ||
Celiac disease lateral-flow immunochromatographic assay (CD-LFIA) result compared to diagnosis of celiac disease based on elevated titers of immunoglobulin A-tissue transglutaminase (IgA-tTG) in a population including gluten-free diet (GFD), first-degree relatives (FDR) and patients with celiac disease (CD)-related symptoms and FDR and patients with CD-related symptoms only. ELISA: Enzyme-linked immunosorbent assay.
Although the CD-LFIA is dependent upon the user-operator’s semi-quantitative assessment of the colors of the reactive bands, the results were very reproducible between devices and user-operators. The concordance between user-operators and devices was indicated by the Kappa coefficients of 0.96 (SE = 0.06) and 0.92 (SE = 0.05), respectively.
In addition, an LR+ of 18.4 (95%CI: 7.0-51.8) and an LR- of 0.22 (95%CI: 0.08-0.46) were found for the CD-LFIA test when compared to the IgA-tTG ELISA (Table 1).
Evaluation of the diagnostic performance of CD-LFIA on a high-risk population
Exclusion of the CD GFD patients from the ROC analysis brought the AUC up to 0.989 (95%CI: 0.971-1.000), depending on the device and user-operator (Figure 3).
The kappa coefficient was 0.96 (SE = 0.07), indicating an excellent concordance between devices and user-operators.
In addition, when the CD GFD patients were excluded and the Rann cut-off of 2 was used, the sensitivity was of 100% (95%CI: 63.1-100) and the specificity remained nearly unchanged at 93.1% (95%CI: 83.3-98.1) (Table 1).
The LR+ became 14.5 (95%CI: 5.8-49.0) and the LR- became 0.00 (95%CI: 0.00-0.39), respectively (Table 1).
DISCUSSION
Diagnostic tests play a vital role in medicine, not only to confirm the presence of diseases but also to rule them out[14]. Diagnosis of CD has improved significantly in the past 20 years, as highly sensitive and specific biomarkers were identified[15]. Nevertheless, the prevalence of CD has dramatically increased over this same period (rising from a previously assumed 0.1% to up to 1.0%)[16,17]. This increase is probably largely due to identification of patients suffering from mild or atypical forms of CD. Moreover, large epidemiological screening studies have revealed that CD is a worldwide health concern[18]. Besides the improved detection methods, other etiological factors appear to have contributed to the increased prevalence[16], and, similar to other autoimmune conditions, these may include different environmental factors, such as gluten, antigens in breast milk, or from other pathogenic infections[19,20].
Unfortunately, CD remains one of the most common underdiagnosed medical conditions, with estimates of more than 90% of the patients being unrecognized[19,21]. Due to mild and atypical symptoms, the diagnosis of CD is often a challenge for many physicians, resulting in delays in diagnosis (up to 11 years[21]) and high rates of patient dissatisfaction and discomfort.
A large retrospective study of a managed-care population demonstrated that timely CD diagnosis was associated with a significant overall cost reduction that was attributable to reduced amounts of office visits, laboratory services, diagnostic and imaging support services, and endoscopy procedures[22]. Several simple, visual assays have been developed to promote the feasibility of CD screening programs[4,23-27]. However, while these assays have been demonstrated as reliable and easy-to-use, they are limited in sensitivity and lack the ability to concomitantly detect IgA deficiency[11].
Therefore, there is a clear unmet clinical need for a rapid and discriminative point-of-care test that could facilitate the management of patients consulting in primary care centers for CD-related symptoms. To this end, in this study, we compared the validity of the newly developed rapid point-of-care diagnostic device for detecting both human IgA and IgG anti-DGP to the measurements of serological IgA and IgG anti-tTG levels detected by routine laboratory ELISA. Sensitivity and specificity are two features of a diagnostic test that measure the validity of a new test as compared to a gold standard test, such as the ELISA. The ROC curves, as well as the corresponding AUCs, are effective measures of the inherent validity of a diagnostic test. Here, we found that the CD-LFIA test had a sensitivity of 100% for the detection of new CD cases, and result interpretation appeared unambiguous between multiple devices and multiple user-operators. The ROC curve indicated that, at a cut-off of 2 Rann, the device has a good discriminative ability between patients with CD and those without CD. The high values of the AUCs (up to 0.989) indicated an excellent accuracy of the CD-LFIA test. LR+ and LR- values represent measures of the performance of a diagnostic method, independent of disease prevalence[18,28]. The CD-LFIA test in this study achieved a LR+ of 14.5, indicating that patients having CD are 15 times more likely to have a positive test than those who are healthy. Moreover, the LR- of 0.0 indicated that the CD-LFIA test is very good at ruling out the disease.
The particular challenges to this test concerned interpretation of samples with weak reactivity that were exclusively representative of the CD GFD patients and would affect monitoring of CD status in this patient population. For this specific group, another approach may be required.
Here, we showed that CD-LFIA is highly accurate in detecting untreated celiac patients. It can be easily performed during the course of a consultation in primary care to test patients with symptoms suggestive of CD, and may represent a reliable alternative to the traditional laboratory assays. With specificity and sensitivity of 93.1% and 100%, respectively, and a LR-value of 0.0, CD-LFIA appears highly suitable for ruling out CD, representing an interesting tool in an exclusion diagnostic strategy. In case of positive serology, the physician can proceed to further investigations by the traditional laboratory assay. Therefore, CD-LFIA can be used as a rapid and accurate test to rule out CD in patients presenting with CD-related symptoms to primary care centers.
ACKNOWLEDGMENTS
We would like to thank Mrs. Carole Salomon for her help and technical expertise.
COMMENTS
Background
Traditionally thought to be a rare childhood disease, celiac disease (CD) is currently recognized as a frequent condition both in adults and children and has become a widespread public health concern. CD diagnosis can be quite challenging for physicians since only a minority of celiac patients suffer from specific gastrointestinal symptoms. The majority of patients present with an atypical extra-intestinal manifestation that may not raise the physician’s suspicion of CD. Laboratory-based methods, such as enzyme-linked immunosorbent assays (ELISA), remain the primary screening tool for CD. However, these tests are labor intensive and relatively high cost. Development and implementation of simple immunoassays will be a first step toward reducing the turnaround time for result delivery and patient counseling and treatment.
Research frontiers
Serology markers of CD have evolved over the years with the identification of more disease-specific antibodies. Endomysial and anti-tissue transglutaminase (tTG) autoantibodies are currently considered among the most reliable of the CD-related markers. Although these markers exhibit a high sensitivity and specificity, their accuracy in very young children, in patients with a minor degree of mucosal damage, and for the follow-up of CD patients under a gluten-free diet remains controversial. Very recently, a new generation of assays based on the detection of antibodies against deamidated gliadin peptides (DGP) has demonstrated very high sensitivity for CD, as well as diagnostic accuracy that is at least equivalent to the traditional immunoassays.
Innovations and breakthroughs
A new rapid point-of-care serologic screening test based on detection of anti- DGP antibodies (immunoglobulin, IgA and IgG) and total IgA by a lateral flow immunochromatographic assay was evaluated in a pediatric and adult population and compared to ELISA reference laboratory serology assays. The new test was found to be rapid and highly accurate for ruling out CD in patients with CD-related symptoms.
Applications
The test can be easily performed during the course of a consultation visit and may represent a reliable alternative to the traditional laboratory assays, and appears to be highly suitable for ruling out CD in primary care centers in patients with CD-related symptoms.
Peer review
The manuscript evaluates the use of a new point-of-care assay for diagnosing CD in a clinical setting and compares its use to traditional tTG ELISA measurements. The test is based on simultaneous detection of IgA and IgG DGP antibodies and total IgA. The test shows a good accuracy in diagnosing CD. This is important as it suggests the test as a reliable alternative to laboratory assays for ruling out CD in patients with CD-related symptoms.
Footnotes
Supported by the Swiss Celiac Association, Association Romande de Coeliakie, No. ME 8309 awarded to Schäppi MG
P- Reviewers Ivanovski PI, Ji JQ, Rostami-Nejad M S- Editor Gou SX L- Editor A E- Editor Zhang DN
References
- 1.Husby S, Koletzko S, Korponay-Szabó IR, Mearin ML, Phillips A, Shamir R, Troncone R, Giersiepen K, Branski D, Catassi C, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–160. doi: 10.1097/MPG.0b013e31821a23d0. [DOI] [PubMed] [Google Scholar]
- 2.Reeves GE, Squance ML, Duggan AE, Murugasu RR, Wilson RJ, Wong RC, Gibson RA, Steele RH, Pollock WK. Diagnostic accuracy of coeliac serological tests: a prospective study. Eur J Gastroenterol Hepatol. 2006;18:493–501. doi: 10.1097/00042737-200605000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Giersiepen K, Lelgemann M, Stuhldreher N, Ronfani L, Husby S, Koletzko S, Korponay-Szabó IR. Accuracy of diagnostic antibody tests for coeliac disease in children: summary of an evidence report. J Pediatr Gastroenterol Nutr. 2012;54:229–241. doi: 10.1097/MPG.0b013e318216f2e5. [DOI] [PubMed] [Google Scholar]
- 4.Tursi A, Brandimarte G, Giorgetti G, Gigliobianco A, Lombardi D, Gasbarrini G. Low prevalence of antigliadin and anti-endomysium antibodies in subclinical/silent celiac disease. Am J Gastroenterol. 2001;96:1507–1510. doi: 10.1111/j.1572-0241.2001.03744.x. [DOI] [PubMed] [Google Scholar]
- 5.Vahedi K, Mascart F, Mary JY, Laberenne JE, Bouhnik Y, Morin MC, Ocmant A, Velly C, Colombel JF, Matuchansky C. Reliability of antitransglutaminase antibodies as predictors of gluten-free diet compliance in adult celiac disease. Am J Gastroenterol. 2003;98:1079–1087. doi: 10.1111/j.1572-0241.2003.07284.x. [DOI] [PubMed] [Google Scholar]
- 6.Dahle C, Hagman A, Ignatova S, Ström M. Antibodies against deamidated gliadin peptides identify adult coeliac disease patients negative for antibodies against endomysium and tissue transglutaminase. Aliment Pharmacol Ther. 2010;32:254–260. doi: 10.1111/j.1365-2036.2010.04337.x. [DOI] [PubMed] [Google Scholar]
- 7.Schwertz E, Kahlenberg F, Sack U, Richter T, Stern M, Conrad K, Zimmer KP, Mothes T. Serologic assay based on gliadin-related nonapeptides as a highly sensitive and specific diagnostic aid in celiac disease. Clin Chem. 2004;50:2370–2375. doi: 10.1373/clinchem.2004.036111. [DOI] [PubMed] [Google Scholar]
- 8.Liu E, Li M, Emery L, Taki I, Barriga K, Tiberti C, Eisenbarth GS, Rewers MJ, Hoffenberg EJ. Natural history of antibodies to deamidated gliadin peptides and transglutaminase in early childhood celiac disease. J Pediatr Gastroenterol Nutr. 2007;45:293–300. doi: 10.1097/MPG.0b013e31806c7b34. [DOI] [PubMed] [Google Scholar]
- 9.Niveloni S, Sugai E, Cabanne A, Vazquez H, Argonz J, Smecuol E, Moreno ML, Nachman F, Mazure R, Kogan Z, et al. Antibodies against synthetic deamidated gliadin peptides as predictors of celiac disease: prospective assessment in an adult population with a high pretest probability of disease. Clin Chem. 2007;53:2186–2192. doi: 10.1373/clinchem.2006.081364. [DOI] [PubMed] [Google Scholar]
- 10.Nemec G, Ventura A, Stefano M, Di Leo G, Baldas V, Tommasini A, Ferrara F, Taddio A, Città A, Sblattero D, et al. Looking for celiac disease: diagnostic accuracy of two rapid commercial assays. Am J Gastroenterol. 2006;101:1597–1600. doi: 10.1111/j.1572-0241.2006.00597.x. [DOI] [PubMed] [Google Scholar]
- 11.Godfrey JD, Murray JA. A rapid antibody test had high specificity but low sensitivity for diagnosing coeliac disease. Evid Based Med. 2008;13:118. doi: 10.1136/ebm.13.4.118. [DOI] [PubMed] [Google Scholar]
- 12.Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185–1194. doi: 10.1097/00042737-199910000-00019. [DOI] [PubMed] [Google Scholar]
- 13.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 14.Kumar R, Indrayan A. Receiver operating characteristic (ROC) curve for medical researchers. Indian Pediatr. 2011;48:277–287. doi: 10.1007/s13312-011-0055-4. [DOI] [PubMed] [Google Scholar]
- 15.Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, Schuppan D. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 16.Rubio-Tapia A, Kyle RA, Kaplan EL, Johnson DR, Page W, Erdtmann F, Brantner TL, Kim WR, Phelps TK, Lahr BD, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009;137:88–93. doi: 10.1053/j.gastro.2009.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaukinen K, Lindfors K, Collin P, Koskinen O, Mäki M. Coeliac disease--a diagnostic and therapeutic challenge. Clin Chem Lab Med. 2010;48:1205–1216. doi: 10.1515/CCLM.2010.241. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong MJ, Robins GG, Howdle PD. Recent advances in coeliac disease. Curr Opin Gastroenterol. 2009;25:100–109. doi: 10.1097/MOG.0b013e32831ef20d. [DOI] [PubMed] [Google Scholar]
- 19.Ravikumara M, Nootigattu VK, Sandhu BK. Ninety percent of celiac disease is being missed. J Pediatr Gastroenterol Nutr. 2007;45:497–499. doi: 10.1097/MPG.0b013e31812e5710. [DOI] [PubMed] [Google Scholar]
- 20.Stene LC, Honeyman MC, Hoffenberg EJ, Haas JE, Sokol RJ, Emery L, Taki I, Norris JM, Erlich HA, Eisenbarth GS, et al. Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study. Am J Gastroenterol. 2006;101:2333–2340. doi: 10.1111/j.1572-0241.2006.00741.x. [DOI] [PubMed] [Google Scholar]
- 21.Green PHR SN, Panagi SG, Goldstein SL, Mcmahon DJ, Absan H, Neugut AI. Characteristics of adult celiac disease in the USA: results of a national survey. Am J Gastroenterol. 2001;96:126–131. doi: 10.1111/j.1572-0241.2001.03462.x. [DOI] [PubMed] [Google Scholar]
- 22.Green PH, Neugut AI, Naiyer AJ, Edwards ZC, Gabinelle S, Chinburapa V. Economic benefits of increased diagnosis of celiac disease in a national managed care population in the United States. J Insur Med. 2008;40:218–228. [PubMed] [Google Scholar]
- 23.Baldas V, Tommasini A, Trevisiol C, Berti I, Fasano A, Sblattero D, Bradbury A, Marzari R, Barillari G, Ventura A, et al. Development of a novel rapid non-invasive screening test for coeliac disease. Gut. 2000;47:628–631. doi: 10.1136/gut.47.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorell L, Garrote JA, Acevedo B, Arranz E. One-step immunochromatographic assay for screening of coeliac disease. Lancet. 2002;359:945–946. doi: 10.1016/S0140-6736(02)08046-7. [DOI] [PubMed] [Google Scholar]
- 25.Ferre-López S, Ribes-Koninckx C, Genzor C, Gamen S, Peña L, Ortigosa L, Méndez E. Immunochromatographic sticks for tissue transglutaminase and antigliadin antibody screening in celiac disease. Clin Gastroenterol Hepatol. 2004;2:480–484. doi: 10.1016/s1542-3565(04)00166-1. [DOI] [PubMed] [Google Scholar]
- 26.Raivio T, Kaukinen K, Nemes E, Laurila K, Collin P, Kovács JB, Mäki M, Korponay-Szabó IR. Self transglutaminase-based rapid coeliac disease antibody detection by a lateral flow method. Aliment Pharmacol Ther. 2006;24:147–154. doi: 10.1111/j.1365-2036.2006.02957.x. [DOI] [PubMed] [Google Scholar]
- 27.Garrote JA, Sorell L, Alfonso P, Acevedo B, Ortigosa L, Ribes-Koninckx C, Gavilondo J, Méndez E. A novel visual immunoassay for coeliac disease screening. Eur J Clin Invest. 1999;29:697–699. doi: 10.1046/j.1365-2362.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- 28.Attia J. Moving beyond sensitivity and specificity: using likelihood ratios to help interpret diagnostic tests. Aust Prescr. 2003;26:111–113. [Google Scholar]


