Abstract
AIM: To investigate the lymph node metastasis patterns of gallbladder cancer (GBC) and evaluate the optimal categorization of nodal status as a critical prognostic factor.
METHODS: From May 1995 to December 2010, a total of 78 consecutive patients with GBC underwent a radical resection at Liaocheng People’s Hospital. A radical resection was defined as removing both the primary tumor and the regional lymph nodes of the gallbladder. Demographic, operative and pathologic data were recorded. The lymph nodes retrieved were examined histologically for metastases routinely from each node. The positive lymph node count (PLNC) as well as the total lymph node count (TLNC) was recorded for each patient. Then the metastatic to examined lymph nodes ratio (LNR) was calculated. Disease-specific survival (DSS) and predictors of outcome were analyzed.
RESULTS: With a median follow-up time of 26.50 mo (range, 2-132 mo), median DSS was 29.00 ± 3.92 mo (5-year survival rate, 20.51%). Nodal disease was found in 37 patients (47.44%). DSS of node-negative patients was significantly better than that of node-positive patients (median DSS, 40 mo vs 17 mo, χ2 = 14.814, P < 0.001), while there was no significant difference between N1 patients and N2 patients (median DSS, 18 mo vs 13 mo, χ2 = 0.741, P = 0.389). Optimal TLNC was determined to be four. When node-negative patients were divided according to TLNC, there was no difference in DSS between TLNC < 4 subgroup and TLNC ≥ 4 subgroup (median DSS, 37 mo vs 54 mo, χ2 = 0.715, P = 0.398). For node-positive patients, DSS of TLNC < 4 subgroup was worse than that of TLNC ≥ 4 subgroup (median DSS, 13 mo vs 21 mo, χ2 = 11.035, P < 0.001). Moreover, for node-positive patients, a new cut-off value of six nodes was identified for the number of TLNC that clearly stratified them into 2 separate survival groups (< 6 or ≥ 6, respectively; median DSS, 15 mo vs 33 mo, χ2 = 11.820, P < 0.001). DSS progressively worsened with increasing PLNC and LNR, but no definite cut-off value could be identified. Multivariate analysis revealed histological grade, tumor node metastasis staging, TNLC and LNR to be independent predictors of DSS. Neither location of positive lymph nodes nor PNLC were identified as an independent variable by multivariate analysis.
CONCLUSION: Both TLNC and LNR are strong predictors of outcome after curative resection for GBC. The retrieval and examination of at least 6 nodes can influence staging quality and DSS, especially in node-positive patients.
Keywords: Gallbladder neoplasms, Lymphatic metastasis, Lymph node excision, Lymph node ratio, Prognosis
Core tip: The presence or absence of lymph node metastasis is an important prognostic factor in patients with curatively resected gallbladder cancer (GBC). The present study evaluates the prognostic impact of number, location and ratio of involved lymph nodes, in addition to well described prognostic parameters, in patients with curatively resected GBC. The results demonstrate that total lymph node count and lymph node ratio are more appropriate to stratify GBC patients with regards to prognosis; removal and pathological examination of at least six lymph nodes can influence staging quality and disease-specific survival especially in node-positive patients.
INTRODUCTION
Gallbladder cancer (GBC) is one of the most common malignancies of the biliary tract with poor prognosis, because it is usually detected at an advanced stage due to no specific symptoms. Treatment options for GBC have evolved over the last decade, as it has become well accepted that patients benefit from radical resection[1-4]. The spread modes of GBC are direct, lymphatic, vascular, neural, intraperitoneal and intraductal. Lymph node is one of the most common sites of metastasis of GBC. The presence or absence of lymph node metastasis is an important prognostic factor in patients with curatively resected GBC[5-8]. However, the method of optimally categorizing lymph nodal involvement in GBC remains controversial[9,10]. It is increasingly being recognized that an inadequate number of lymph nodes examined may adversely influence survival and lead to understaging of GBC[11]. Some investigators have highlighted the importance of metastatic lymph node count as a means of stratification, while others rely on the location of involved nodes[12]. Some investigators have emphasized the total number of lymph nodes resected during operation[13,14]. Recent studies have also demonstrated the influence of involved lymph node count and metastatic to examined lymph nodes ratio (LNR) on survival of patients with GBC[15,16]. The present study evaluates the prognostic impact of number, location and ratio of involved lymph nodes, in addition to well described prognostic parameters, in patients with curatively resected gallbladder cancer.
MATERIALS AND METHODS
Patient population
From May 1995 to December 2010, a total of 78 consecutive patients with GBC underwent a radical resection at Liaocheng People’s Hospital. A radical resection was defined as removing both the primary tumor and the regional lymph nodes of the gallbladder. Cancer arising in the cystic duct was also included as gallbladder cancer. Eight patients with early pT stages (Tis or T1) were excluded due to their resection of only simple cholecystectomy without lymphadenectomy. Eleven patients were excluded due to incomplete clinicopathologic data or follow-up loss. As a result, 78 patients were retrospectively reviewed; these included 46 women and 32 men ranging in age from 33 years to 82 years (median, 59 years).
Radical resection procedures
Radical resection procedures consisted of cholecystectomy, en bloc hepatic resection, and lymphadenectomy with or without bile duct excision. Lymphadenectomy included en bloc clearance of cystic duct, pericholedochal, hepatic artery, portal vein, periduodenal and peripancreatic lymph nodes. Celiac artery, perigastric, superior mesenteric artery and para-aortic nodal clearances were not performed routinely in every patient, but if there was any evidence of tumor infiltration or metastasis to the near organ or tissues, these nodes would be cleared by an extended radical operation such as pancreaticoduodenectomy. The extent of liver resection was guided by the extent of the tumor’s liver infiltration, and the guiding principle is acquiring a negative surgical margin while at the same time preserving the maximal amount of liver parenchyma. A 2-cm non-anatomical wedge of gallbladder fossa was performed if the tumor was confined to gallbladder, and formal resection of segments V and IV a was performed if there was gross liver involvement.
The operative procedures are shown in Table 1. All patients underwent lymphadenectomy. The operative procedures included cholecystectomy (n = 8), wedge resection (n = 29), resection of segments IVa and V (n = 30), resection of the bile duct (n = 20), extended hepatectomy (n = 5), hepatopancreaticoduodenectomy (n = 6), with other organ tissue resection (n = 7), portal vein resection and reconstruction (n = 2), proper or right hepatic artery resection (n = 3).
Table 1.
Number of radical resection procedures and their relationship with tumor node metastasis stages
| TNM stage procedure | 0 | I | II | IIIA | IIIB | IVA | IVB | Sum |
| C + N | 1 | 2 | 11 | 3 | 7 | |||
| C + WR + N | 2 | 4 | 6 | 5 | 2 | 19 | ||
| C + S4aS5 + N | 8 | 9 | 1 | 18 | ||||
| C + ELH + N | 1 | 1 | ||||||
| C + ERH + N | 2 | 2 | ||||||
| C + BD + N | 12 | 1 | ||||||
| C + WR + BD + N | 1 | 3 | 1 | 3 | 1 | 1 | 10 | |
| C + S4As5 + BD + N | 1 | 3 | 1 | 1 | 6 | |||
| C + CH + BD + N | 1 | 1 | ||||||
| C + S4aS5 + other + N | 3 | 1 | 1 | 5 | ||||
| C + S4As5 + BD + other + N | 1 | 1 | ||||||
| C + ERH + BD + other + N | 1 | 1 | ||||||
| HPD + N | 2 | 4 | 6 | |||||
| Sum | 1 | 6 | 8 | 19 | 24 | 10 | 10 | 78 |
Tumor of the patient infiltrated the serosa at the visceral surface of the gallbladder bottom;
This patient was an incidental gallbladder cancer with a diagnosis confirmed during the initial operation by frozen section with a preoperative diagnosis of choledochal cyst. C: Cholecystectomy; N: Lymphadenectomy; WR: Wedge resection of the gallbladder fossae; S4aS5: Liver resection of segments IVa and V; ELH: Extended left hepatectomy; ERH: Extended right hepatectomy; CH: Central hepatectomy; BD: Resection of the bile duct; HPD: Hepatopancreaticoduodenectomy; Other: Other organ tissue resection; TNM: Tumor node metastasis.
Pathological examination
Immediately after resection, the operating surgeon separated the lymph nodes from the node-bearing adipose tissues of the fresh surgical specimen, which were then divided by the surgeon into individual node groups according to their locations. The specimen was then fixed in 10% buffered formaldehyde solution. Primary tumor was examined to determine the histologic type, tumor grade, depth of infiltration, tumor involvement of excised contiguous viscera and resection margins. Histologic grade was determined based on the areas of tumor with highest grade. Lymph node metastasis was defined as tumor cells detected on histopathologic examination using hematoxylin and eosin stain.
The lymph nodes retrieved were examined histologically for metastases routinely from each node. The positive lymph node count (PLNC) as well as the total lymph node count (TLNC) was recorded for each patient. Here, PLNC and TLNC represented the sum of regional, celiac artery, perigastric, superior mesenteric artery and para-aortic nodes evaluated in the patient. Then the metastatic to examined LNR was calculated.
Patient follow-up after resection
Of 78 patients, one died during the hospital stay because of liver failure after the definitive resection, giving an in-hospital mortality rate of 1.28%. Patients discharged to home were followed up regularly every 1-6 mo, with a median follow-up time of 26.50 mo (range, 2-132 mo). Adjuvant chemoradiation therapy was administered to 23 patients at the discretion of the individual surgeons. Only deaths from tumor recurrence were treated as failure cases in the analysis of disease-specific survival (DSS), whereas those from other causes were recorded as censored cases. The survival time in each patient was defined as the interval between the date of definitive resection and the date of last follow up or death.
Statistical analysis
Categorical variables were compared using the Pearson χ2 test. Numerical variables were compared using paired samples t test. Survival curves were constructed using the Kaplan-Meier method, and differences in survival were evaluated with the log rank test. Cox regression analysis was used to identify independent predictors of disease-specific survival using factors found to be significant by univariate analysis. The IBM SPSS 16.0 software (SPSS Inc., Chicago, IL, United States) was used for all statistical evaluations. All tests were two-tailed and P values less than 0.05 were considered statistically significant.
RESULTS
Pathologic features
Pathological findings were documented using the American Joint Committee on Cancer (AJCC) cancer staging manual (7th edition)[17]. Resection margin status was judged as no residual tumor (R0) in all 78 patients. The primary tumor was pTis in 1 patient, pT1 in 7 patients, pT2 in 12 patients, pT3 in 44 patients, and pT4 in 14 patients. The lymph node stage was N0 in 41 patients, N1 in 31 patients and N2 in 6 patients. The M stage was M0 in 74 patients and M1 in 4 patients. Of the metastasis patients, 1 was a single metastasis lesion on the visceral peritoneum and the other 3 were liver metastases. Then the patients were classified according to tumor node metastasis (TNM) staging: stage 0 (n = 1), stage I (n = 6), stage II (n = 8), stage IIIA (n = 19), stage IIIB (n = 24), stage IVA (n = 10) and stage IVB (n = 10).
Distribution of lymph nodes metastasis
A total of 465 lymph nodes taken from the 78 studied patients were evaluated. TLNC ranged from 1 to 24 (median, 4) per patient. According to the AJCC cancer staging manual (7th edition)[17], the topographical distribution of the analyzed lymph nodes included 361 first-station nodes and 104 second-station nodes (Table 2). There were significantly more first-station nodes per patient (median = 4; range: 1-12) than second-station nodes (median = 0; range: 0-12) (t = 10.46, P < 0.001).
Table 2.
Topographical distribution of 465 lymph nodes evaluated in 78 patients with gallbladder cancer n (%)
| Node group | Patients with node group | Lymph nodes evaluated | Patients with positive | Positive nodes |
| evaluated | nodes | |||
| Cystic duct1 | 41 (53.95) | 46 (9.89) | 18 (23.08) | 19 (19.39) |
| Pericholedochal1 | 68 (81.18) | 146 (31.40) | 20 (25.64) | 29 (29.59) |
| Periportal1 | 47 (60.26) | 74 (15.91) | 12 (15.38) | 18 (18.37) |
| Hepatic artery1 | 48 (61.54) | 69 (14.84) | 10 (12.82) | 12 (12.24) |
| Posterosuperior pancreaticoduodenal2 | 22 (28.21) | 56 (12.04) | 6 (7.69) | 12 (12.24) |
| Hilar1 | 18 (23.08) | 26 (5.59) | 4 (5.13) | 6 (6.12) |
| Right celiac2 | 8 (10.26) | 21 (4.52) | 1 (1.28) | 2 (2.04) |
| Perigastric2 | 4 (5.13) | 6 (1.29) | 0 (0.00) | 0 (0.00) |
| Superior mesenteric artery2 | 6 (7.69) | 11 (2.37) | 0 (0.00) | 0 (0.00) |
| Paraaortic2 | 6 (7.69) | 10 (2.15) | 0 (0.00) | 0 (0.00) |
| Sum | 78 (100) | 465 (100) | 37 (47.44) | 98 (100) |
First-station nodes;
Second-station nodes; according to the American Joint Committee on Cancer cancer staging manual (7th edition). Here, hilar lymph nodes classified as first-station nodes and perigastric lymph nodes classified as second-station nodes.
Of the 78 studied patients, 37 (47.44%) had a total of 98 positive lymph nodes. The number of positive nodes per patient ranged from 1 to 9 (median = 2). There were 5 (25.00%) of 20 patients with pTis to pT2 stage who had positive nodal disease, whereas 32 (55.17%) of 58 patients with pT3 to pT4 stage had positive nodal disease. The occurrence of lymph node metastasis was increased obviously with the advance of pT stage (χ2 = 5.430, P = 0.020).
The topographical distribution of all positive lymph nodes is shown in Table 2. Among the 37 node-positive patients, the prevalence of nodal disease was highest in the pericholedochal (n = 20, 54.05%) or the cystic duct (n = 18, 48.65%) node group, followed by the periportal (n = 12, 32.43%), hepatic artery (n = 10, 27.03%), posterosuperior pancreaticoduodenal (n = 6, 16.22%), hilar (n = 4, 10.81%), and right celiac (n = 1, 2.70%) node groups. The paraaortic, superior mesenteric artery and perigastric nodes were not involved in any of our patients.
Of 13 patients with a single positive node, 11 (84.62%) had nodal disease in either the pericholedochal (n = 6) or cystic duct (n = 5) node group, suggesting that initial nodal involvement occurred primarily in these node groups.
Analysis of the topographical distribution of positive lymph nodes may be helpful to derive the route of lymphatic spread from GBC (Table 2). In this study, GBC primarily spread to the first-station nodes, then to the second-station nodes.
Survival after regional lymphadenectomy
Of the overall patients, there were 22 patients who survived more than 3 years and 16 patients survived more than 5 years by the end of the follow-up; the median DSS was 29.00 ± 3.92 mo (5-year survival rate, 20.51%). The postoperative DSS of node-negative patients was significantly better than that of node-positive patients (median DSS, 40 mo vs 17 mo, χ2 = 14.814, P < 0.001, Figure 1A). Most node-negative patients achieved long-term survival after R0 resection (5-year survival rate, 26.83%). Of the 37 node-positive patients after an R0 resection, only 5 patients survived more than 5 years (5-year survival rate, 13.51%).
Figure 1.
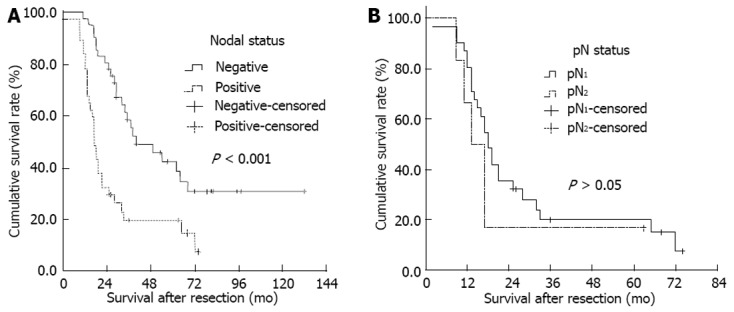
Kaplan-Meier survival estimates stratified. A: Lymph node status (negative vs positive; median disease-specific survival, 40 mo vs 17 mo); B: pN status in nodal positive patients (pN1 vs pN2; median disease-specific survival, 18 mo vs 13 mo).
We then focused on a subgroup of 37 node-positive patients who had undergone an R0 resection for survival analysis; they comprised 31 N1 stage patients and 6 N2 stage patients. The postoperative DSS was not significantly different between N1 node-positive patients (median survival time, 18 mo; 5-year survival rate, 12.90%) and N2 node-positive patients (median survival time, 13 mo; 5-year survival rate, 16.67%) (χ2 = 0.741, P = 0.389, Figure 1B). Of the 5 patients with node-positive disease who survived for more than 5 years, there were two patients who underwent a pancreaticoduodenal lymph node dissection with hepatopancreaticoduodenectomy for suspected N2 nodal disease. These findings suggested that regional lymphadenectomy could achieve an acceptable rate of long-term survival even in patients with advanced stage of nodal metastasis, provided that an R0 resection is feasible.
Cut-off values for the TNLC, PNLC and LNR
Based on the magnitude of the Log-rank test χ2 statistic, the optimal cut-off value was four nodes for the number of TLNC. Based on these results, the number of TLNC was placed into two categories in subsequent analyses (< 4 or ≥ 4, respectively). DSS of TLNC < 4 group was worse than that of TLNC ≥ 4 group (median DSS, 18 mo vs 33 mo, χ2 = 5.606, P = 0.018, Figure 2A). When node-negative patients were divided according to TLNC, there was no difference in DSS between TLNC < 4 subgroup (n = 60) and TLNC ≥ 4 subgroup (n = 21) (median DSS, 37 mo vs 54 mo, χ2 = 0.715, P = 0.398, Figure 2B). For node-positive patients, DSS of TLNC < 4 subgroup was worse than that of TLNC ≥ 4 subgroup (median DSS, 13 mo vs 21 mo, χ2 = 11.035, P < 0.001, Figure 3A). Moreover, for node-positive patients, a new cut-off value of six nodes for the number of TLNC clearly stratified them into 2 separate survival groups (< 6 or ≥ 6, respectively; median DSS, 15 mo vs 33 mo, χ2 = 11.820, P < 0.001, Figure 3B).
Figure 2.
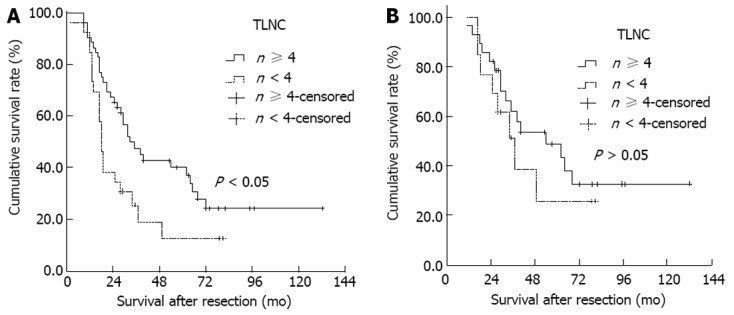
Kaplan-Meier survival estimates stratified for total lymph node count status (< 4 or ≥ 4, respectively). A: In 78 patients who underwent an R0 resection (median disease-specific survival, 18 mo vs 33 mo); B: In 41 node-negative patients (median disease-specific survival, 37 mo vs 54 mo). TLNC: Total lymph node count.
Figure 3.
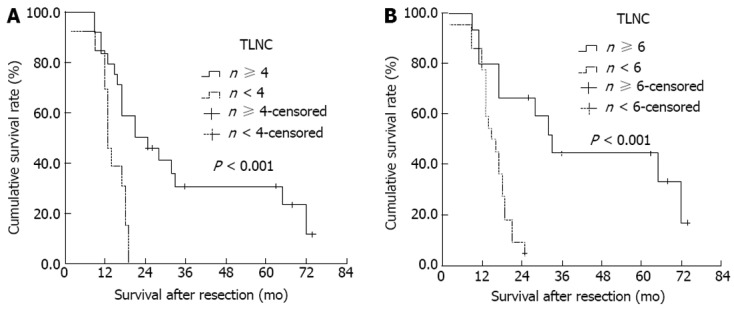
Kaplan-Meier survival estimates stratified for total lymph node count status in 37 node-positive patients. A: n < 4 or ≥ 4, respectively; median disease-specific survival, 13 mo vs 21 mo; B: n < 6 or ≥ 6, respectively; median disease-specific survival, 15 mo vs 33 mo. TLNC: Total lymph node count.
DSS progressively worsened with increasing PLNC and LNR, but no definite cut-off value could be identified. Based on the previous literature, we left the cut-off value as 3 nodes for PLNC and 50% for LNR separately[13,16].
Factors influencing disease-specific survival after resection
Univariate analyses identified liver invasion, venous invasion, pT classification, pN classification, pM classification, TNM staging, lymph node invasion, TLNC, PLNC, LNR and histological grade as significant prognostic factors (Table 3).
Table 3.
Univariate analysis of clinical and histopathologic variables
| Variable | Number of patients |
Survival rate |
P value | |
| 3-yr | 5-yr | |||
| Age (yr) | 0.222 | |||
| < 60 | 41 | 34.15% | 17.07% | |
| ≥ 60 | 37 | 24.32% | 18.92% | |
| Sex | 0.523 | |||
| Female | 46 | 28.26% | 17.39% | |
| Male | 32 | 31.25% | 18.75% | |
| Cholelithiasis | 0.374 | |||
| Present | 25 | 24.00% | 16.00% | |
| Absent | 53 | 32.08% | 18.87% | |
| Type of radical resection | 0.179 | |||
| Extended cholecystectomy | 7 | 42.86% | 42.86% | |
| Partial hepatectomy1 | 37 | 32.43% | 13.51% | |
| Partial hepatectomy and EBD resection | 23 | 30.43% | 26.09% | |
| Extended hepatectomy2 | 5 | 0.00% | 0.00% | |
| Hepatopancreaticoduodenectomy | 6 | 50.00% | 33.33% | |
| Hepatic infiltration | 0.005 | |||
| Present | 41 | 14.63% | 4.88% | |
| Absent | 37 | 51.35% | 37.84% | |
| Bile duct infiltration | 0.238 | |||
| Present | 17 | 29.41% | 23.53% | |
| Absent | 61 | 32.79% | 19.67% | |
| Venous invasion | 0.001 | |||
| Present | 10 | 0.00% | 0.00% | |
| Absent | 68 | 36.76% | 23.53% | |
| Perineural invasion | 0.539 | |||
| Present | 9 | 22.22% | 22.22% | |
| Absent | 69 | 33.33% | 20.29% | |
| Lymph node involvement | < 0.001 | |||
| Present | 37 | 16.22% | 13.51% | |
| Absent | 41 | 46.34% | 26.83% | |
| pT classification3 | 0.001 | |||
| pT0-pT2 | 20 | 60.00% | 45.00% | |
| pT3-pT4 | 58 | 22.41% | 12.07% | |
| pN classification3 | < 0.001 | |||
| pN0 | 41 | 46.34% | 26.83% | |
| pN1 | 31 | 16.13% | 12.90% | |
| pN2 | 6 | 16.67% | 16.67% | |
| pM classification3 | 0.002 | |||
| pM0 | 74 | 33.78% | 21.62% | |
| pM1 | 4 | 0.00% | 0.00% | |
| TNM staging3 | < 0.001 | |||
| 0-II | 15 | 80.00% | 60.00% | |
| III | 43 | 23.26% | 11.63% | |
| IV | 20 | 15.00% | 10.00% | |
| TLNC | 0.018 | |||
| < 4 | 26 | 15.38% | 7.69% | |
| ≥ 4 | 52 | 40.38% | 26.92% | |
| Number of positive lymph nodes | < 0.001 | |||
| 0 | 41 | 46.34% | 26.83% | |
| < 3 | 24 | 16.67% | 16.67% | |
| ≥ 3 | 13 | 15.38% | 7.69% | |
| LNR | < 0.001 | |||
| 0 | 41 | 46.34% | 26.83% | |
| < 50 | 15 | 33.33% | 33.33% | |
| ≥ 50 | 22 | 4.55% | 0.00% | |
| Histological type | 0.706 | |||
| Adenocarcinoma | 69 | 33.33% | 20.29% | |
| Others | 9 | 22.22% | 22.22% | |
| Histological grade | 0.042 | |||
| G1-G2 | 58 | 36.21% | 24.14% | |
| G3-G4 | 19 | 15.79% | 5.26% | |
Includes wedge resection and resection of segments IVa and V;
Includes extended right hepatectomy, extended left hepatectomy and central hepatectomy;
According to the American Joint Committee on Cancer cancer staging manual (7th edition). G1: Well differentiated; G2: Moderately differentiated; G3: Poorly differentiated; G4: Undifferentiated; EBD: Endoscopic balloon dilatation; TNM: Tumor node metastasis; TLNC: Total lymph node count; LNR: Lymph node ratio.
The univariately significant variables were then entered into multivariate analysis. Histological grade, TNM staging, TLNC and LNR remained as independently significant variables (Table 4). Neither location of positive lymph nodes nor PLNC were identified as an independent variable by multivariate analysis.
Table 4.
Results of Cox multivariate regression analysis
| Variable | Parameter estimate | Wald χ2 | P | Hazard ratio | 95%CI |
| Tumor node metastasis staging | 20.559 | < 0.001 | |||
| 0-II | 1.000 | ||||
| III | -3.112 | 19.846 | < 0.001 | 0.045 | 0.011-0.175 |
| IV | -1.044 | 9.341 | 0.002 | 0.352 | 0.180-0.688 |
| Lymph node ratio | 2.424 | 20.247 | < 0.001 | 11.293 | 3.929-32.465 |
| Total lymph node count | -0.147 | 14.273 | < 0.001 | 0.864 | 0.800-0.932 |
| Histological grade | -0.755 | 5.512 | 0.019 | 0.470 | 0.250-0.883 |
DISCUSSION
Studies have demonstrated that the presence or absence of lymph node metastasis is an important prognostic factor in patients with curatively resected GBC[5,13,18-20]. Patients with lymph node metastasis have significantly worse survival than those with negative nodes[1,21]. With the increasing safety of hepatic and pancreatic surgery, various radical procedures have been advocated to improve the curative outcome for advanced GBC[22-24]. Recent data also suggest that aggressive resection may improve long-term survival, even in patients with advanced stage disease[3,12,25].
It had been confirmed that the main lymphatic pathway of the gallbladder descends along the common bile duct and into the retroportal nodes, then to the posterosuperior of the head of the pancreas or around the hepatic artery, and finally to the paraaortic nodes near the left renal vein[26-28]. Based on these detailed anatomical studies, it has been suggested that lymphatic metastasis from GBC spreads widely through the hepatoduodenal ligament towards the peripancreatic region and beyond. In this study, initial nodal involvement occurred primarily in the cystic duct or pericholedochal nodes, followed by periportal and hepatic artery nodes. Posterosuperior pancreaticoduodenal and right celiac lymph nodes were involved in 16.22% of node-positive patients and were classified as N2 disease, according to the 7th edition of AJCC classification. However, we observed that the categorization of patients as having N2 disease did not adversely influence DSS as compared to those with N1 disease. Hence, we believe that even patients with N2 lymph node metastasis can achieve an ideal survival if radical lymphadenectomy is performed. An addition of pancreaticoduodenectomy could result in an R0 resection by removing extensive peripancreatic nodal disease in a select group of patients[22,23,29]. Furthermore, Murakami et al[30] suggested that it is lymph node metastasis but not para-aortic lymph node metastasis that is associated independently with longer survival by multivariate analysis. In this study, six patients were treated with pancreaticoduodenectomy and two patients survived more than five years.
The high propensity for lymphatic spread in GBC renders adequate lymphadenectomy indispensable for improving patient outcomes after resection[8,19]. However, because of the rarity of disease and low resectability rates, which limit the ability to perform large cohort studies or prospective randomized trials, the optimal extent of lymphadenectomy remains unresolved and there are no uniform evidence-based guidelines on the issue[9,10]. Accuracy of nodal staging depends on a critical number of lymph nodes analyzed; insufficient number of nodes retrieved during surgery or examined pathologically leads to underestimation of disease stage[14]. Although the 6th edition of AJCC suggests a minimum of three lymph nodes to be assessed for appropriate pathologic nodal staging of gallbladder cancer, the basis of recommendation is not clear, and there are no established standards. A large population-based study on the SEER database demonstrated that of the 2835 resected patients with T1-T3 M0 GBC, only 5.3% had a lymphadenectomy of three or more lymph nodes[31]. Also, Ito et al[14] independently suggested that retrieval and evaluation of at least six lymph nodes improves risk stratification after resection in node-negative patients. These observations indicate that retrieval of a larger number of lymph nodes than previously practiced is warranted not only for accurately staging the nodal status, but also for improving survival due to better clearance of nodal disease[13].
Although a greater number of examined nodes might improve the survival of the disease, the results of our study suggest that retrieval and evaluation of at least four nodes is perhaps optimal. Furthermore, TLNC significantly correlated with DSS in node-positive patients and allowed better prognostic substratification of these patients. For node-positive patients in this study, we can get a new cut-off value of six nodes for the number of TLNC that clearly stratifies them into 2 separate survival groups, which is more optimal than four nodes. But no definite cut-off value of TLNC could be identified for node-negative patients. Since the TLNC-survival relationship was observed only in node-positive patients and not in those node-negative patients, we believe that a higher count not only helps in stage purification but also helps improve therapeutic benefit, which is more serious in node-positive patients. These findings should heighten awareness about the importance of TLNC amongst surgeons performing lymphadenectomy for suspected node-positive patients. We believe that adequate lymphadenectomy is indispensable for improving the prognosis after radical resection in patients with GBC.
Endo et al[32] first suggested that the PLNC is more useful in assessing nodal status than the location of positive nodes in GBC. Sakata et al[12] additionally showed that the number, but not location, of positive nodes independently determined prognosis after resection. The burden of nodal disease (PLNC) also had an impact on prognosis; there was significantly reduced DSS observed in this study with involved nodes. The DSS progressively worsened with increasing PLNC; however, we were not able to identify any specific cut-off value. The use of PLNC as a prognostic factor might be limited by inherent bias of inadequate number of lymph nodes retrieved or histologically examined which leads to the phenomenon of “stage migration”. However, many recent studies (including this study) have reported a number of long-term survivors after resection for GBC with multiple positive lymph nodes[11,29,30,33]. These observations indicate that regional lymph node dissection for GBC provides long term survival for selected patients with multiple positive lymph nodes, provided that R0 resection is feasible.
LNR has been shown to be an important predictor of survival for many gastrointestinal tract cancers after surgery because it is a better and reproducible method of stratifying nodal status which incorporates not only the burden and biology of disease (PLNC) but also the quality of lymphadenectomy and pathologic examination (TLNC)[34-36]. Negi et al[16] first found that LNR, and not PLNC, was an independent prognostic factor in their study cohort comprising 57 patients with a relatively small TLNC. Our study suggests that, along with tumor TNM staging, LNR is an independent prognostic factor and another important lymph nodal variable in patients undergoing curative resection for GBC. The prognostic impact of LNR was observed in the entire group, including the subgroup of patients with positive nodes, even though we could not find an optimal cut-off value in this study. LNR is of particular value in patients who cannot adequately be staged because of the limited number of lymph nodes evaluated. In the case of insufficient lymph node evaluation, LNR will more accurately reflect the nodal status than the number of positive nodes in GBC. Patients with high LNR after radical resection might need adjuvant chemoradiation therapy to improve their prognosis.
The strengths of our study include the reasonably sized cohort of patients managed in a single institution using a standardized treatment approach. The current study has several limitations: the retrospective nature of the analysis, the relatively small number of patients spanning a long period of time, some variability in the degree of nodal dissection, and the short follow-up time for some patients. The observations need to be confirmed in larger, especially population-based, cohort. We believe, however, that these limitations did not greatly affect the results of the study as the differences between groups were too marked to have resulted from bias. In addition, the role of TLNC and LNR in assessing the nodal status for GBC is now more clearly defined than previously, based on the current study. Our results thus provide useful information for accurately staging nodal disease, predicting prognosis after resection, and selecting candidates for adjuvant chemoradiation therapy after resection.
The results of the present study demonstrate that, rather than categorizing GBC patients based on PLNC or location of involved nodes, TLNC and LNR are more appropriate tools to stratify patients with regards to prognosis. Our data also suggest that removal and pathological examination of at least six lymph nodes can influence staging quality and disease-specific survival especially in node-positive patients. This knowledge should heighten awareness amongst surgeons about the importance of performing lymphadenectomy for suspected node-positive patients, aiming to retrieve and examine an adequate number of lymph nodes.
COMMENTS
Background
Lymph node is one of the most common sites of metastasis of gallbladder cancer (GBC). The presence or absence of lymph node metastasis is an important prognostic factor in patients with curatively resected GBC. However, the method of optimally categorizing lymph nodal involvement in GBC remains controversial.
Research frontiers
It is increasingly being recognized that an inadequate number of lymph nodes examined may adversely influence survival and lead to understaging of GBC. Some investigators have highlighted the importance of metastatic lymph node count as a means of stratification while others rely on the location of involved nodes. Some investigators emphasized the total number of lymph nodes resected during operation. Recent studies have also demonstrated the influence of involved lymph node count and metastatic to examined lymph nodes ratio (LNR) on survival of patients with GBC.
Innovations and breakthroughs
The presence or absence of lymph node metastasis is an important prognostic factor in patients with curatively resected GBC. The present study evaluates the prognostic impact of number, location and ratio of involved lymph nodes, in addition to well described prognostic parameters, in patients with curatively resected GBC. The results demonstrate that total lymph node count (TLNC) and LNR are more appropriate to stratify GBC patients with regards to prognosis, and removal and pathological examination of at least six lymph nodes can influence staging quality and disease-specific survival especially in node-positive patients.
Applications
The study results suggest that TLNC and LNR are more appropriate to predict the prognosis of GBC patients, while surgeons need to achieve clearance and pathologically examine at least six lymph nodes to improve staging quality and disease-specific survival especially in node-positive patients.
Peer review
The lymph node is one of the most common sites of metastasis of GBC. The presence or absence of lymph node metastasis is an important prognostic factor in patients with curatively resected GBC.
Footnotes
P- Reviewers Gumbs A, Han TQ, Pavlidis TE, Radojcic BS S- Editor Gou SX L- Editor Logan S E- Editor Li JY
References
- 1.Kondo S, Takada T, Miyazaki M, Miyakawa S, Tsukada K, Nagino M, Furuse J, Saito H, Tsuyuguchi T, Yamamoto M, et al. Guidelines for the management of biliary tract and ampullary carcinomas: surgical treatment. J Hepatobiliary Pancreat Surg. 2008;15:41–54. doi: 10.1007/s00534-007-1279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kai M, Chijiiwa K, Ohuchida J, Nagano M, Hiyoshi M, Kondo K. A curative resection improves the postoperative survival rate even in patients with advanced gallbladder carcinoma. J Gastrointest Surg. 2007;11:1025–1032. doi: 10.1007/s11605-007-0181-4. [DOI] [PubMed] [Google Scholar]
- 3.Choi SB, Han HJ, Kim CY, Kim WB, Song TJ, Suh SO, Kim YC, Choi SY. Fourteen year surgical experience of gallbladder cancer: validity of curative resection affecting survival. Hepatogastroenterology. 2012;59:36–41. doi: 10.5754/hge10297. [DOI] [PubMed] [Google Scholar]
- 4.Marsh Rde W, Alonzo M, Bajaj S, Baker M, Elton E, Farrell TA, Gore RM, Hall C, Nowak J, Roy H, et al. Comprehensive review of the diagnosis and treatment of biliary tract cancer 2012. Part II: multidisciplinary management. J Surg Oncol. 2012;106:339–345. doi: 10.1002/jso.23027. [DOI] [PubMed] [Google Scholar]
- 5.Kim WS, Choi DW, You DD, Ho CY, Heo JS, Choi SH. Risk factors influencing recurrence, patterns of recurrence, and the efficacy of adjuvant therapy after radical resection for gallbladder carcinoma. J Gastrointest Surg. 2010;14:679–687. doi: 10.1007/s11605-009-1140-z. [DOI] [PubMed] [Google Scholar]
- 6.Principe A, Del Gaudio M, Ercolani G, Golfieri R, Cucchetti A, Pinna AD. Radical surgery for gallbladder carcinoma: possibilities of survival. Hepatogastroenterology. 2006;53:660–664. [PubMed] [Google Scholar]
- 7.Miura F, Asano T, Amano H, Toyota N, Wada K, Kato K, Takada T, Takami H, Ohira G, Matsubara H. New prognostic factor influencing long-term survival of patients with advanced gallbladder carcinoma. Surgery. 2010;148:271–277. doi: 10.1016/j.surg.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 8.Parvez T, Parvez B, Alharbi TM. Advanced carcinoma gallbladder. J Coll Physicians Surg Pak. 2007;17:175–179. [PubMed] [Google Scholar]
- 9.Pilgrim CH, Usatoff V, Evans P. Consideration of anatomical structures relevant to the surgical strategy for managing gallbladder carcinoma. Eur J Surg Oncol. 2009;35:1131–1136. doi: 10.1016/j.ejso.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Reid KM, Ramos-De la Medina A, Donohue JH. Diagnosis and surgical management of gallbladder cancer: a review. J Gastrointest Surg. 2007;11:671–681. doi: 10.1007/s11605-006-0075-x. [DOI] [PubMed] [Google Scholar]
- 11.Shirai Y, Wakai T, Hatakeyama K. Radical lymph node dissection for gallbladder cancer: indications and limitations. Surg Oncol Clin N Am. 2007;16:221–232. doi: 10.1016/j.soc.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Sakata J, Shirai Y, Wakai T, Ajioka Y, Hatakeyama K. Number of positive lymph nodes independently determines the prognosis after resection in patients with gallbladder carcinoma. Ann Surg Oncol. 2010;17:1831–1840. doi: 10.1245/s10434-009-0899-1. [DOI] [PubMed] [Google Scholar]
- 13.Schwarz RE, Smith DD. Lymph node dissection impact on staging and survival of extrahepatic cholangiocarcinomas, based on U.S. population data. J Gastrointest Surg. 2007;11:158–165. doi: 10.1007/s11605-006-0018-6. [DOI] [PubMed] [Google Scholar]
- 14.Ito H, Ito K, D’Angelica M, Gonen M, Klimstra D, Allen P, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Accurate staging for gallbladder cancer: implications for surgical therapy and pathological assessment. Ann Surg. 2011;254:320–325. doi: 10.1097/SLA.0b013e31822238d8. [DOI] [PubMed] [Google Scholar]
- 15.Shirai Y, Sakata J, Wakai T, Ohashi T, Ajioka Y, Hatakeyama K. Assessment of lymph node status in gallbladder cancer: location, number, or ratio of positive nodes. World J Surg Oncol. 2012;10:87. doi: 10.1186/1477-7819-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Negi SS, Singh A, Chaudhary A. Lymph nodal involvement as prognostic factor in gallbladder cancer: location, count or ratio? J Gastrointest Surg. 2011;15:1017–1025. doi: 10.1007/s11605-011-1528-4. [DOI] [PubMed] [Google Scholar]
- 17.Benson AB, Abrams TA, Ben-Josef E, Bloomston PM, Botha JF, Clary BM, Covey A, Curley SA, D’Angelica MI, Davila R, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw. 2009;7:350–391. doi: 10.6004/jnccn.2009.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyakawa S, Ishihara S, Horiguchi A, Takada T, Miyazaki M, Nagakawa T. Biliary tract cancer treatment: 5,584 results from the Biliary Tract Cancer Statistics Registry from 1998 to 2004 in Japan. J Hepatobiliary Pancreat Surg. 2009;16:1–7. doi: 10.1007/s00534-008-0015-0. [DOI] [PubMed] [Google Scholar]
- 19.Mayo SC, Shore AD, Nathan H, Edil B, Wolfgang CL, Hirose K, Herman J, Schulick RD, Choti MA, Pawlik TM. National trends in the management and survival of surgically managed gallbladder adenocarcinoma over 15 years: a population-based analysis. J Gastrointest Surg. 2010;14:1578–1591. doi: 10.1007/s11605-010-1335-3. [DOI] [PubMed] [Google Scholar]
- 20.Yagi H, Shimazu M, Kawachi S, Tanabe M, Aiura K, Wakabayashi G, Ueda M, Nakamura Y, Kitajima M. Retrospective analysis of outcome in 63 gallbladder carcinoma patients after radical resection. J Hepatobiliary Pancreat Surg. 2006;13:530–536. doi: 10.1007/s00534-006-1104-6. [DOI] [PubMed] [Google Scholar]
- 21.Lin HT, Liu GJ, Wu D, Lou JY. Metastasis of primary gallbladder carcinoma in lymph node and liver. World J Gastroenterol. 2005;11:748–751. doi: 10.3748/wjg.v11.i5.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miwa S, Kobayashi A, Akahane Y, Nakata T, Mihara M, Kusama K, Ogawa S, Soeda J, Miyagawa S. Is major hepatectomy with pancreatoduodenectomy justified for advanced biliary malignancy? J Hepatobiliary Pancreat Surg. 2007;14:136–141. doi: 10.1007/s00534-006-1107-3. [DOI] [PubMed] [Google Scholar]
- 23.Lim CS, Jang JY, Lee SE, Kang MJ, Kim SW. Reappraisal of hepatopancreatoduodenectomy as a treatment modality for bile duct and gallbladder cancer. J Gastrointest Surg. 2012;16:1012–1018. doi: 10.1007/s11605-012-1826-5. [DOI] [PubMed] [Google Scholar]
- 24.Liang JW, Dong SX, Zhou ZX, Tian YT, Zhao DB, Wang CF, Zhao P. Surgical management for carcinoma of the gallbladder: a single-institution experience in 25 years. Zhonghua Yixve Zazhi (Engl) 2008;121:1900–1905. [PubMed] [Google Scholar]
- 25.Nasu Y, Tanaka E, Hirano S, Tsuchikawa T, Kato K, Matsumoto J, Shichinohe T, Kondo S. The prognosis after curative resection of gallbladder cancer with hilar invasion is similar to that of hilar cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2012;19:274–280. doi: 10.1007/s00534-011-0439-9. [DOI] [PubMed] [Google Scholar]
- 26.Shirai Y, Yoshida K, Tsukada K, Ohtani T, Muto T. Identification of the regional lymphatic system of the gallbladder by vital staining. Br J Surg. 1992;79:659–662. doi: 10.1002/bjs.1800790721. [DOI] [PubMed] [Google Scholar]
- 27.Uesaka K, Yasui K, Morimoto T, Torii A, Yamamura Y, Kodera Y, Hirai T, Kato T, Kito T. Visualization of routes of lymphatic drainage of the gallbladder with a carbon particle suspension. J Am Coll Surg. 1996;183:345–350. [PubMed] [Google Scholar]
- 28.Ito M, Mishima Y, Sato T. An anatomical study of the lymphatic drainage of the gallbladder. Surg Radiol Anat. 1991;13:89–104. doi: 10.1007/BF01623880. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki R, Itabashi H, Fujita T, Takeda Y, Hoshikawa K, Takahashi M, Funato O, Nitta H, Kanno S, Saito K. Significance of extensive surgery including resection of the pancreas head for the treatment of gallbladder cancer--from the perspective of mode of lymph node involvement and surgical outcome. World J Surg. 2006;30:36–42. doi: 10.1007/s00268-005-0181-z. [DOI] [PubMed] [Google Scholar]
- 30.Murakami Y, Uemura K, Sudo T, Hashimoto Y, Nakashima A, Kondo N, Sakabe R, Kobayashi H, Sueda T. Is para-aortic lymph node metastasis a contraindication for radical resection in biliary carcinoma? World J Surg. 2011;35:1085–1093. doi: 10.1007/s00268-011-1036-4. [DOI] [PubMed] [Google Scholar]
- 31.Coburn NG, Cleary SP, Tan JC, Law CH. Surgery for gallbladder cancer: a population-based analysis. J Am Coll Surg. 2008;207:371–382. doi: 10.1016/j.jamcollsurg.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 32.Endo I, Shimada H, Tanabe M, Fujii Y, Takeda K, Morioka D, Tanaka K, Sekido H, Togo S. Prognostic significance of the number of positive lymph nodes in gallbladder cancer. J Gastrointest Surg. 2006;10:999–1007. doi: 10.1016/j.gassur.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Shirai Y, Wakai T, Sakata J, Hatakeyama K. Regional lymphadenectomy for gallbladder cancer: rational extent, technical details, and patient outcomes. World J Gastroenterol. 2012;18:2775–2783. doi: 10.3748/wjg.v18.i22.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pawlik TM, Gleisner AL, Cameron JL, Winter JM, Assumpcao L, Lillemoe KD, Wolfgang C, Hruban RH, Schulick RD, Yeo CJ, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007;141:610–618. doi: 10.1016/j.surg.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 35.Ito K, Ito H, Allen PJ, Gonen M, Klimstra D, D’Angelica MI, Fong Y, DeMatteo RP, Brennan MF, Blumgart LH, et al. Adequate lymph node assessment for extrahepatic bile duct adenocarcinoma. Ann Surg. 2010;251:675–681. doi: 10.1097/SLA.0b013e3181d3d2b2. [DOI] [PubMed] [Google Scholar]
- 36.Maduekwe UN, Lauwers GY, Fernandez-Del-Castillo C, Berger DL, Ferguson CM, Rattner DW, Yoon SS. New metastatic lymph node ratio system reduces stage migration in patients undergoing D1 lymphadenectomy for gastric adenocarcinoma. Ann Surg Oncol. 2010;17:1267–1277. doi: 10.1245/s10434-010-0914-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


