Abstract
The patient was a 30-year-old female who had undergone excision of the extrahepatic bile duct and Roux-en-Y hepaticojejunostomy for congenital biliary dilatation at the age of 7. Thereafter, she suffered from recurrent acute pancreatitis due to pancreaticobiliary maljunction and received subtotal stomach-preserving pancreaticoduodenectomy. She developed a pancreatic fistula and an intra-abdominal abscess after the operation. These complications were improved by percutaneous abscess drainage and antibiotic therapy. However, upper abdominal discomfort and the elevation of serum pancreatic enzymes persisted due to stenosis from the pancreaticojejunostomy. Because we could not accomplish dilation of the stenosis by endoscopic retrograde cholangiopancreatography, we tried an endoscopic ultrasonography (EUS) guided rendezvous technique for pancreatic duct drainage. After transgastric puncture of the pancreatic duct using an EUS-fine needle aspiration needle, the guidewire was inserted into the pancreatic duct and finally reached to the jejunum through the stenotic anastomosis. We changed the echoendoscope to an oblique-viewing endoscope, then grasped the guidewire and withdrew it through the scope. The stenosis of the pancreaticojejunostomy was dilated up to 4 mm, and a pancreatic stent was put in place. Though the pancreatic stent was removed after three months, the patient remained symptom-free. Pancreatic duct drainage using an EUS-guided rendezvous technique was useful for the treatment of a stenotic pancreaticojejunostomy after pancreaticoduodenectomy.
Keywords: Balloon dilatation, Endoscopic ultrasound-guided fine needle aspiration, Pancreaticobiliary maljunction, Pancreaticoduodenectomy, Pancreatitis, Postoperative complication
Core tip: The usefulness of pancreatic duct drainage using endoscopic ultrasonography-guided rendezvous technique for stenotic pancreaticojejunostomy after pancreaticoduodenectomy. However, this procedure requires technically skill and the success rate is low. The main reason for failure is the inability to pass through the stenotic anastomosis due to its tightness. In our case, the stenosis was not so tight because the stenosis developed about a month after the operation. A case of stenotic pancreaticojejunostomy that occurs at any early stage after pancreaticoduodenectomy with a dilated pancreatic duct is possibly a good indication.
INTRODUCTION
The surgical mortality rate after pancreaticoduodenectomy (PD) has decreased due to advances in surgical technique, but surgical morbidity has not yet decreased[1]. Stenotic pancreaticoenteric anastomosis is one of the complications after PD[2]. Endoscopic retrograde cholangiopancreatography (ERCP) is performed to treat this complication, but the success rate has been low[3]. Surgeons prefer to avoid re-operation for pancreaticoenteric stenosis due to the operative risks. Recently, interventional endoscopic ultrasonography (EUS) has greatly advanced in terms of the available devices and techniques[4-11]. Pancreaticobiliary cases treated by interventional EUS have been reported, but the usefulness of interventional EUS-fine needle aspiration (FNA) for the treatment of post-operative complications is not yet known. We here report a case with stenotic pancreaticojejunostomy that was efficiently treated by an EUS-guided rendezvous technique.
CASE REPORT
The patient was a 30-year-old female who had undergone excision of the extrahepatic bile duct and Roux-en-Y hepaticojejunostomy for congenital biliary dilatation at the age of 7 (Figure 1A). She was referred to our hospital for upper abdominal and dorsal pain. Pancreatic enzymes and inflammatory reactions (serum amylase: 210 IU/L, serum lipase: 390 IU/L, C-reactive protein: 2.4 mg/dL) were elevated, and abdominal computed tomography (CT) revealed peripancreatic fluid collection and pancreatic swelling. This patient was diagnosed as acute pancreatitis based on these findings and admitted to our hospital in 2007. Magnetic resonance imaging (MRI) and ERCP showed protein plugs in the main pancreatic duct (MPD) and pancreaticobiliary maljunction with a dilated common channel and dilated residual intra-pancreatic bile duct. The pancreaticobiliary maljunction was suspected to be the cause of the protein plugs in the MPD. We performed endoscopic pancreatic sphincterotomy and removed the protein plugs, but she suffered from acute pancreatitis due to recurrent protein plugs until 2011. PD was thought to be an adequate operation for preventing recurrent acute pancreatitis by protein plugs. After obtaining informed consent, the patient received subtotal stomach-preserving PD. Regarding reconstruction, pancreaticojejunostomy and gastrojejunostomy were performed, and Roux-en-Y hepaticojejunostomy was re-established (Figure 1B).
Figure 1.
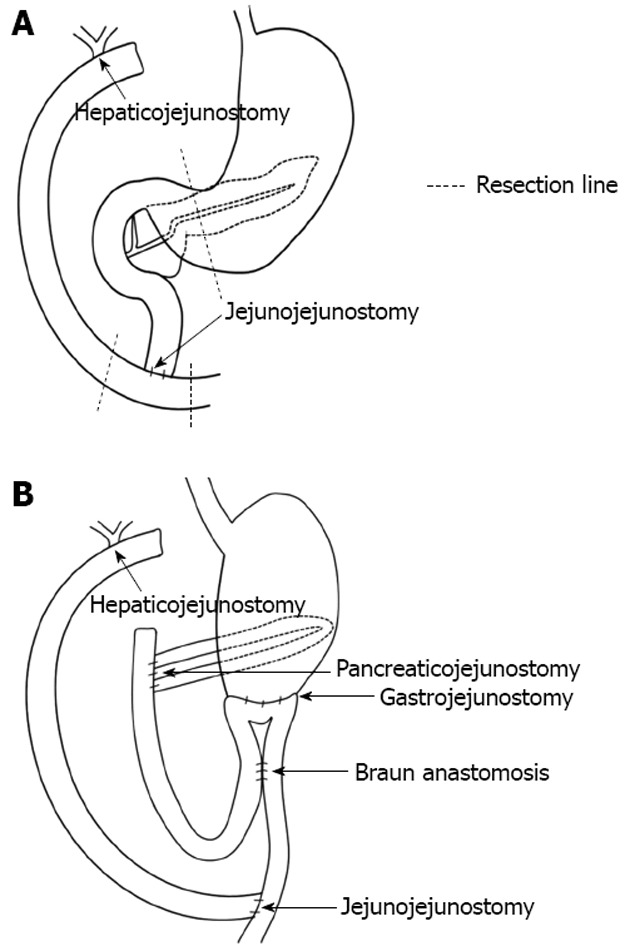
Re-construction after subtotal stomach-preserving pancreaticoduodenectomy. A: Before the operation (after excision of extrahepatic bile duct and Roux-en-Y hepaticojejunostomy; B: After the operation, pancreaticojejunostomy and gastrojejunostomy were performed. Roux-en-Y hepaticojejunostomy was re-established.
On the 16th post-operative day, the patient developed a high fever after accidental removal of an external drainage tube placed in the pancreatic duct. Abdominal CT revealed fluid collection and an intra-abdominal abscess near the anastomotic site of the pancreaticojejunostomy. After percutaneous drainage of the abscess and antibiotic therapy, her condition improved. However, upper abdominal discomfort and the elevation of serum pancreatic enzymes persisted. CT and EUS revealed a pancreatic duct dilatation (6 mm) (Figure 2). These symptoms were due to the stenosis of the pancreaticojejunostomy, and we tried ERCP using an oblique-viewing endoscope (GIF-XK240; Olympus, Tokyo, Japan). The anastomotic site of the pancreaticojejunostomy was identified, but we could not perform either pancreatography or a guidewire insertion into the pancreatic duct. We then tried EUS-guided rendezvous technique for drainage of the pancreatic duct on the 53rd post-operative day. We used a convex array echoendoscope (GF-UCT240-AL5; Olympus, Japan) and identified the echo image of the dilated pancreatic duct from the stomach. A vascular structure was confirmed by color Doppler imaging and successfully avoided. The MPD was punctured using a 19-gauge needle (Echo Tip; Cook, Wilston-Salem, NC, United States). Pancreatography was obtained by the injection of contrast medium (Figure 3A), and a 0.025-inch guidewire (VisiGlide; Olympus, Japan) was inserted into the MPD and finally reached to the jejunum through the stenotic anastomosis (Figure 3B). The echoendoscope was removed, leaving the guidewire. After introducing an oblique-viewing endoscope (GIF-XK240; Olympus, Japan) up to the pancreaticojejunostomy, we grasped the guidewire by a snare and withdrew it through the working channel (Figure 3C). The stenosis of the pancreaticojejunostomy was dilated up to 4 mm by a wire-guided balloon catheter (MaxForce; Boston Scientific, Natric, MA, United States) (Figure 3D), and replaced with a 5-Fr endoscopic nasopancreatic drainage (ENPD) tube (GADELIUS, Tokyo, Japan) (Figure 3E). Eight days later, we replaced the ENPD tube with a 7-Fr pancreatic stent (GADELIUS). Her symptom was improved and the serum amylase and lipase values returned to the normal range. When we performed ERCP after 3 mo, we removed the pancreatic stent and confirmed good pancreatic juice drainage. At 1 year after the last endoscopic treatment, she was symptom-free and the blood chemistry test results were normal.
Figure 2.
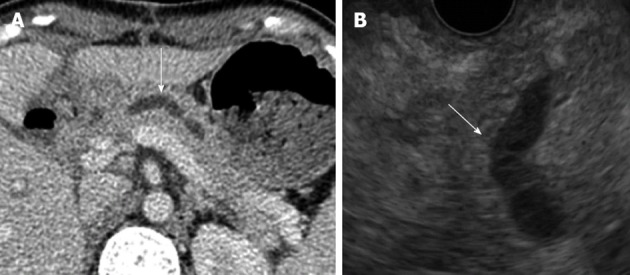
Computed tomography (A) and and endoscopic ultrasonography (B) revealed a dilated pancreatic duct (white arrows).
Figure 3.
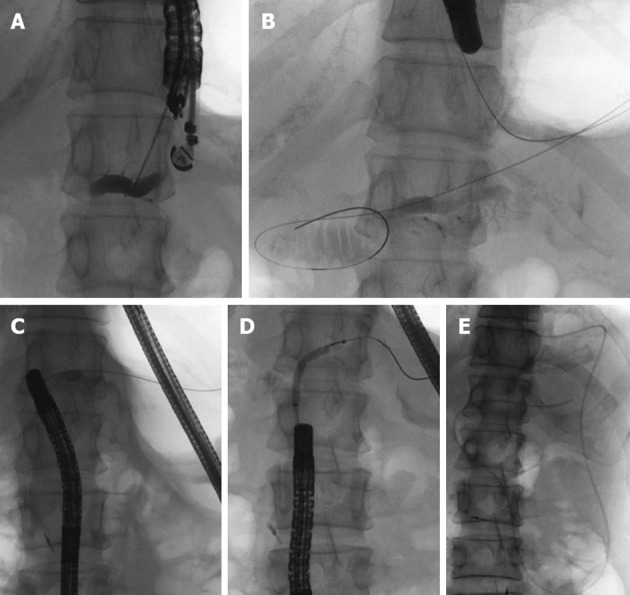
Pancreatic duct drainage procedures using endoscopic ultrasonograph-guided rendezvous technique. A: Pancreatic duct puncture and pancreatography using endoscopic ultrasonography; B: Introducing the guidewire into the jejunum through the pancreatic duct and the stenotic anastomosis; C: After exchanging echoendoscope for oblique-viewing endoscope, the guidewire was withdrawn into the working channel; D: Balloon dilatation of the stenotic anastomosis; E: Placement of an endoscopic naso-pancreatic drainage tube.
DISCUSSION
Pancreatoenteric anastomotic site stenosis can be a problematic complication after PD. Reid-Lombardo et al. reported that stenotic pancreaticojejunostomy requiring intervention was observed in 4.6% of PD patients[2]. The Patients with stenotic pancreaticojejunostomy after PD tended to be treated with ERCP-related procedures. However, the success rates were not often high due to the inability to reach or to identify the pancreaticojejunostomy through the afferent loop[3]. A long afferent loop and postoperative adhesions might hamper endoscopic treatment. Whether lateral-viewing, forward-viewing or oblique-viewing endoscope was chosen, it has been unclear which type is the appropriate choice. Kikuyama et al[4] suggested that oblique-viewing endoscope was a good option for ERCP in operated patients, since it was useful for deep cannulation and therapeutic procedures due to the instrument elevator and good angle of view for advancing into the afferent loop. If a conventional endoscope was unable to reach the pancreaticojejunostomy, single or double balloon enteroscopes should be used as an alternative[5]. However, these endoscopes were not appropriate for interventional endoscopic treatment due to the small forceps’ channel. In our case, we performed ERCP using an oblique-viewing endoscope and we could reach and identify the pancreaticojejunostomy because the afferent loop was not long (Figure 1B). However, pancreatography and guidewire insertion into the pancreatic duct were not possible.
Recently, interventional EUS has greatly advanced in terms of available devices and techniques. Bataille et al[6] first reported pancreatic duct drainage with EUS-guided rendezvous technique in 2002. Thereafter, several reports have described this procedure in post-PD patients (Table 1)[4,7-11]. This procedure is technically challenging and has an approximately 50% success rate. The reasons for failure include the impossibility of puncturing the pancreatic duct without dilatation, and the inability to pass through the stenotic anastomosis due to its tightness and less than ideal orientation of the puncture. Although the early course of this patient was favorable, it is important to follow up this patient carefully due to the risk of restenosis of pancreaticojejunostomy.
Table 1.
Reported cases of pancreatic duct drainage using endoscopic ultrasonography-guided rendezvous technique for stenosis of after pancreticojejunostomy
| Ref. | n | Age (yr)/sex | Indication | Pancreatic duct | Success | Reasons for failure | Postoperative period (yr) |
| Kikuyama et al[4] | 19 | 72/M | ARP | N/A | N/A | ||
| 20 | 66/M | ARP | |||||
| 21 | 51/M | ARP | |||||
| Mallery et al[7] | 1 | 35/F | ARP | Non dilated | Yes | 4 | |
| 2 | 55/M | Chronic pancreaticocutaneous fistula | Non dilated | No | Failed guidewire passage | 1 | |
| Kinney et al[8] | 3 | ARP | No | ||||
| 4 | ARP | No | |||||
| 5 | ARP, pancreatic stone | Yes | Failed pancreatic | ||||
| 6 | ARP, pancreatic stone | Yes | duct puncture | ||||
| 7 | N/A | ARP, chronic pain | N/A | Yes | (in two patients) | N/A | |
| 8 | ARP, chronic pain | Yes | |||||
| 9 | ARP, chronic pain | No | Failed guidewire passage | ||||
| 10 | Chronic pancreaticocutaneous fistula | No | (in three patients) | ||||
| 11 | Inwardly migrated surgical stent with recurrent pancreatitis | No | |||||
| Barkay et al[9] | 12 | 36/F | IPMN | Dilated | No | Failed guidewire passage | |
| 13 | 60/M | ARP | Dilated | Yes | |||
| 14 | 22/F | CP, pancreatic divisum | Dilated | No | Failed guidewire passage | ||
| 15 | 54/F | CP | Non dilated | No | Failed guidewire passage | N/A | |
| 16 | 53/M | IPMN | Dilated | No | Failed guidewire passage | ||
| 17 | 67/M | IPMN | Dilated | No | Failed guidewire passage | ||
| 18 | 59/F | CP | Dilated | Yes | |||
| DeWitt et al[10] | 22 | 25/F | Chronic pain | Dilated | No | Failed guidewire passage | N/A |
| Itoi et al[11] | 23 | 66/M | ARP | Dilated | Yes | 12 | |
| 24 | 51/M | ARP | Dilated | Yes | 8 |
F: Female; M: Male; ARP: Acute recurrent pancreatitis; N/A: Not available; IPMN: Intraductal papillary mucinous neoplasm; CP: Chronic pancreatitis.
Kikuyama et al[4] have described difficulty in passing the stenotic anastomosis due to a tight stenosis, since stenosis of the pancreaticoenteric anastomosis usually happens as a late complication[2]. In our case, we expected that the stenosis was not tight since the stenosis developed about a month after PD due to a pancreatic fistula and an intra-abdominal abscess. We supposed that the length of time after the operation and the severity of other complications such as a pancreatic fistula might affect the outcomes, in terms of fibrosis, edema and compression occupying the space around the anastomotic site.
The diameter of the pancreatic duct is an important factor in avoiding complications as well as success. We could achieve successful pancreatic duct drainage in this case since the MPD was dilated enough to puncture (6 mm). Fatal complications have never been reported with this procedure, while a few complications such as abscess, mild pancreatitis or transient fever were reported, and these complications mostly happened to patients with pancreatic ducts of normal diameter[7,9]. Accordingly, for the EUS-guided rendezvous techniques we should select patients who satisfy these conditions.
EUS-guided pancreaticogastrostomy was an option for the treatment of this case. EUS-guided pancreaticogastrostomy has the risk of stent dysfunctions such as obstruction and migration[12], whereas dilatation of the stenotic anastomosis by a balloon catheter has a small risk of restenosis[4]. We therefore selected balloon dilatation for stenotic pancreaticojejunostomy using the rendezvous technique. Our case suggests that stenotic pancreaticojejunostomy ocurring at any early stage after PD with a dilated pancreatic duct might be a good indication for this technique.
Footnotes
Supported by Grant-in-Aid to the Research Committee of the Intractable Pancreatic Diseases (Chairman, Shimosegawa T), provided from the Ministry of Health, Labour and Welfare of Japan
P- Reviewers Bradley EL, Hoeppner J, Mizuno S, Wakai T S- Editor Zhai HH L- Editor A E- Editor Li JY
References
- 1.Yang SH, Dou KF, Sharma N, Song WJ. The methods of reconstruction of pancreatic digestive continuity after pancreaticoduodenectomy: a meta-analysis of randomized controlled trials. World J Surg. 2011;35:2290–2297. doi: 10.1007/s00268-011-1159-7. [DOI] [PubMed] [Google Scholar]
- 2.Reid-Lombardo KM, Ramos-De la Medina A, Thomsen K, Harmsen WS, Farnell MB. Long-term anastomotic complications after pancreaticoduodenectomy for benign diseases. J Gastrointest Surg. 2007;11:1704–1711. doi: 10.1007/s11605-007-0369-7. [DOI] [PubMed] [Google Scholar]
- 3.Farrell J, Carr-Locke D, Garrido T, Ruymann F, Shields S, Saltzman J. Endoscopic retrograde cholangiopancreatography after pancreaticoduodenectomy for benign and malignant disease: indications and technical outcomes. Endoscopy. 2006;38:1246–1249. doi: 10.1055/s-2006-944970. [DOI] [PubMed] [Google Scholar]
- 4.Kikuyama M, Itoi T, Ota Y, Matsumura K, Tsuchiya T, Itokawa F, Sofuni A, Yamao K. Therapeutic endoscopy for stenotic pancreatodigestive tract anastomosis after pancreatoduodenectomy (with videos) Gastrointest Endosc. 2011;73:376–382. doi: 10.1016/j.gie.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Shimatani M, Matsushita M, Takaoka M, Koyabu M, Ikeura T, Kato K, Fukui T, Uchida K, Okazaki K. Effective “short” double-balloon enteroscope for diagnostic and therapeutic ERCP in patients with altered gastrointestinal anatomy: a large case series. Endoscopy. 2009;41:849–854. doi: 10.1055/s-0029-1215108. [DOI] [PubMed] [Google Scholar]
- 6.Bataille L, Deprez P. A new application for therapeutic EUS: main pancreatic duct drainage with a “pancreatic rendezvous technique”. Gastrointest Endosc. 2002;55:740–743. doi: 10.1067/mge.2002.123621. [DOI] [PubMed] [Google Scholar]
- 7.Mallery S, Matlock J, Freeman ML. EUS-guided rendezvous drainage of obstructed biliary and pancreatic ducts: Report of 6 cases. Gastrointest Endosc. 2004;59:100–107. doi: 10.1016/s0016-5107(03)02300-9. [DOI] [PubMed] [Google Scholar]
- 8.Kinney TP, Li R, Gupta K, Mallery S, Hunter D, Jensen E, Vickers S, Freeman ML. Therapeutic pancreatic endoscopy after Whipple resection requires rendezvous access. Endoscopy. 2009;41:898–901. doi: 10.1055/s-0029-1215081. [DOI] [PubMed] [Google Scholar]
- 9.Barkay O, Sherman S, McHenry L, Yoo BM, Fogel EL, Watkins JL, DeWitt J, Al-Haddad MA, Lehman GA. Therapeutic EUS-assisted endoscopic retrograde pancreatography after failed pancreatic duct cannulation at ERCP. Gastrointest Endosc. 2010;71:1166–1173. doi: 10.1016/j.gie.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 10.DeWitt J, Sherman S, Lillemoe KD. Fracture of an EUS-guided FNA needle during an attempted rendezvous for an inaccessible pancreatic duct. Gastrointest Endosc. 2011;73:171–173. doi: 10.1016/j.gie.2010.04.045. [DOI] [PubMed] [Google Scholar]
- 11.Itoi T, Kikuyama M, Ishii K, Matsumura K, Sofuni A, Itokawa F. EUS-guided rendezvous with single-balloon enteroscopy for treatment of stenotic pancreaticojejunal anastomosis in post-Whipple patients (with video) Gastrointest Endosc. 2011;73:398–401. doi: 10.1016/j.gie.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Tessier G, Bories E, Arvanitakis M, Hittelet A, Pesenti C, Le Moine O, Giovannini M, Devière J. EUS-guided pancreatogastrostomy and pancreatobulbostomy for the treatment of pain in patients with pancreatic ductal dilatation inaccessible for transpapillary endoscopic therapy. Gastrointest Endosc. 2007;65:233–241. doi: 10.1016/j.gie.2006.06.029. [DOI] [PubMed] [Google Scholar]


