Abstract
The technological advances in real-time ultrasound image guidance for high dose rate (HDR) prostate brachytherapy places this treatment modality at the forefront of innovation in radiotherapy. This review article will explore the rationale for HDR brachytherapy as a highly conformal method of dose delivery and safe dose escalation to the prostate, in addition to the particular radiobiological advantages it has over low dose rate and external beam radiotherapy. The encouraging outcome data and favourable toxicity profile will be discussed before looking at emerging applications for the future and how this procedure will feature alongside stereotactic radiosurgery.
Prostate cancer is the most common male malignancy in the Western world. This is largely attributable to earlier detection and screening following the introduction of prostate-specific antigen (PSA) testing into routine clinical practice in the late 1980s, and as life expectancy increases the prevalence will also increase in a more aged population [1]. As a result prostate cancer is likely to continue to be a significant health problem with potentially more localised disease being detected, and a demand for active treatment in a more elderly patient cohort of whom at least 20% are likely to fail an initial period of surveillance [2].
The traditional treatment options for those with localised prostate cancer—radical prostatectomy, external beam radiotherapy (EBRT) and low dose rate (LDR) brachytherapy—are considered to have similar efficacy and have contributed to a decline in what still remains a substantial mortality from prostate cancer [3]. Thus, factors such as the impact on quality of life, treatment time, convenience and cost will all play an increasingly important role in deciding which therapeutic modality the patient chooses.
Radiotherapy is an important therapeutic modality for the treatment of patients with localised or locally advanced prostate cancer [4]. The past few decades have seen significant advances in radiation techniques, and in particular high dose rate (HDR) brachytherapy, that together with radiosurgery are now at the forefront of innovation in the field of radiotherapy.
This overview will focus on the role of HDR brachytherapy in the treatment of prostate cancer, looking at the rationale and the advantages over LDR brachytherapy, three-dimensional conformal radiotherapy (3D-CRT) and intensity-modulated radiotherapy (IMRT). The clinical indications and experiences of its use thus far will be reviewed before considering newer applications for the future and how it compares with radiosurgery using CyberKnife® (Accuray Incorporated, Sunnyvale, CA).
The dose–response for prostate cancer and the use of three-dimensional conformal radiotherapy and intensity-modulated radiotherapy
Local tumour control is directly related to the radiation dose that it receives, with pre-clinical evidence suggesting that at least a dose of 70 Gy is needed to control prostate cancer [5]. Moreover, clinical studies have also shown that disease control rates for intermediate- to high-risk disease can be improved with higher doses of EBRT. For example, a sequential dose-escalating study from Zelefsky et al [6] indicated a steady reduction in the post-treatment positive biopsy rate from 54% at 64.8 Gy to 34% at 70.2 Gy, 23% at 75.6 Gy and 10% at 81 Gy. Similarly, a randomised study from the MD Anderson Cancer Center comparing 70 Gy with 78 Gy showed an improved disease-free survival with the higher radiation dose, with the 6-year freedom-from-failure rate increasing from 64% to 70% [7]. However, such dose escalation came at a price of increasing gastrointestinal and genitourinary toxicity with the actuarial risk of grade ≥2 rectal toxicity at 6 years of 12% and 26% for the 70 and 78 Gy arms, respectively (p=0.001) [7]. Subgroup analysis suggested that the benefit of dose escalation was greater for those with a pre-treatment PSA level >10 ng ml–1. The data from these trials contributed to the general consensus that many patients with localised prostate cancer should receive at least 74 Gy [8].
The efficacy of radiotherapy depends on trying to achieve optimal biological and clinical effects on the tumour on one hand, while sparing normal tissue on the other. Considerable advances in external beam radiation technology over the last decade have led to the development of 3D-CRT and IMRT, which closely match and modulate the high-dose volume to the tumour target while reducing the radiation to dose-limiting normal tissues. Such “shaping” of the radiotherapy beam is achieved using linear accelerators with multileaf collimators. A randomised Phase 3 trial conducted at the Royal Marsden Hospital, London, UK, has shown that a significant reduction in rectal toxicity can be achieved when compared with standard non-conformal/conventional EBRT, with a reduction in the rate of proctitis from 18% to 8% [9]. Such reduction in toxicity formed the basis of testing the concept of dose escalation, which might improve treatment efficacy [10]. There is now good clinical evidence to confirm that IMRT can reduce acute and late occurring toxicities, and thereby serve as a tool for dose escalation. For example, Zelefsky and colleagues [11] followed a series of 772 patients who were treated with a dose of either 81 Gy or more for a median of 24 months. This resulted in grade ≥2 toxicity of just 4% at 3 years.
The advantages of brachytherapy over external beam radiotherapy
Prostate brachytherapy is a form of radiotherapy in which radiation delivery is targeted directly at the prostate gland via a radiation source that is implanted or temporarily placed within the gland. Brachytherapy has a long heritage in cancer treatment, first being used over 100 years ago [12]. Traditionally, brachytherapy was delivered by using radioactive seeds that were permanently implanted into the prostate using an open retropubic approach, delivering radiation at a continuous LDR [13]. This has now been succeeded by a transperineal approach using transrectal ultrasound. There are now good robust durable data for the efficacy of LDR brachytherapy with results similar to both surgery and EBRT [14]. As such, it is now well established as a treatment modality for localised prostate cancer.
More recently, HDR brachytherapy has emerged into the clinical arena. This procedure involves using a radiation source (iridium) that is delivered from a chamber through a series of catheters that are temporarily placed within the prostate transperineally using ultrasound guidance.
Brachytherapy represents the ultimate in conformal therapy [15,16], because the dose decreases exponentially with increasing distance from the radioactive source according to the inverse-square law. This allows the dose to surrounding normal tissues to be minimised, thereby permitting safe dose escalation (>140 Gy) to the prostate [17,18]. Unlike EBRT, any movement of the prostate during treatment does not need to be taken into account as the implanted radioactive sources move with the prostate. It is these two factors that make brachytherapy a potentially more attractive option than either 3D-CRT or IMRT.
The conformality achievable with brachytherapy results in a significant reduction in the volume of healthy tissue receiving unnecessary radiation, with the potential to reduce the incidence of urinary and sexual function side-effects compared with surgery, and a lower incidence of bowel side-effects than EBRT [19]. The reduction in radiation dose exposure to nearby normal tissue also reduces the risk of radiation-induced secondary malignancies, notably bowel and bladder cancer, the risk of which increases with time [20]. This is an important factor when considering radiotherapy as a radical treatment option in younger patients. Brachytherapy also has significantly shorter treatment times than EBRT—day(s) rather than weeks—and shorter recovery times than surgery [17]. In addition, brachytherapy is highly cost-effective with lower set-up and maintenance costs than modern conformal EBRT [21].
The rationale for high dose rate brachytherapy: radiobiological advantage over low dose rate
The attractiveness of HDR over LDR brachytherapy lies in the understanding of the radiobiology of prostate cancer. There is a growing belief that prostate cancer is particularly sensitive to radiation delivered at a high dose per fraction, and this radiation fractionation sensitivity is reflected by a low α/β ratio. Tissues with a lower α/β ratio will experience greater cell killing by larger fraction size than tissues with a higher ratio. Although the α/β ratio for prostate cancer is unknown, most investigators believe it to be around 1.4–1.8 [22,23]. This is even lower than the typical α/β ratio of late normal tissue (around 3) [24-26], and much lower than the α/β ratio of acutely reacting normal tissues and most cancers (around 10).
The significance of this is shown in Figure 1. If the α/β ratio of normal tissue is less than that of a tumour, then radiation delivered at high dose per fraction, such as HDR brachytherapy, will theoretically result in greater killing of normal tissue and hence more toxicity. If, however, the reverse were the case, and the tumour had a lower α/β ratio than that of normal tissue, high dose per fraction EBRT or HDR brachytherapy would result in relative sparing of normal tissues. This suggests that for prostate cancer either HDR brachytherapy or hypofractionated external beam regimes offer the potential for better tumour control rates than conventional (EBRT using 2 Gy per fraction) treatments with a reduction in late sequelae [27].
Figure 1.
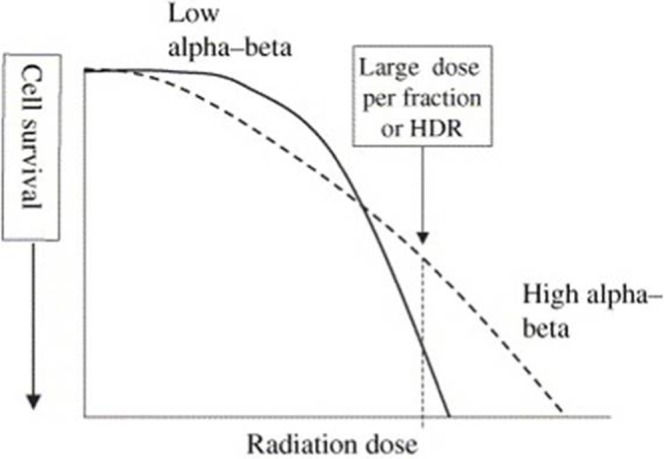
Idealised cell survival curves of a tissue with a low α/β ratio (solid line) and one with a high α/β ratio (dashed). A higher dose per fraction or high dose rate (HDR) brachytherapy will result in lower cell survival for tissues with a lower α/β ratio.
Table 1 illustrates how much extra radiation dose can be delivered using HDR when combined with EBRT, by comparing the biological equivalent doses at 2 Gy per fraction, for different fractionation regimes and assuming different α/β ratios. A hypofractionated regime (55 Gy over 20 fractions) and a standard regime of 74 Gy over 37 fractions are compared with a dose regime of 44 Gy over 22 fractions to the pelvis, with 17 Gy over 2 fractions of HDR boost to the prostate. Assuming an α/β ratio of 10 for early responding tissues (i.e. bowel) we see that similar dose equivalents are achieved with either HDR or EBRT (and therefore the potential for similar early toxicity), but if we assume a low α/β ratio for prostate cancer then the biological equivalent dose is substantially greater than with standard or hypofractionated EBRT alone, supporting safe dose escalation with HDR.
Table 1. Comparison of biologically equivalent doses (BEDs) as total doses at 2 Gy per fraction for different dose fractionation regimens.
| Dose schedule | BED10 (early responding tissues) | BED1.5 (late responding tissue/prostate cancer) |
| EBRT alone schedule (hypofractionation) | ||
| 55 Gy in 20 daily fractions | 70.1 | 155.8 |
| EBRT+HDR brachytherapy boost | ||
| 44 Gy in 22 daily fractions | 52.8 | 102.7 |
| 17 Gy in two fractions | 31.5 | 113.3 |
| Total dose | 84.3 | 216.0 |
| EBRT alone schedule (conventional) | ||
| 74 Gy in 37 daily fractions | 87.3 | 162.8 |
EBRT, external beam radiation therapy; HDR, high dose rate.
Indications for high dose rate brachytherapy
Currently, the main indication for HDR brachytherapy is in the context of safe dose escalation to the prostate, for intermediate- and high-risk disease (Table 2) in which pelvic treatment is required and the radiation dose is limited by bowel tolerance. In these circumstances, the HDR is given as a boost treatment to the prostate together with EBRT to the pelvis. This has gained National Institute for Health and Clinical Excellence approval since 2005 [28]. Although there are reports of using HDR brachytherapy as monotherapy, this tends to be reserved for lower-risk disease and should be considered investigational at this stage.
Table 2. Risk groups for localised prostate cancer.
| Parameter | Low risk | Intermediate risk | High risk |
| Tumour stage | T1–T2a and | T2b–T2c or | T3a or |
| Gleason score | 2–6 and | 7 or | 8–10 or |
| Pre-treatment PSA | ≤10 ng ml–1 | 10–20 ng ml–1 | >20 ng ml–1 |
PSA, prostate-specific antigen.
The main contraindications for HDR are those patients who are not medically fit to tolerate a general anaesthetic, and those with significant urinary symptoms (scoring >18 on the International Prostate Symptom Score) or who have had a previous transurethral resection of the prostate (TURP) (increased risk of developing urinary incontinence) [29]. In addition, a prostate volume of >60 cm3 is likely to be associated with an increased risk of pubic arch interference, thus preventing the insertion of the needles to adequately cover the prostate. However, these factors are relative rather than absolute contraindications; for example, it is possible to perform HDR under spinal anaesthesia, and the increased urinary toxicity associated with those who have had a previous TURP reduces after a time interval of 6 months. Such judgement will depend on the experience of each brachytherapy centre.
Procedure
There are a number of sophisticated treatment planning systems and remote afterloaders available from various commercial suppliers that allow real-time image-guided HDR prostate brachytherapy.
A typical real-time image-guided HDR procedure is carried out under general anaesthetic with the patient in the dorsal lithotomy position. Under transrectal ultrasound, guidance needles are inserted into the prostate via transperineal implantation through a grid, as shown in Figure 2a. Transrectal ultrasound provides real-time imaging, good image quality of the prostate boundary and clear visualisation of the needles. One of the limitations of transrectal ultrasound, however, is poor soft-tissue resolution; therefore, a marker wire or aerated gel is inserted into the urinary catheter in order to visualise the bladder and urethra. The anterior of the rectum is visualised in contact with the ultrasound probe and image quality is improved with the aid of a saline-filled endorectal balloon on the ultrasound probe.
Figure 2.
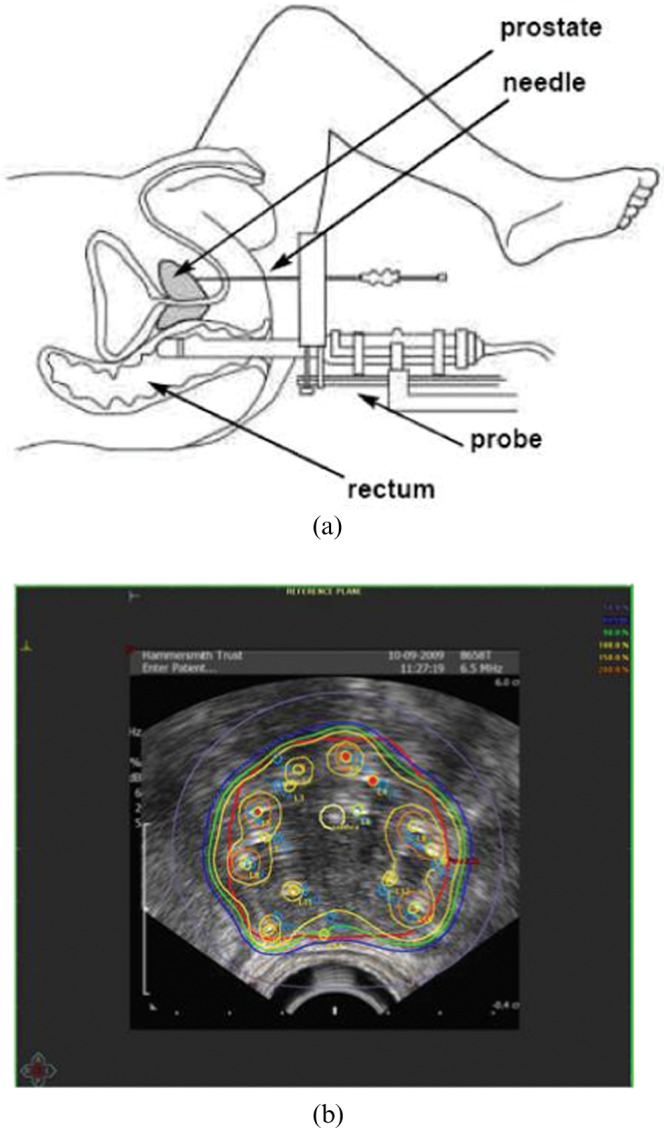
(a) Needle insertion is carried out via transperineal implantation under ultrasound guidance. (b) Typical high dose rate dose distribution.
CT and MRI, where available, can provide suitable images for brachytherapy planning; however, unlike ultrasound, they are not real time, but most treatment planning systems allow fusion of various imaging modalities to provide further imaging information.
Needle positions are chosen prior to their insertion in the form of a pre-plan; typically, anywhere between 12 and 20 needles will be needed to cover prostate gland volumes of 25–60 cm3, and the exact needle configuration will depend on the individual prostate anatomy. In order to ensure coverage of the base of the prostate, the needles are overinserted and therefore pushed beyond the prostate base, typically to a fixed depth (1.2–2.0 cm beyond the prostate base). Source positions and source dwell times (length of time the source remains at the dwell position) are determined using inverse planning algorithms to provide an optimal dose distribution to the prostate gland while minimising the dose to critical structures such as the bladder, urethra and rectum, as shown in Figure 2b.
Under ultrasound guidance each needle is tracked into the planning system during insertion, and therefore any deviations from the pre-planned ideal paths are modelled appropriately. When all of the needles have been tracked and their true locations entered into the planning system, source positions and source dwell times are recalculated and modified when necessary.
This information is then transferred to the remote afterloader, which delivers the source to the appropriate needles in turn, for the dwell positions and dwell times determined in the live plan.
A key benefit of this method over LDR prostate brachytherapy is its flexibility. Under real-time ultrasound guidance dwell positions and dwell times can be modified to provide truly precise, dynamic, real-time image-guided brachytherapy, suggesting that HDR prostate brachytherapy offers improved dose distributions and better dosimetric selectivity while sparing critical structures.
Evidence for the efficacy of high dose rate brachytherapy boost
The use of HDR brachytherapy as a boost to EBRT is now well established, with studies reporting durable 10-year freedom from biochemical failure in excess of 60% for high-risk groups and more favourable results for low- and intermediate-risk prostate cancer (Table 3). This is especially encouraging if these results are compared with historical data of using whole pelvic EBRT and hormonal treatment, as carried out in the Radiation Therapy Oncology Group trial RTOG 9413, for essentially intermediate- to high-risk prostate cancer (patients having a lymph node risk of >15%) in which progression-free survival does not reach beyond 50% at 10 years [30].
Table 3. Summary of studies showing freedom from biochemical relapse after high dose rate (HDR) brachytherapy combined with external beam radiotherapy (EBRT), according to risk group.
| Reference | Dose schedule | No. of patients | Low risk (%) | Intermediate risk (%) | High risk (%) | End point (years) |
| Aström et al [34] | EBRT: 50 Gy @ 2 Gy per fraction | 214 | 100 | 100 | 86 | 4 |
| HDR: 2×10 Gy per fraction | ||||||
| Flynn et al [38] | NAHT: 86% | 674 | 97 | 92 | 72 | 5 |
| EBRT: 45 Gy @ 1.8 Gy per fraction | ||||||
| HDR: 15.5–21.0 Gy in 3 or 4 fractions | ||||||
| Galalae et al [33] | EBRT: 45.6−50.0 Gy @ 1.8–2.0 Gy per fraction | 611 | 96 | 88 | 69 | 5 |
| HDR: BED 79.6–123.0 Gy | ||||||
| Galalae et al [39] | NAHT: 0% | 324 | – | 85 | 81 | 5 |
| BED: <94 Gy vs >94 Gy | ||||||
| Guix et al [41] | EBRT: 46–66 Gy @ 2 Gy per fraction | 445 | – | 95 | 94 | 5 |
| HDR: 2×5–8 Gy | ||||||
| Izard et al [43] | NAHT: median 6 months | 165 | 100 | 95 | 67 | 5 |
| EBRT: 45.0–59.4 Gy @ 1.8 Gy per fraction | ||||||
| PDR BRT: 18 Gy in 3 fractions | ||||||
| Martinez et al [44] | NAHT: no | 207 | – | 85 | 75 | 5 |
| HDR: 5.5–11.5 Gy per fraction | ||||||
| Phan et al [46] | NAHT: 36% | 309 | 100 | 100 | 97 | 5 |
| EBRT: 36.0–50.4 @ 1.8–2.0 Gy per fraction | ||||||
| HDR: 22–24 Gy | ||||||
| Yamada et al [47] | EBRT: 45.0–50.4 Gy @ 1.8 Gy per fraction | 105 | 100 | 98 | 92 | 5 |
| HDR: 5.5–7.0 Gy in single fraction | ||||||
| Pellizzon et al [45] | EBRT: 45 Gy median | 209 | 91 | 90 | 89 | 5 |
| HDR: 20 Gy median | ||||||
| Agoston et al [36] | EBRT: 60 Gy median | 280 | – | 84 | 82 | 5 |
| HDR: 10 Gy in single fraction | ||||||
| Demanes et al [37] | NAHT: no | 209 | 93 | 82 | 62 | 10 |
| EBRT: 36 Gy @ 1.8 Gy per fraction | ||||||
| HDR: 22–24 Gy in 4 fractions | ||||||
| Ghilezan et al [40] | NAHT: 43% | 1577 | – | 88 | 74 | 10 |
| EBRT: 40 Gy median | ||||||
| HDR: 24 Gy median | ||||||
| Hasan et al [42] | 886 | 98 | 92 | 71 | 10 |
BED, biologically equivalent dose; NAHT, neoadjuvant hormone therapy; PDR, pulse dose rate brachytherapy.
Two randomised trials have helped to establish the superiority of HDR brachytherapy boost over EBRT alone. In the randomised study by Kestin et al [31], the overall 5-year actuarial survival with HDR brachytherapy plus EBRT was 86%, compared with 54% with EBRT alone (p<0.001). In the trial by the Mount Vernon Group [32], 220 patients were randomised to receive either EBRT alone (55 Gy over 20 fractions) or a hypofractionated EBRT schedule (35.75 Gy over 13 fractions during 2.5 weeks) together with an HDR boost (17 Gy over 2 fractions during 24 h). The trial was stratified for important prognostic parameters, including tumour stage, presenting PSA, Gleason score and use of adjuvant hormonal therapy. With a median follow-up of 30 months (range 3–91) a significant improvement in actuarial biochemical relapse-free survival was seen in favour of the combined brachytherapy schedule (p=0.03) [32]. These randomised trials support the outcome data from a number of case series with 5-year overall survival figures of 85–95% [33-35], and 1 reporting a 10-year overall survival of 65% [33].
A wide range of HDR and fractionation schedules have been published [33,34,36-47] (Table 3). Typically, 1–4 fractions are usually combined with EBRT at a dose of 36–54 Gy, either with a single implant or with 2 implants separated by 1–2 weeks. From a review of the published literature, the median reported total brachytherapy dose is 20 Gy over 2 fractions, and the median external beam dose is 45 Gy. There is an increasing trend, however, for logistical reasons to use a single implant of 12.5–15.0 Gy, which has a comparable efficacy [4,48].
In a more recent systematic review on radiotherapy for prostate cancer, Pieters et al [49] looked at over 40 studies using dose-escalated EBRT alone or EBRT combined either with radioactive seeds (LDR) or with HDR brachytherapy. Using the pooled data of this meta-analysis and accepting the limitations of different definitions of biochemical failure, the combination of EBRT and HDR brachytherapy seemed more favourable in terms of both biochemical control and overall survival, probably because of the higher doses of radiation delivered to the prostate with the HDR.
The safety profile
Just as there is evidence supporting the efficacy of combined modality treatment using an HDR boost, there are also reasonable data to suggest that it has an acceptable toxicity profile compared with that of EBRT alone [31,34-38,41,43,44,46,47,50-62] (Table 4).
Table 4. Summary of studies showing toxicity after high dose rate (HDR) brachytherapy combined with external beam radiotherapy.
| Reference | Sample size | Genitourinary toxicity | Gastrointestinal toxicity | Erectile dysfunction | Follow-up (months) |
| Borghede et al [50] | n=50 | 8% G3 | 0% G3 | 10% | 45 |
| Mate et al [58] | n=104 | 6.7% urethral stricture | |||
| Kestin et al [31] | n=161 | 5% G3, 0% G4 | 29% | 36 | |
| 4% urethral stricture | |||||
| Syed et al [61] | n=200 | 20% G3, 10% G4, late 2% G3 | 1.5% | 30% | 30 |
| 1.5% urethral stricture | |||||
| <1% urinary incontinence | |||||
| Deger et al [35] | n=230 | 12.2% G3 and 4 | 1.7% recto-urethral fistula | 40 | |
| 7.4% urethral strictures | |||||
| 3% G2–3 urinary incontinence | |||||
| Galalae et al [52] | n=144 | 2.3% G3 | 4.1% G3 | 96 | |
| Martinez et al [44] | n=207 | 8% G3, 0% G4 | 0.5% G3, 0.5% G4 | 51% | 60 |
| 1.4% urethral stricture | |||||
| Martin et al [56] | n=2004 | 5% G3 | 1% G3 | 32 | |
| Hiratsuka et al [53] | n=71 | 6% urethral structure | 44 | ||
| Aström et al [34] | n=214 | 17% transient haematuria | 0% G3 | 14% | 48 |
| 7% urethral stricture | |||||
| Demanes et al [37] | n=209 | 6.7% G3, 1% G4 | 0% | 33% | 120 |
| 6.7% urethral stricture | |||||
| 3.8% urinary incontinence | |||||
| Izard et al [43] | n=165 | 3% G3, 1.4% G4 | 2.6% G3, 0% G4 | 39% | 60 |
| 1.2% urinary incontinence | |||||
| Yamada et al [47] | n=105 | 47% | 60 | ||
| Chin et al [51] | n=65 | 4.6% G3 | 0% | 42 | |
| 6.2% G3, 1.5% G4 | |||||
| 4.6% urethral stricture | |||||
| Flynn et al [38] | n=674 | 2.5% and 15.8% urinary incontinence without and with previous TURP | 0% | 35% at 1 year, 40% at 2 years | 60 |
| Guix et al [41] | n=445 | 17.2% G2 | 21.6% G2 | 60 | |
| Phan et al [46] | n=309 | 4% G3 | 1% G3 | 38% | 60 |
| 1% urinary incontinence | |||||
| 4.5% urethral stricture | |||||
| Rades et al [59] | n=41 | No G3 early or late toxicity | 24–35% | ||
| Kalkner et al [55] | n=154 | 4% G3 | 1% G3 | 72 | |
| Sato et al [60] | n=53 | 0% G2 | 3.8% G2 | 22.6% | 60 |
| Zwahlen et al [62] | n=196 | 7.1% G3 | 0% G3 | 66 | |
| No G4 toxicity | |||||
| Hsu et al [54] | n=112 | 2.6% G3 | 3.6% G3 | 27.7% | 30 |
| 0.9% urethral stricture | |||||
| 0.9% urinary incontinence | |||||
| Martinez et al [57] | n=472 | 2–3% G3 | <0.5% G3 | 120 | |
| Agoston et al [36] | n=280 | 14.4% G3 | 2.1% G3 | 60 | |
| 29.1% G3 previous TURP |
G, grade; TURP, transurethral resection of prostate.
Figure 3 shows the median late toxicity rates of the studies listed in Table 4. The median grade ≥3 genitourinary toxicity was 4.5% [range 0–14.4%; standard deviation (SD) 3.795], and grade ≥3 gastrointestinal toxicity was 0.5% (range 0–4.1%; SD 1.304). The rates of chronic urinary incontinence ranged from <1% to 3.8% without a previous TURP [35,37,46,54,61], which increased to 15.8–29.0% if patients had previous history of TURP [36,38]. When it was reported separately from other urological complications, the urethral stricture rate following HDR brachytherapy varied between 0.9% and 7.4% (median 4.5%), with a median time to occurrence of 2–4 years following completion of treatment [34,44,53,58].
Figure 3.
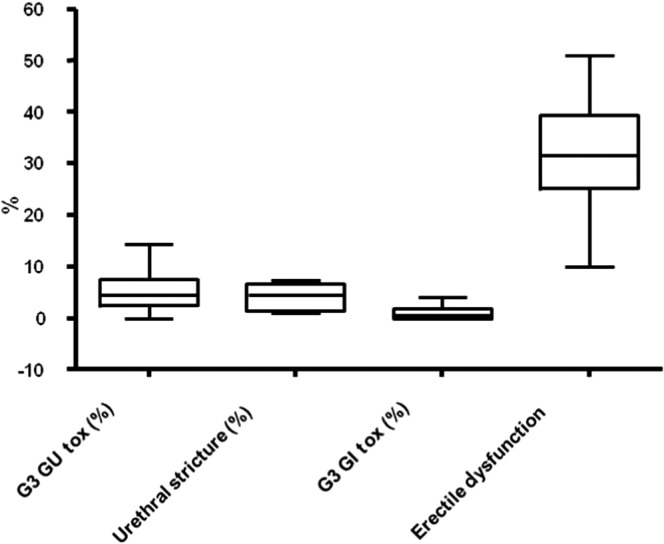
Toxicity profile of high dose rate brachytherapy boost from the studies listed in Table 4. G3, grade 3; GI, gastrointestinal; GU, genitourinary.
The potency rates following HDR brachytherapy are variable, reflecting the non-standardised definitions used to measure potency outcome across studies, and also compounded by androgen deprivation. The rates varied from 10% to 51% (median 31.5%), as shown in Table 4. The median time to occurrence was 0.9 years (range 0–6 years) [31,44]. Robinson et al [63] looked at the rates of erectile function after treatment of localised prostate cancer from 54 studies in a meta-analysis. They predicted a probability of erectile dysfunction of 24% when brachytherapy was used alone, which was significantly lower than HDR boost after EBRT (40%), EBRT alone (45%), nerve sparing (66%) and standard radical prostatectomy (75%) and cryotherapy (87%) [63].
Quality of life
Urinary incontinence and erectile dysfunction are recognised as common sequelae of radical prostatectomy (RP) [64] and have been evaluated by using physician-reported data. However, adverse urinary and sexual health-related quality of life (HRQOL) effects are more prevalent when using patient-reported data [65,66]. Prospective assessment of post-treatment quality of life (QOL) using a validated HRQOL questionnaire allows the outcomes to be expressed in robust, valid summary scores. Physician-reported data suggest that males undergoing prostate brachytherapy are also prone to urinary, bowel and sexual adverse effects. However, as with RP, physician-reported morbidity may underestimate adverse HRQOL effects when compared with patient-reported data.
Crook et al [67] assessed the HRQOL at a mean of 5.3 years for 168 males who were treated with RP or brachytherapy. The response rate was 88.4%. There was no difference in bowel or hormonal domains for RP or brachytherapy, but patients treated with brachytherapy scored significantly better in urinary and sexual domains and in patient satisfaction. Vordermark et al [68] compared the effects of two modalities (3D-CRT and HDR brachytherapy boost) on HRQOL using EORTC Quality of Life Questionnaire (QLQ-C30) and prostate-specific (PR25) modules with a median of 19 and 14 months after treatment, respectively. With response rates over 90%, diarrhoea and insomnia scores were significantly increased in both groups. In the PR25 module, scores of 3D-CRT and HDR boost patients for urinary, bowel and treatment-related symptoms were similar. The investigators concluded that dose escalation in prostate cancer by either 3D-CRT or HDR brachytherapy boost appears to result in similar HRQOL profiles.
Galalae et al [69] reported HRQOL outcomes 6.5 years after whole pelvic radiotherapy followed by HDR brachytherapy boost and found that this treatment regime was both well tolerated and curative, as indicated by patient-administered questionnaires. All scores suggested excellent QOL levels similar to patients' baseline before therapy.
Future directions for high dose rate prostate brachytherapy
The radiobiological advantage of delivering a HDR per fraction to the prostate while sparing the rectum and bladder, together with the ability to make real-time adjustments to both source positioning and dwell times of the radioactive sources thereby allowing for optimal dose distribution, has led to interest in other indications in which HDR prostate brachytherapy might be applied. One of the main practical attractions of HDR over EBRT is the shorter treatment times, and thus more recent work has focused on HDR monotherapy. This has been predominantly applied for more favourable intermediate- and low-risk disease, in which LDR has been well established. Such treatment requires multiple fractions/implants, typically given over 2–3 days, with some centres also repeating the implant 1–2 weeks later. Thus, although the number of hospital visits is lower than with EBRT, they are more intense and require inpatient stay. While the results are not as mature as with HDR boost, monotherapy studies have reported freedom from biochemical relapse rates of 89–100% in low- and intermediate-risk patients, which compare favourably with findings with LDR brachytherapy [70-76]. At present this approach is not recommended routinely, but should the long-term outcome data become favourable, this will undoubtedly help to establish HDR monotherapy as a credible option for localised prostate patients.
There is recognition that approximately 25% of all patients failing EBRT will have recurrent disease confined to the prostate [77]. These patients are normally candidates for local salvage therapies, such as cryoablation and high-intensity focused ultrasound, which were designed to replace salvage prostatectomy because of its adverse toxicity profile and the technical difficulty in removing the prostate after EBRT. Although these techniques have shown promising figures for biochemical control [78], all are still associated with high risks of urinary incontinence, erectile dysfunction and recto-urethral fistula [79,80]. LDR brachytherapy has also been used in this setting, producing similar biochemical progression-free survival data, but again is not without urinary and rectal complications [81]. In addition, notable limitations of LDR in this setting include the inability to reliably implant the seminal vesicles or allow for extracapsular extension, the inability to reposition seeds once implanted and variable seed migration [82,83].
HDR can potentially overcome these limitations, and in particular decrease the dose to the urethra (the dose-limiting structure for LDR) while maintaining adequate coverage to the prostate. Although it is technically more difficult to implant a smaller prostate gland in which there is already fibrosis from previous EBRT, there are emerging data that show early promise with better biochemical control and decreased toxicity than cryotherapy or LDR [84].
The development of stereotactic radiotherapy using CyberKnife technology is based on the principles of HDR brachytherapy (virtual HDR), aiming to deliver high doses per fraction (typically five or fewer outpatient visits) and utilising continuous image guidance to automatically track, detect and correct for intrafraction prostate movement [85]. It is an attractive option and is steadily gaining momentum as it is non-invasive (although fiducial markers for image guidance are mandatory) and does not require transperineal catheters, the requirement of a urinary catheter or hospital admission. While CyberKnife radiosurgery has the ability to recapitulate HDR dosimetry [86], it is important to note, however, that at present there are no durable outcome data, with relatively few centres in the UK able to provide this service. Given the tight “surgical” therapeutic margin associated with its use, it is not recommended for more advanced disease such as extracapsular extension [85,87] or seminal vesicle involvement, and at present the data are extrapolated mainly from the experiences of HDR to justify its use in the context of a boost treatment. In addition, the risk of secondary malignancy as a result of the multiple external beams used remains unknown, but is theoretically likely to be of more concern than with HDR.
The outlook for HDR prostate brachytherapy remains promising. Based on the premise of exploiting the low α/β ratio of prostate cancer and the high conformality associated with delivering radiation from within makes this an appealing option, especially in the context of safe dose escalation. The continuing improvements in image guidance and the flexibility of real-time planning will allow much greater precision in dosimetry without the need for taking into account, or tracking for, prostate motion. Its development as monotherapy and in the salvage setting means that this will be an invaluable tool in the armamentarium for the radiation treatment of prostate cancer.
References
- 1.Quon H, Loblaw A, Nam R. Dramatic increase in prostate cancer cases by 2021. BJU Int 2011;108:1734–8 [DOI] [PubMed] [Google Scholar]
- 2.Klotz L. Active surveillance with selective delayed intervention: using natural history to guide treatment in good risk prostate cancer. J Urol 2004;172(5 Pt 2):S48–50; discussion S50–1 [DOI] [PubMed] [Google Scholar]
- 3.Institute for Clinical and Economic Review. Management options for low-risk prostate cancer: a report on comparative effectiveness and value. ICER [serial on the Internet] 2010 [cited on 15 June 2011]. Available from: http://www.icer-review.org/index.php/mgmtoptionlrpc.html. [Google Scholar]
- 4.Mangar SA, Huddart RA, Parker CC, Dearnaley DP, Khoo VS, Horwich A. Technological advances in radiotherapy for the treatment of localised prostate cancer. Eur J Cancer 2005;41:908–21 [DOI] [PubMed] [Google Scholar]
- 5.Hanks GE, Martz KL, Diamond JJ. The effect of dose on local control of prostate cancer. Int J Radiat Oncol Biol Phys 1988;15:1299–305 [DOI] [PubMed] [Google Scholar]
- 6.Zelefsky MJ, Fuks Z, Hunt M, Lee HJ, Lombardi D, Ling CC, et al. High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer. J Urol 2001;166:876–81 [PubMed] [Google Scholar]
- 7.Pollack A, Zagars GK, Starkschall G, Antolak JA, Lee JJ, Huang E, et al. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys 2002;53:1097–105 [DOI] [PubMed] [Google Scholar]
- 8.Lukka H, Warde P, Pickles T, Morton G, Brundage M, Souhami L. Controversies in prostate cancer radiotherapy: consensus development. Can J Urol 2001;8:1314–22 [PubMed] [Google Scholar]
- 9.Dearnaley DP, Khoo VS, Norman AR, Meyer L, Nahum A, Tait D, et al. Comparison of radiation side-effects of conformal and conventional radiotherapy in prostate cancer: a randomised trial. Lancet 1999;353:267–72 [DOI] [PubMed] [Google Scholar]
- 10.Dearnaley DP, Sydes MR, Graham JD, Aird EG, Bottomley D, Cowan RA, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol 2007;8:475–87 [DOI] [PubMed] [Google Scholar]
- 11.Zelefsky MJ, Fuks Z, Hunt M, Yamada Y, Marion C, Ling CC, et al. High-dose intensity modulated radiation therapy for prostate cancer: early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys 2002;53:1111–16 [DOI] [PubMed] [Google Scholar]
- 12.Gupta VK. Brachytherapy: past, present and future. J Med Phys 1995;20:31–8 [Google Scholar]
- 13.Whitmore WF, Jr, Hilaris B, Grabstald H. Retropubic implantation of iodine 125 in the treatment of prostatic cancer. Trans Am Assoc Genitourin Surg 1972;64:55–7 [PubMed] [Google Scholar]
- 14.Moule RN, Hoskin PJ. Non-surgical treatment of localised prostate cancer. Surg Oncol 2009;18:255–67 [DOI] [PubMed] [Google Scholar]
- 15.Fatyga M, Williamson JF, Dogan N, Todor D, Siebers JV, George R, et al. A comparison of HDR brachytherapy and IMRT techniques for dose escalation in prostate cancer: a radiobiological modeling study. Med Phys 2009;36:3995–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hermesse J, Biver S, Jansen N, Lenaerts E, De Patoul N, Vynckier S, et al. A dosimetric selectivity intercomparison of HDR brachytherapy, IMRT and helical tomotherapy in prostate cancer radiotherapy. Strahlenther Onkol 2009;185:736–42 [DOI] [PubMed] [Google Scholar]
- 17.Pisansky TM, Gold DG, Furutani KM, Macdonald OK, McLaren RH, Mynderse LA, et al. High-dose-rate brachytherapy in the curative treatment of patients with localized prostate cancer. Mayo Clin Proc 2008;83:1364–72 [DOI] [PubMed] [Google Scholar]
- 18.Stewart AJ, Jones B. Radiobiologic concepts for brachytherapy. Philadelphia, PA: Lippincott, Williams & Wilkins; 2002 [Google Scholar]
- 19.Ferrer M, Suarez JF, Guedea F, Fernandez P, Macias V, Marino A, et al. Health-related quality of life 2 years after treatment with radical prostatectomy, prostate brachytherapy, or external beam radiotherapy in patients with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 2008;72:421–32 [DOI] [PubMed] [Google Scholar]
- 20.Takam R, Bezak E, Yeoh EE. Risk of second primary cancer following prostate cancer radiotherapy: DVH analysis using the competitive risk model. Phys Med Biol 2009;54:611–25 [DOI] [PubMed] [Google Scholar]
- 21.Perlroth DJ, Goldman DP, Garber AM. The potential impact of comparative effectiveness research on U.S. health care expenditures. Demography 2010;47Suppl.:S173–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leborgne F, Fowler J, Leborgne JH, Mezzera J. Later outcomes and alpha/beta estimate from hypofractionated conformal three-dimensional radiotherapy versus standard fractionation for localized prostate cancer. Int J Radiat Oncol Biol Phys 2012; 82:1200–7 [DOI] [PubMed] [Google Scholar]
- 23.Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: alpha/beta=1.4 (0.9–2.2) Gy. Int J Radiat Oncol Biol Phys 2012; 82:e17–24 [DOI] [PubMed] [Google Scholar]
- 24.Wang JZ, Li XA, Yu CX, DiBiase SJ. The low alpha/beta ratio for prostate cancer: what does the clinical outcome of HDR brachytherapy tell us? Int J Radiat Oncol Biol Phys 2003;57:1101–8 [DOI] [PubMed] [Google Scholar]
- 25.Brenner DJ, Martinez AA, Edmundson GK, Mitchell C, Thames HD, Armour EP. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys 2002;52:6–13 [DOI] [PubMed] [Google Scholar]
- 26.Fowler J, Chappell R, Ritter M. Is alpha/beta for prostate tumors really low? Int J Radiat Oncol Biol Phys 2001;50:1021–31 [DOI] [PubMed] [Google Scholar]
- 27.Fowler JF, Ritter MA, Chappell RJ, Brenner DJ. What hypofractionated protocols should be tested for prostate cancer? Int J Radiat Oncol Biol Phys 2003;56:1093–104 [DOI] [PubMed] [Google Scholar]
- 28.National Institute for Health and Clinical Excellence Interventional procedure overview of high dose rate brachytherapy for prostate cancer. London, UK: NICE; 2005. Available from: http://www.nice.org.uk/nicemedia/live/11215/31528/31528.pdf [Google Scholar]
- 29.Kovacs G, Potter R, Loch T, Hammer J, Kolkman-Deurloo IK, de laRosette JJ, et al. GEC/ESTRO-EAU recommendations on temporary brachytherapy using stepping sources for localised prostate cancer. Radiother Oncol 2005;74:137–48 [DOI] [PubMed] [Google Scholar]
- 30.Roach M, 3rd, DeSilvio M, Lawton C, Uhl V, Machtay M, Seider MJ, et al. Phase III trial comparing whole-pelvic versus prostate-only radiotherapy and neoadjuvant versus adjuvant combined androgen suppression: Radiation Therapy Oncology Group 9413. J Clin Oncol 2003;21:1904–11 [DOI] [PubMed] [Google Scholar]
- 31.Kestin LL, Martinez AA, Stromberg JS, Edmundson GK, Gustafson GS, Brabbins DS, et al. Matched-pair analysis of conformal high-dose-rate brachytherapy boost versus external-beam radiation therapy alone for locally advanced prostate cancer. J Clin Oncol 2000;18:2869–80 [DOI] [PubMed] [Google Scholar]
- 32.Hoskin PJ, Motohashi K, Bownes P, Bryant L, Ostler P. High dose rate brachytherapy in combination with external beam radiotherapy in the radical treatment of prostate cancer: initial results of a randomised phase three trial. Radiother Oncol 2007;84:114–20 [DOI] [PubMed] [Google Scholar]
- 33.Galalae RM, Martinez A, Mate T, Mitchell C, Edmundson G, Nuernberg N, et al. Long-term outcome by risk factors using conformal high-dose-rate brachytherapy (HDR-BT) boost with or without neoadjuvant androgen suppression for localized prostate cancer. Int J Radiat Oncol Biol Phys 2004;58:1048–55 [DOI] [PubMed] [Google Scholar]
- 34.Aström L, Pedersen D, Mercke C, Holmang S, Johansson KA. Long-term outcome of high dose rate brachytherapy in radiotherapy of localised prostate cancer. Radiother Oncol 2005;74:157–61 [DOI] [PubMed] [Google Scholar]
- 35.Deger S, Boehmer D, Turk I, Roigas J, Wernecke KD, Wiegel T, et al. High dose rate brachytherapy of localized prostate cancer. Eur Urol 2002;41:420–6 [DOI] [PubMed] [Google Scholar]
- 36.Agoston P, Major T, Frohlich G, Szabo Z, Lovey J, Fodor J, et al. Moderate dose escalation with single-fraction high-dose rate brachytherapy boost for clinically localized intermediate- and high-risk prostate cancer: 5-year outcome of the first 100 consecutively treated patients. Brachytherapy 2011;10:376–84 [DOI] [PubMed] [Google Scholar]
- 37.Demanes DJ, Rodriguez RR, Schour L, Brandt D, Altieri G. High-dose-rate intensity-modulated brachytherapy with external beam radiotherapy for prostate cancer: California endocurietherapy's 10-year results. Int J Radiat Oncol Biol Phys 2005;61:1306–16 [DOI] [PubMed] [Google Scholar]
- 38.Flynn JP, Kelly DA, Brookover T. High dose rate (HDR) brachytherapy boost for prostate cancer gives high control rate. Int J Radiat Oncol Biol Phys 2007;69Suppl. 1:S323–S24 [Google Scholar]
- 39.Galalae RM, Martinez A, Nuernberg N, Edmundson G, Gustafson G, Gonzalez J, et al. Hypofractionated conformal HDR brachytherapy in hormone naive men with localized prostate cancer. Is escalation to very high biologically equivalent dose beneficial in all prognostic risk groups? Strahlenther Onkol 2006;182:135–41 [DOI] [PubMed] [Google Scholar]
- 40.Ghilezan M, Galalae R, Demanes J, Schour L, Gustafson G, Nuernberg N, et al. 10-Year results in 1577 intermediate/high risk prostate cancer patients treated with external beam RT (EBRT) and hypofractionated high dose rate (HDR) brachytherapy boost. Int J Radiat Oncol Biol Phys 2007;69Suppl. 1:S83–417848303 [Google Scholar]
- 41.Guix B, Bartrina J, Henriquez I, Tello J, Vendrell J, Serrate R. Combined treatment 3D-conformal radiotherapy plus HDR brachytherapy as treatment for intermediate- or high-risk prostate cancer: early toxicity and biochemical outcome of a dose-escalation prospective randomized trial. Int J Radiat Oncol Biol Phys 2007;69Suppl. 1:S85 [Google Scholar]
- 42.Hasan Y, Mitchell C, Wilson G, Demanes DJ, Gillian AA, Martinez AA. Long-term outcome for high-dose-rate brachytherapy boost treatment of prostate cancer. Brachytherapy 2007;6:85 [Google Scholar]
- 43.Izard MA, Haddad RL, Fogarty GB, Rinks A, Dobbins T, Katelaris P. Six year experience of external beam radiotherapy, brachytherapy boost with a 1Ci (192)Ir source, and neoadjuvant hormonal manipulation for prostate cancer. Int J Radiat Oncol Biol Phys 2006;66:38–47 [DOI] [PubMed] [Google Scholar]
- 44.Martinez A, Gonzalez J, Spencer W, Gustafson G, Kestin L, Kearney D, et al. Conformal high dose rate brachytherapy improves biochemical control and causes specific survival in patients with prostate cancer and poor prognostic factors. J Urol 2003;169:974–9; discussion 979–80 [DOI] [PubMed] [Google Scholar]
- 45.Pellizzon AC, Salvajoli J, Novaes P, Maia M, Fogaroli R, Gides D, et al. The relationship between the biochemical control outcomes and the quality of planning of high-dose rate brachytherapy as a boost to external beam radiotherapy for locally and locally advanced prostate cancer using the RTOG-ASTRO Phoenix definition. Int J Med Sci 2008;5:113–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phan TP, Syed AM, Puthawala A, Sharma A, Khan F. High dose rate brachytherapy as a boost for the treatment of localized prostate cancer. J Urol 2007;177:123–7; discussion 127 [DOI] [PubMed] [Google Scholar]
- 47.Yamada Y, Bhatia S, Zaider M, Cohen G, Donat M, Eastham J, et al. Favorable clinical outcomes of three-dimensional computer-optimized high-dose-rate prostate brachytherapy in the management of localized prostate cancer. Brachytherapy 2006;5:157–64 [DOI] [PubMed] [Google Scholar]
- 48.Morton GC, Loblaw DA, Sankreacha R, Deabreu A, Zhang L, Mamedov A, et al. Single-fraction high-dose-rate brachytherapy and hypofractionated external beam radiotherapy for men with intermediate-risk prostate cancer: analysis of short- and medium-term toxicity and quality of life. Int J Radiat Oncol Biol Phys 2010;77:811–17 [DOI] [PubMed] [Google Scholar]
- 49.Pieters BR, de Back DZ, Koning CC, Zwinderman AH. Comparison of three radiotherapy modalities on biochemical control and overall survival for the treatment of prostate cancer: a systematic review. Radiother Oncol 2009;93:168–73 [DOI] [PubMed] [Google Scholar]
- 50.Borghede G, Hedelin H, Holmang S, Johansson KA, Aldenborg F, Pettersson S, et al. Combined treatment with temporary short-term high dose rate iridium-192 brachytherapy and external beam radiotherapy for irradiation of localized prostatic carcinoma. Radiother Oncol 1997;44:237–44 [DOI] [PubMed] [Google Scholar]
- 51.Chin YS, Bullard J, Bryant L, Bownes P, Ostler P, Hoskin PJ. High dose rate iridium-192 brachytherapy as a component of radical radiotherapy for the treatment of localised prostate cancer. Clin Oncol (R Coll Radiol) 2006;18:474–9 [DOI] [PubMed] [Google Scholar]
- 52.Galalae RM, Kovacs G, Schultze J, Loch T, Rzehak P, Wilhelm R, et al. Long-term outcome after elective irradiation of the pelvic lymphatics and local dose escalation using high-dose-rate brachytherapy for locally advanced prostate cancer. Int J Radiat Oncol Biol Phys 2002;52:81–90 [DOI] [PubMed] [Google Scholar]
- 53.Hiratsuka J, Jo Y, Yoshida K, Nagase N, Fujisawa M, Imajo Y. Clinical results of combined treatment conformal high-dose-rate iridium-192 brachytherapy and external beam radiotherapy using staging lymphadenectomy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2004;59:684–90 [DOI] [PubMed] [Google Scholar]
- 54.Hsu IC, Bae K, Shinohara K, Pouliot J, Purdy J, Ibbott G, et al. Phase II trial of combined high-dose-rate brachytherapy and external beam radiotherapy for adenocarcinoma of the prostate: preliminary results of RTOG 0321. Int J Radiat Oncol Biol Phys 2010;78:751–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalkner KM, Wahlgren T, Ryberg M, Cohn-Cedermark G, Castellanos E, Zimmerman R, et al. Clinical outcome in patients with prostate cancer treated with external beam radiotherapy and high dose-rate iridium 192 brachytherapy boost: a 6-year follow-up. Acta Oncol 2007;46:909–17 [DOI] [PubMed] [Google Scholar]
- 56.Martin T, Baltas D, Kurek R, Roddiger S, Kontova M, Anagnostopoulos G, et al. 3-D conformal HDR brachytherapy as monotherapy for localized prostate cancer. A pilot study. Strahlenther Onkol 2004;180:225–32 [DOI] [PubMed] [Google Scholar]
- 57.Martinez AA, Gonzalez J, Ye H, Ghilezan M, Shetty S, Kernen K, et al. Dose escalation improves cancer-related events at 10 years for intermediate- and high-risk prostate cancer patients treated with hypofractionated high-dose-rate boost and external beam radiotherapy. Int J Radiat Oncol Biol Phys 2011;79:363–70 [DOI] [PubMed] [Google Scholar]
- 58.Mate TP, Gottesman JE, Hatton J, Gribble M, Van Hollebeke L. High dose-rate afterloading 192Iridium prostate brachytherapy: feasibility report. Int J Radiat Oncol Biol Phys 1998;41:525–33 [DOI] [PubMed] [Google Scholar]
- 59.Rades D, Schwarz R, Todorovic M, Thurmann H, Graefen M, Walz J, et al. Experiences with a new high-dose-rate brachytherapy (HDR-BT) boost technique for T3b prostate cancer. Strahlenther Onkol 2007;183:398–402 [DOI] [PubMed] [Google Scholar]
- 60.Sato M, Mori T, Shirai S, Kishi K, Inagaki T, Hara I. High-dose-rate brachytherapy of a single implant with two fractions combined with external beam radiotherapy for hormone-naive prostate cancer. Int J Radiat Oncol Biol Phys 2008;72:1002–9 [DOI] [PubMed] [Google Scholar]
- 61.Syed AM, Puthawala A, Sharma A, Gamie S, Londrc A, Cherlow JM, et al. High-dose-rate brachytherapy in the treatment of carcinoma of the prostate. Cancer Control 2001;8:511–21 [DOI] [PubMed] [Google Scholar]
- 62.Zwahlen DR, Andrianopoulos N, Matheson B, Duchesne GM, Millar JL. High-dose-rate brachytherapy in combination with conformal external beam radiotherapy in the treatment of prostate cancer. Brachytherapy 2010;9:27–35 [DOI] [PubMed] [Google Scholar]
- 63.Robinson JW, Moritz S, Fung T. Meta-analysis of rates of erectile function after treatment of localized prostate carcinoma. Int J Radiat Oncol Biol Phys 2002;54:1063–8 [DOI] [PubMed] [Google Scholar]
- 64.Dalkin BL, Christopher BA, Shawler D. Health related quality of life outcomes after radical prostatectomy: attention to study design and the patient-based importance of single-surgeon studies. Urol Oncol 2006;24:28–32 [DOI] [PubMed] [Google Scholar]
- 65.Potosky AL, Legler J, Albertsen PC, Stanford JL, Gilliland FD, Hamilton AS, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst 2000;92:1582–92 [DOI] [PubMed] [Google Scholar]
- 66.Schapira MM, Lawrence WF, Katz DA, McAuliffe TL, Nattinger AB. Effect of treatment on quality of life among men with clinically localized prostate cancer. Med Care 2001;39:243–53 [DOI] [PubMed] [Google Scholar]
- 67.Crook JM, Gomez-Iturriaga A, Wallace K, Ma C, Fung S, Alibhai S, et al. Comparison of health-related quality of life 5 years after SPIRIT: Surgical Prostatectomy Versus Interstitial Radiation Intervention Trial. J Clin Oncol 2011;29:362–8 [DOI] [PubMed] [Google Scholar]
- 68.Vordermark D, Wulf J, Markert K, Baier K, Kolbl O, Beckmann G, et al. 3-D conformal treatment of prostate cancer to 74 Gy vs. high-dose-rate brachytherapy boost: a cross-sectional quality-of-life survey. Acta Oncol 2006;45:708–16 [DOI] [PubMed] [Google Scholar]
- 69.Galalae RM, Loch T, Riemer B, Rzehak P, Kuchler T, Kimmig B, et al. Health-related quality of life measurement in long-term survivors and outcome following radical radiotherapy for localized prostate cancer. Strahlenther Onkol 2004;180:582–9 [DOI] [PubMed] [Google Scholar]
- 70.Demanes DJ, Gilhezan M, Schour L, Gustafson G, Hill DR, Marvin K, et al. High dose rate brachytherapy (HDR-BT) as monotherapy for favorable prostate cancer: excellent 5-year control rates and low toxicity. Int J Radiat Oncol Biol Phys 2007;69Suppl. 1:S8317848303 [Google Scholar]
- 71.Demanes DJ, Martinez AA, Ghilezan M, Hill DR, Schour L, Brandt D, et al. High-dose-rate monotherapy: safe and effective brachytherapy for patients with localized prostate cancer. Int J Radiat Oncol Biol Phys 2011; 81:1286–92 [DOI] [PubMed] [Google Scholar]
- 72.Ghilezan M, Gustafson G, Vargas C, Kestin L, Martinez A. High-dose rate (HDR) brachytherapy (BT) in favorable risk prostate cancer patients is equivalent to low-dose-rate (LDR) BT in terms of 5-year clinical outcome. Brachytherapy 2006;5:111 [Google Scholar]
- 73.Grills IS, Martinez AA, Hollander M, Huang R, Goldman K, Chen PY, et al. High dose rate brachytherapy as prostate cancer monotherapy reduces toxicity compared to low dose rate palladium seeds. J Urol 2004;171:1098–104 [DOI] [PubMed] [Google Scholar]
- 74.Yoshioka Y, Konishi K, Sumida I, Takahashi Y, Isohashi F, Ogata T, et al. Monotherapeutic high-dose-rate brachytherapy for prostate cancer: five-year results of an extreme hypofractionation regimen with 54 Gy in nine fractions. Int J Radiat Oncol Biol Phys 2011;80:469–75 [DOI] [PubMed] [Google Scholar]
- 75.Rogers L, Hayes J, Childs L, Hansen R, Sweet J, Spearman J, et al. High dose rate brachytherapy as monotherapy for clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 2006;66Suppl. 1:S377–8 [Google Scholar]
- 76.Mark RJ, Akins RS, Anderson PJ, Neumann TR, Nair M, White D, et al. Interstitial high dose rate (HDR) brachytherapy as monotherapy for early stage prostate cancer: a report of 206 cases. Int J Radiat Oncol Biol Phys 2007;69Suppl. 1:S329 [Google Scholar]
- 77.Lee WR, Hanks GE, Hanlon A. Increasing prostate-specific antigen profile following definitive radiation therapy for localized prostate cancer: clinical observations. J Clin Oncol 1997;15:230–8 [DOI] [PubMed] [Google Scholar]
- 78.Katz AE, Rewcastle JC. The current and potential role of cryoablation as a primary therapy for localized prostate cancer. Curr Oncol Rep 2003;5:231–8 [DOI] [PubMed] [Google Scholar]
- 79.Han KR, Cohen JK, Miller RJ, Pantuck AJ, Freitas DG, Cuevas CA, et al. Treatment of organ confined prostate cancer with third generation cryosurgery: preliminary multicenter experience. J Urol 2003;170(4 Pt 1):1126–30 [DOI] [PubMed] [Google Scholar]
- 80.Murat FJ, Poissonnier L, Pasticier G, Gelet A. High-intensity focused ultrasound (HIFU) for prostate cancer. Cancer Control 2007;14:244–9 [DOI] [PubMed] [Google Scholar]
- 81.Traudt K, Ciezki J, Klein EA. Low-dose-rate brachytherapy as salvage treatment of local prostate cancer recurrence after radical prostatectomy. Urology 2011;77:1416–19 [DOI] [PubMed] [Google Scholar]
- 82.Merrick GS, Butler WM, Dorsey AT, Lief JH, Benson ML. Seed fixity in the prostate/periprostatic region following brachytherapy. Int J Radiat Oncol Biol Phys 2000;46:215–20 [DOI] [PubMed] [Google Scholar]
- 83.Cox JM, Busby JE. Salvage therapy for prostate cancer recurrence after radiation therapy. Curr Urol Rep 2009;10:199–205 [DOI] [PubMed] [Google Scholar]
- 84.Lee B, Shinohara K, Weinberg V, Gottschalk AR, Pouliot J, Roach M, 3rd, et al. Feasibility of high-dose-rate brachytherapy salvage for local prostate cancer recurrence after radiotherapy: the University of California-San Francisco experience. Int J Radiat Oncol Biol Phys 2007;67:1106–12 [DOI] [PubMed] [Google Scholar]
- 85.King CR, Brooks JD, Gill H, Presti Jr JC. Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys 2012; 82:877–82 [DOI] [PubMed] [Google Scholar]
- 86.Fuller DB, Naitoh J, Lee C, Hardy S, Jin H. Virtual HDR CyberKnife treatment for localized prostatic carcinoma: dosimetry comparison with HDR brachytherapy and preliminary clinical observations. Int J Radiat Oncol Biol Phys 2008;70:1588–97 [DOI] [PubMed] [Google Scholar]
- 87.Aluwini S, van Rooij P, Hoogeman M, Bangma C, Kirkels WJ, Incrocci L, et al. CyberKnife stereotactic radiotherapy as monotherapy for low- to intermediate-stage prostate cancer: early experience, feasibility, and tolerance. J Endourol 2010;24:865–9 [DOI] [PubMed] [Google Scholar]


