Abstract
Erectile dysfunction (ED) represents a common and debilitating condition with a wide range of organic and non-organic causes. Physical aetiologies can be divided into disorders affecting arterial inflow, the venous occlusion mechanism or the penile structure itself. Various imaging modalities can be utilised to investigate the physical causes of ED, but penile Doppler sonography (PDS) is the most informative technique, indicated in those patients with ED who do not respond to oral pharmacological agents (e.g. phosphodiesterase type 5 inhibitors). This review will examine the anatomical and physiological basis of penile erection, the method for performing PDS and features of specific causes of ED, and will also consider the alternative imaging modalities available.
Erectile dysfunction (ED) represents a substantial burden upon public health. Studies have estimated that approximately 50% of the male population aged between 40 and 70 years will suffer from ED at some stage, with 10% of these affected severely [1]. On average, a general practitioner is estimated to see between one and five new cases of ED per month [2], and the impact upon the psychosocial health of the sufferer and his relationships may be considerable. ED is defined as the persistent inability to achieve or maintain penile erections of sufficient value to engage in satisfactory sexual activity [3]. Impotence tends not to be used as a descriptive terminology currently as it is felt to imply failure.
Many physical causes of ED exist, with only 10–20% of sufferers believed to have a solely psychological cause [2]. There are many organic causes for ED, with the majority of these based upon vascular insufficiency. These organic causes are summarised in Table 1.
Table 1. Organic causes of erectile dysfunction.
| Vascular (same risk factors as for cardiovascular disease) | Pre-existing cardiovascular disease |
| Atherosclerosis | |
| Hyperlipidaemia | |
| Hypertension | |
| Diabetes mellitus | |
| Smoking | |
| Anatomical | Penile fibrosis |
| Peyronie's disease | |
| Micropenis | |
| Neurological—central | Traumatic brain injury |
| Parkinson's disease | |
| Cerebrovascular disease | |
| Multiple sclerosis | |
| Brain tumours | |
| Spinal cord disease/injury | |
| Intervertebral disc disease | |
| Neurological—peripheral | Peripheral neuropathy (e.g. diabetic) |
| Uraemia | |
| Alcoholism | |
| Pelvic surgery | |
| Hormonal | Central hypogonadism |
| Thyroid disease | |
| Cushing's disease | |
| Hyperprolactinaemia | |
| Drugs | Beta blockers |
| LHRH analogues | |
| Antidepressants (SSRIs/tricyclics) | |
| Histamine H2-receptor antagonists (cimetidine/ranitidine) | |
| Recreational drugs |
LHRH, lutenising hormone releasing hormone; SSRI, selective serotonin reuptake inhibitor.
Imaging in the investigation of ED is dominated by penile Doppler sonography and the main focus of this article will examine this technique. Alternative modalities such as MRI and angiography will also be explored, and the penile anatomy and the physiology of erection will be outlined.
Penile anatomy
The central erectile structures are bilateral corpora cavernosa, seen as dorsolaterally placed low-reflectivity bodies on ultrasound, surrounded by the thick fibrous tunica albuginea (Figures 1 and 2). The corpora cavernosa are formed by multiple sinusoids composed of endothelium and smooth muscle. These sinusoids are capable of substantial volume expansion. The solitary ventrally located corpus spongiosum is enclosed by a thinner layer of tunica albuginea and surrounds the penile urethra. The spongiosum is anatomically independent of the cavernosa. The three corpora are enclosed by the more superficial Buck's fascia.
Figure 1.
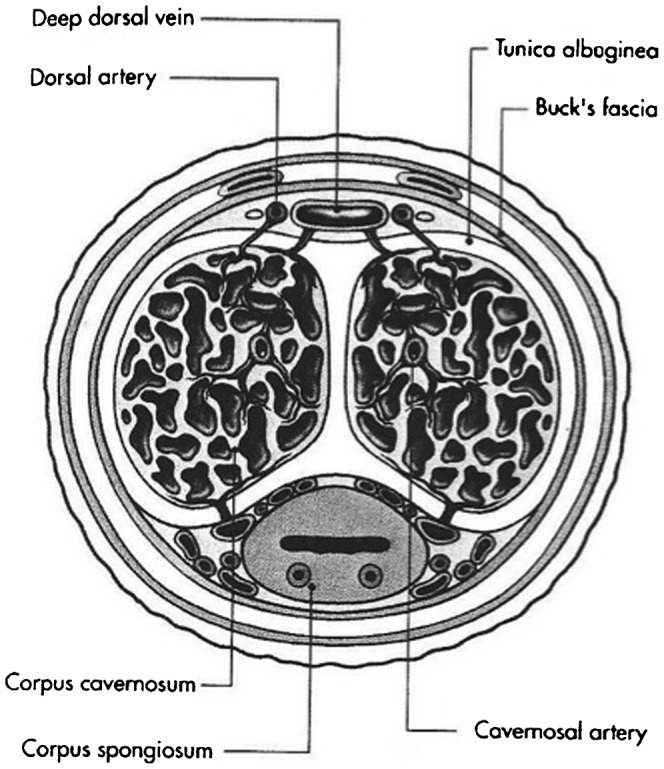
Diagrammatic representation of cross-section through the penis, showing the corpora, facial layers and arteries.
Figure 2.
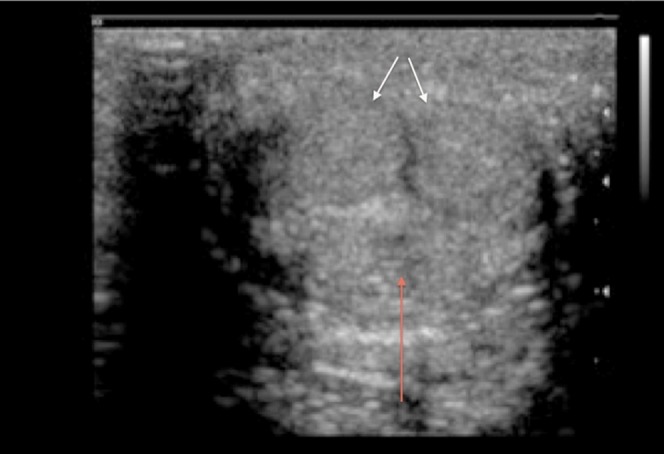
Transverse scan through the undistended penis to demonstrate the sonographic appearances (white arrows, corpora cavernosa; red arrow, corpus spongiosum).
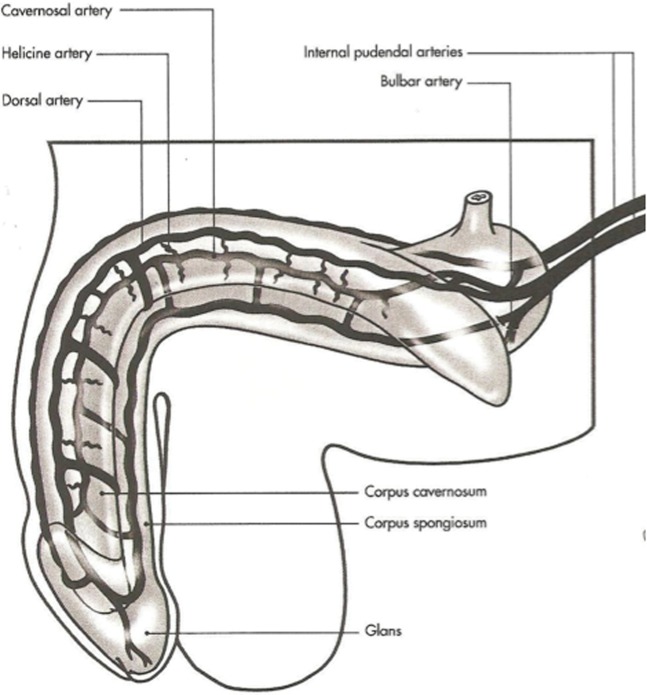
The penile arterial supply displays slight variation in its anatomy [4,5]. The penis is usually supplied by branches of the internal pudendal artery, which continue as the penile artery. The bulbar artery supplies the proximal shaft and is the first branch of the penile artery, which then divides into the dorsal and cavernosal arteries (Figure 3). The cavernosal artery enters and supplies the corpora cavernosal via several helicine arteries, which in turn flow into the sinusoids via multiple arterioles. The intercavernous septum is perforated, allowing for communication of blood (and injected pharmacological agents) across the midline. Emissary veins pierce the tunica albuginea to drain into the deep dorsal vein, via the spongiosal, circumflex and cavernosal veins.
Figure 3.
Normal arterial anatomy of the penis.
Physiology of erection
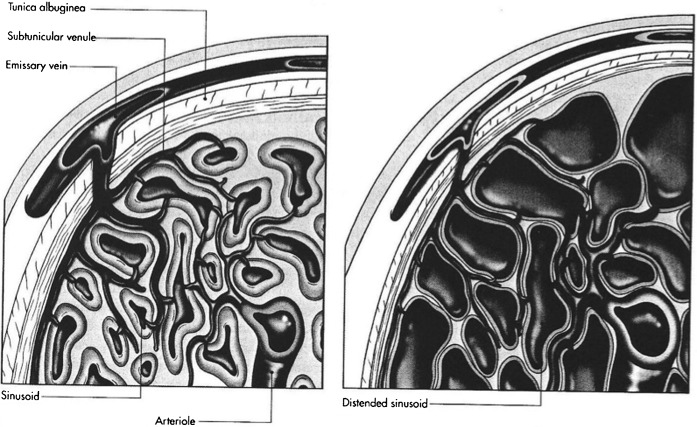
In the flaccid unstimulated penis, the resting smooth muscle tonicity of the cavernosal arterioles and sinusoids is elevated, leading to a high-resistance vascular bed with resultant low volume inflow and outflow. When stimulated to erection, relaxation of the smooth muscle occurs through increased parasympathetic drive from the sacral nervous plexus, resulting in nitric oxide release from endothelial cells within the cavernosa. This relaxation of tone and vascular resistance results in markedly increased cavernosal artery flow throughout the cardiac cycle. The resulting engorgement of the cavernosal sinusoids produces penile lengthening and tumescence. The engorging sinusoids compress the exiting venules and emissary veins, leading to passive limitation of venous outflow (Figure 4). The high inflow and restricted outflow rapidly increases intracorporal pressure, and when this pressure nears systolic pressure reduction in inflow will also normally occur.
Figure 4.
Diagrammatic representation of sinusoidal volume expansion causing passive occlusion of exiting venules.
Ultrasound
Ultrasound examination can be utilised to examine penile structure and vasculature with penile Doppler sonography (PDS), allowing for the examination of the cavernosal and dorsal penile arteries. It was established as an investigative technique by Lue et al in 1985 [6] and recently has evolved through the use of phosphodiesterase type 5 (PDE5) inhibitor agents such as sildenafil. These drugs cause smooth muscle relaxation, vasodilatation and increased inflow of blood into the corpora cavernosa, leading to tumescence. An adequate response to a trial of these agents confirms adequate arterial supply and veno-occlusive mechanism, and precludes the need for further investigation. Therefore, penile Doppler sonography can be reserved for those patients with little or no response to these first-line medications, and in whom arterial or venous insufficiency is suspected. In addition, PDS may be of use in anatomical delineation for patients with post-traumatic/post-surgical abnormalities where curative/reconstructive surgery is being considered.
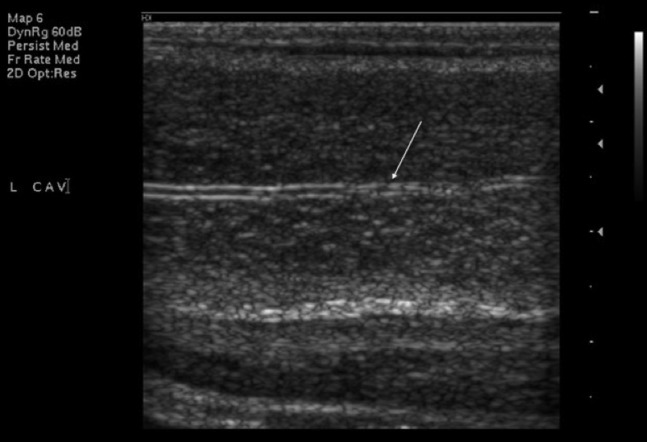
At ultrasound, penile anatomy is readily demonstrated. The corpora cavernosa are depicted as longitudinally orientated vascular beds of mixed echogenicity, with the tunica albuginea visualised as a thin echogenic envelope, usually <2 mm thick. In the absence of cavernosal fibrosis, the interface between the tunica and the underlying cavernosal tissue is quite distinct. The spongiosum is visualised on the ventral surface and is of slightly higher reflectivity than the cavernosa. The cavernosal arteries can be identified within the corpora cavernosa at ultrasound as parallel hyperechoic lines (Figure 5).
Figure 5.
Longitudinal ultrasound image of left cavernosal artery (arrow) within corpus cavernosum in an unstimulated penis.
Variants in arterial anatomy exist in up to 20% (Figure 6), but their relevance to clinical practice is not established.
Figure 6.
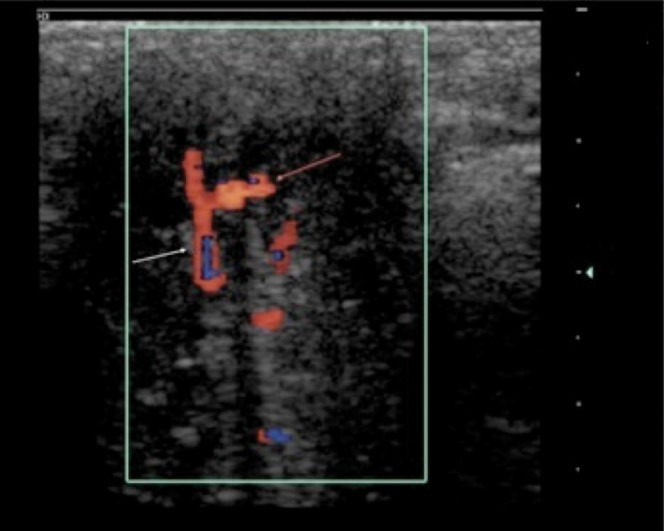
Transverse ultrasound image of right cavernosal artery (white arrow) branching to supply left cavernosum (red arrow) in an unstimulated penis.
Ultrasound method
PDS aims to examine the cavernosal arteries and the response of their spectral Doppler waveforms following intracavernosal injection of a pharmacostimulant agent, commonly a prostaglandin E1 derivative such as alprostadil. The fundamental principle is repeated sampling of these waveforms in a stepwise manner until maximal peak systolic and minimal diastolic velocities have been reached [7-10].
Owing to the nature of the problem under investigation, a quiet, private and comfortable environment is essential for satisfactory PDS. Many patients will be anxious, and a detailed explanation of the procedure is required prior to commencing. Informed consent should be obtained, especially with regard to the low risk of priapism following intracavernosal injection [11]. The patient is positioned supine upon the examination bed and an initial injection of alprostadil is tailored according to the patient. If the patient is pharmacologically naïve, then a small dose (5 μg of alprostadil) is initially given. If there has been a poor response to the PDE5 agents previously, then up to the full dose (20 μg) may be given at the outset. The injection is gently massaged into one of the corpora cavernosa. The authors' standard approach is dorsal, although ventral scanning may also be undertaken. Initially transverse views are obtained, utilising a high-frequency linear probe, and once tumescence commences an oblique–longitudinal approach may be necessary. Angulation of 20–30° cephalad in the transverse plane enables visualisation of the cavernosal artery at its root, running towards the probe, and the artery can be insonated at a Doppler angle of 0°. As usual, angle correction is essential and should be implemented. A velocity gradient exists within the artery from the base to the tip, and reproducible and accurate measurements are best obtained at the penile base towards the peno-scrotal junction [12]. 2–3 min after injection, the cavernosal arteries should become more visible, and spectral measurement and image acquisition should begin at this stage. In addition, the quality of the erection should be assessed both objectively by the operator and subjectively by the patient, and recorded. If the quality of erection is insufficient, repeated injection can be made. Other authors advocate self- or visual stimulation [13-15].
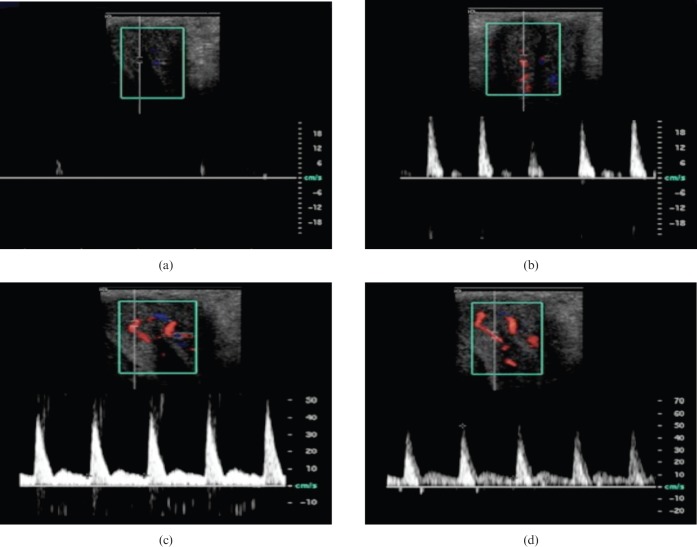
Repeated Doppler measurements should occur at 5-min intervals until the maximal peak systolic velocity (PSV) and end-diastolic velocity (EDV) are judged to have been reached. The PSV is normal if it is >35 cm s –1 and EDV is usually normal if negative or close to 0 cm s–1.
The PDS waveform during erection is multiphasic (Figures 7 and 8). Following injection of pharmacostimulant, initial waveforms display elevation of velocities, especially the diastolic velocity, which reflects smooth muscle relaxation (Phase 2, Figure 7). The PSV then usually stabilises after this and is sustained for at least 5 min. After this, intracavernosal pressure (IP) increases, reflected by a steady reduction in the end-diastolic velocity as increasing sinusoidal distension elevates IP and impedes venous outflow (Phase 3, Figure 7). As the IP continues to rise, the diastolic velocity diminishes and the systolic waveform narrows. When IP exceeds diastolic pressure, the diastolic waveform will reverse (Phase 4, Figure 7). As maximal penile rigidity is achieved in the final phase, the IP is equal to or greater than systolic, producing further narrowing of the systolic peak. In some cases the systolic velocity may reduce to such an extent that there is a transient interruption to systolic flow.
Figure 7.
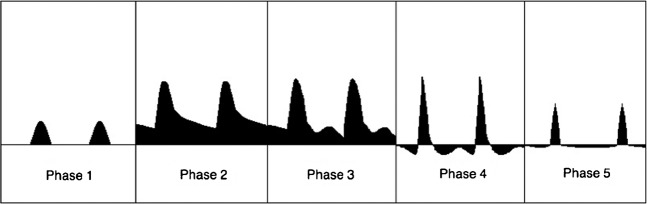
Diagrammatic representation of multiphasic Doppler waveforms encountered during normal erection in the cavernosal arteries. Phase 1—initial low flow arterial waveform. Phase 2—elevation of systolic and diastolic velocities approximately 5 min following injection. Phase 3—reduction in end-diastolic velocity as sinusoidal distension causes increased intracavernosal pressure and reduction in venous outflow. Phase 4—reversal in diastolic waveform as intracavernosal pressure exceeds diastolic pressure. Phase 5—intracavernosal pressure exceeds systolic pressure at maximal tumescence, causing reduction in the systolic velocity and narrowing of the systolic peak.
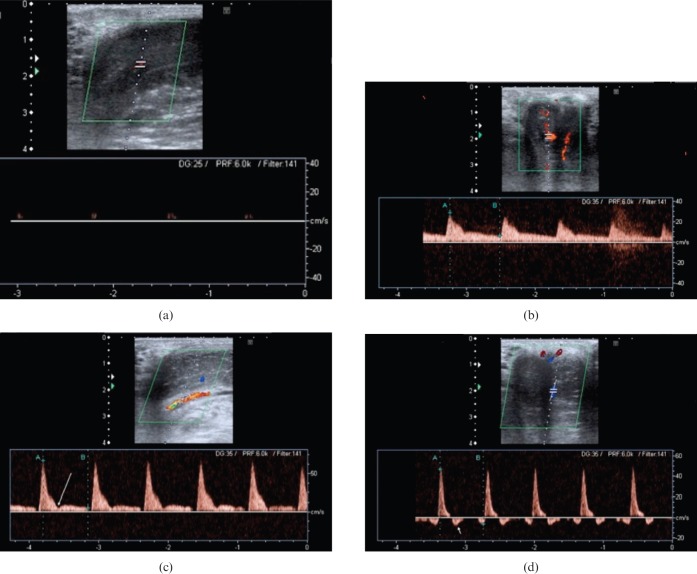
Figure 8.
(a) Longitudinal Doppler image showing cavernosal artery at base of penis (seen in longitudinal section) prior to stimulation. Note the barely recordable arterial flow. (b) The same cavernosal artery trace a few minutes following injection. Note the elevated systolic (28.9 cm s–1) and diastolic (6.7 cm s–1) velocities. (c) Later in the same study, the systolic velocity has peaked (66.9 cm s–1), indicating excellent arterial inflow with a narrowing of the systolic trace. A dicrotic notch is visible (long arrow). (d) Late in the study, diastolic flow reversal can be observed (short arrow). This signifies an intact venous occlusion mechanism. Later still, in a normal study a significant reduction in the systolic velocities may also be observed (not shown).
When the minimal diastolic velocity is attained, the Doppler study is complete, although subsequent examination of the vessel course should be undertaken to exclude distal stenosis and appreciate any variations in the vascular supply.
Standard greyscale imaging is then used to examine for the presence of non-vascular abnormalities such as plaques, fibrosis or tunica albuginea defects.
Diagnostic values
Threshold values for the diagnosis of arterial insufficiency (Figure 9) vary in the literature, with values of 25–35 cm s–1 suggested [6,5,16,17]. A PSV of <25 cm s–1 has been correlated with severe arterial disease on angiographic imaging. PDS not only enables the arterial waveform to be analysed but also allows for visualisation of stenoses, damped waveforms and high-velocity “jets” that may occur as a result of a proximal stenosis elsewhere [9].
Figure 9.
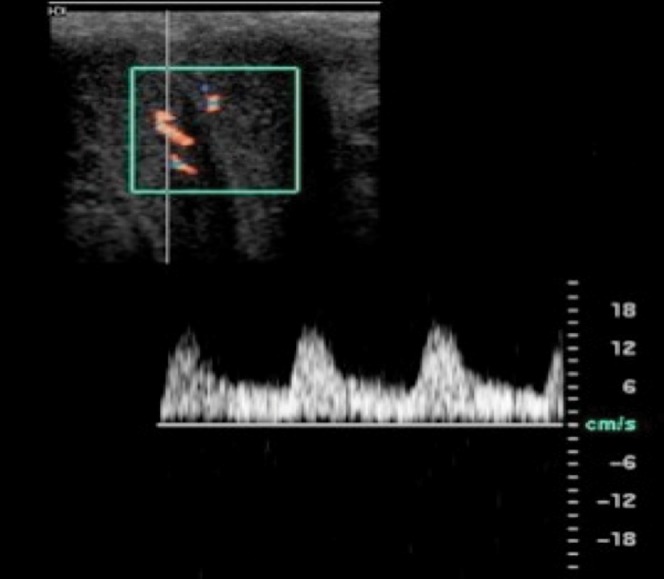
Arterial insufficiency. Patient with a pelvic fracture and subsequent erectile dysfunction who underwent intracavernosal injection of pharmacostimulant; image at 10 min following injection. The pre-stimulation Doppler waveform is not shown. Throughout the study the PSV was below the threshold value of 35 cm s–1 with a wide systolic trace, indicating inadequate arterial inflow to produce rigidity. The persistently elevated end-diastolic flow seen throughout the examination is felt to be a consequence of the arterial insufficiency preventing full sinusoidal dilation (which would normally occlude venous outflow). The patient achieved only soft tumescence. Note only the right cavernosal artery is shown.
Venous incompetence can cause ED through failure of cavernosal engorgement. On PDS this is manifested by persistent diastolic flow and elevated end-diastolic velocity. Again, various threshold EDV values have been suggested between 5 and 7 cm s–1 as diagnostic of venous incompetence [10,16,17]. However, such threshold values for EDV can be misleading if arterial insufficiency is present [9]. In these cases, resistive indices (RI) <0.75 may be helpful in predicting venous leakage (Figure 10) [18,19], although measurement of RI is not usually undertaken in our practice.
Figure 10.
Venous leak. (a) Unstimulated Doppler trace of cavernosal artery. (b) Systolic velocities indicating adequate arterial inflow with elevated diastolic flow (6.2 cm s–1) following intracavernosal injection of pharmacostimulant. (c, d) As the study progresses, the diastolic flow remains abnormally persistently elevated (7.4 cm s–1), signalling venous leak.
Further ultrasound findings
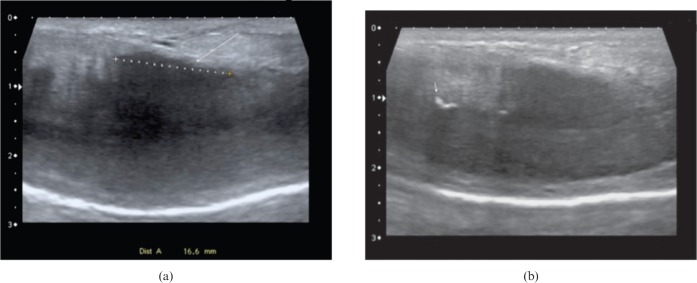
Ultrasound can demonstrate alternative pathologies that may cause ED. In our practice, the penis is assessed for the presence of non-vascular abnormalities such as plaques, areas of fibrosis and defects in the tunica albuginea once full tumescence has been achieved. Peyronie's disease is a localised benign connective tissue disorder with an unknown aetiology that results in fibrous thickening of the penile tunica albuginea. An association with Dupuytren's contracture has been established [20]. The disorder has a prevalence reported to be up to 3% [21], and typical features include pain upon erection, penile curvature, loss of girth and ED. At ultrasound, it is manifested by hyperechoic thickened plaques of the tunica albuginea, which may be calcified (Figure 11) [22]. In the early stage of the disease, inflammation predominates and lasts for 12–18 months, causing erectile discomfort and unstable curvature [23]. In this early phase, hyperperfusion can be demonstrated around the plaques by power Doppler [23]. A more chronic phase follows with stable deformity and plaque size. Up to 80% of patients with Peyronie's will suffer from ED [24,25], although the association is multifactorial, with pain and psychological factors also recognised. In addition, many authors have postulated that ED in Peyronie's is secondary to vascular abnormalities [24,26-28], with both arterial incompetence and venous leakage documented [23].
Figure 11.
(a) Longitudinal ultrasound image showing highly fibrogenic plaque in Peyronie's disease (long arrow) measuring 1.6 cm. The plaque is principally dorsal in location, contributing in this patient to failure of the veno-occlusion mechanism, with normal arterial inflow but elevated diastolic velocities throughout. (b) Longitudinal ultrasound image showing typical calcified plaque (short arrow) encountered in Peyronie's disease.
Penile fractures can result in the long-term complication of ED through the development of fibrous plaque formation, similar to those encountered in Peyronie's disease [29]. In addition, venous leakage and arterial insufficiency can be encountered as a further long-term complication of penile trauma [30], as can defects in the tunica albuginea [30]. These have been reported to lead to herniation of the adjacent cavernosum, causing venous leakage [31].
Penile fibrosis may be encountered in association with Peyronie's disease, but also following trauma, prolonged priapism and after penile prosthesis surgery [32]. On ultrasound it is manifest as focal, often linear, hyperreflective areas within the corpora cavernosa (Figure 12).
Figure 12.
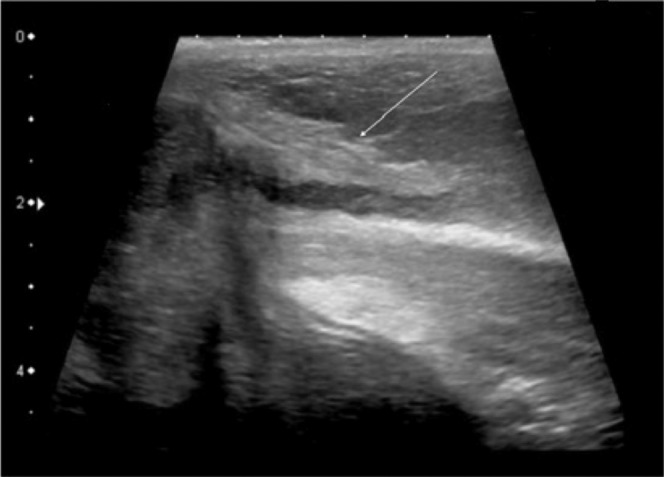
Longitudinal ultrasound image displaying hyperechogenic fibrotic thickening (arrow) at base of unstimulated penis in patient with erectile dysfunction and history of previous penile trauma.
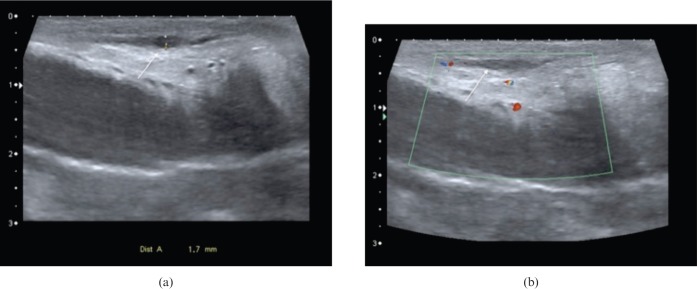
Mondor's disease (Figure 13) refers to thrombophlebitis of the superficial dorsal vein of the penis. The vein is felt as a painful cord and the thrombosed lumen will be clearly seen on ultrasound. It is self-limiting and not a cause of ED, but as the pain is more notable during erection, the patient may present for PDS.
Figure 13.
Mondor's disease affecting distal dorsal superficial penile vein. (a) Longitudinal ultrasound image displaying focal area of thrombosis (arrow) in distal dorsal superficial penile vein. The cavernosa are normal bilaterally. (b) Longitudinal colour Doppler ultrasound image showing lack of flow within thrombosed segment of vein (arrow).
Angiography/cavernosography
In our practice, formal catheter angiography (Figures 14 and 15) is reserved for those patients with a suspected stenotic or occlusive lesion causing arterial insufficiency. It is considered a second-line technique utilised as an adjunct to ultrasound. Catheterisation of the internal pudendal artery allows formal documentation of arterial supply to the penis, and will demonstrate the extent and location of any arterial lesion as preparation for bypass surgery/revascularisation.
Figure 14.
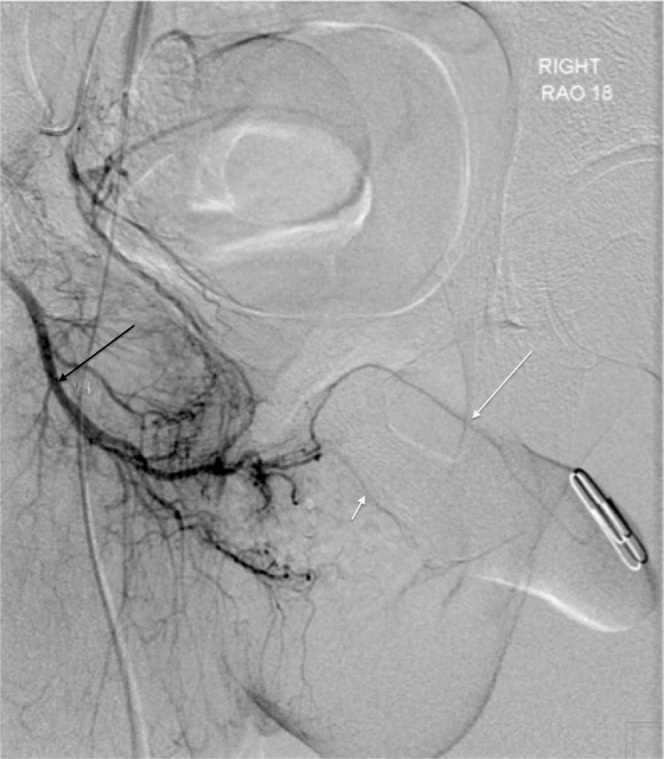
Right internal iliac digital subtraction angiogram showing normal penile arterial anatomy (black arrow, internal pudendal artery; short white arrow, cavernosal artery; long white arrow, dorsal penile artery).
Figure 15.
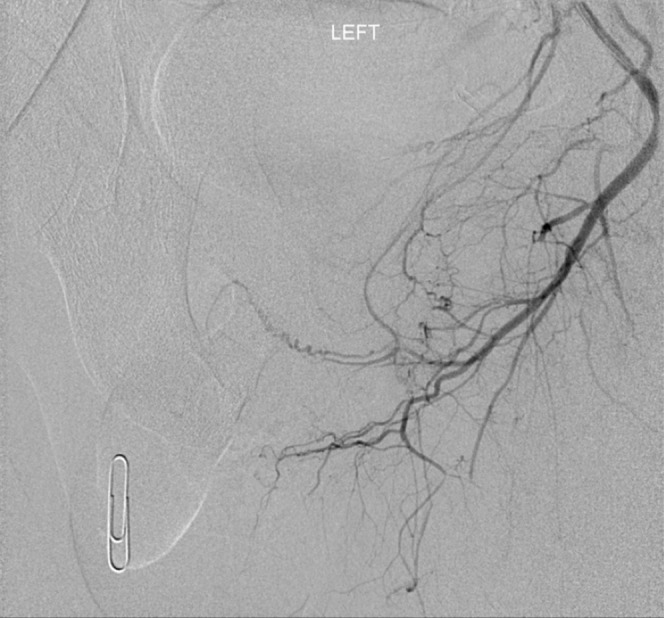
Left internal iliac digital subtraction angiogram in patient with erectile dysfunction. Study confirms the prior ultrasound findings of lack of flow within left cavernosal and dorsal penile arteries.
Cavernosography is a technique utilising injection of contrast medium into the cavernosa as a means of primarily detecting defects in the veno-occlusive mechanism that are causing leaks. It can be combined with pressure manometry, but is now largely regarded as of historical interest only, having been superseded by MRI.
MRI
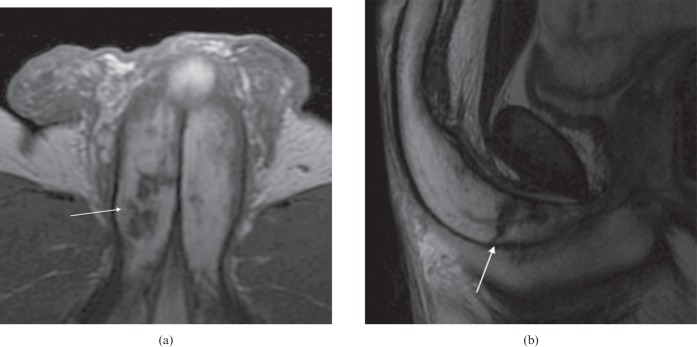
MRI readily demonstrates penile anatomy with both the cavernosa and spongiosum displaying intermediate to high signal on T1 weighted sequences and high signal on T2 weighted sequences. The tunica albuginea manifests as a hypointense structure surrounding the corpora, and in some cases the suspensory ligament can also be identified. Some authors advocate the use of MRI in combination with pharmacostimulant injection to demonstrate tunical dehiscence and cavernosal fibrosis (Figure 16) [32]. In addition, Peyronie's disease plaques and haematoma resulting from penile trauma/fracture can also be well visualised [33]. MRI, in our ED practice, is utilised almost exclusively as a second-line “problem-solving” modality in those cases where structural abnormality has been demonstrated but requires further characterisation.
Figure 16.
T2 weighted MRI images displaying extensive low signal fibrosis (arrows) within both corpora of penile crura in patient with erectile dysfunction and history of previous penile trauma (same patient as in Figure 12). (a) Axial small-field-of-view MR image. (b) Sagittal MR image.
Conclusion
ED is a complex multifactorial condition, which has a potentially heavy impact upon the physical and psychosocial well-being of the patient. Penile Doppler sonography is of vital importance in documenting any vascular abnormalities contributing to ED. The need for meticulous technique and repeated examination is stressed.
References
- 1.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 1994;1511:54–61 [DOI] [PubMed] [Google Scholar]
- 2.European Association of Urology Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Arnhem, Netherlands: European Association of Urology; 2009http://scholar.google.com/ [DOI] [PubMed] [Google Scholar]
- 3.NIH Consensus Conference Impotence. NIH Consensus Development Panel on Impotence. JAMA 1993;270:83–90 [PubMed] [Google Scholar]
- 4.Bähren W, Gall H, Scherb W, Stief C, Thon W. Arterial anatomy and arteriographic diagnosis of arteriogenic impotence. Cardiovasc Intervent Radiol 1988;11:195–210 [DOI] [PubMed] [Google Scholar]
- 5.Quam JP, King BF, James EM, Lewis RW, Brakke DM, Ilstrup DM, et al. Duplex and color Doppler sonographic evaluation of vasculogenic impotence. AJR Am J Roentgenol 1989;153:1141–7 [DOI] [PubMed] [Google Scholar]
- 6.Lue TF, Hricak H, Marich KW, Tanagho EA. Vasculogenic impotence evaluated by high-resolution ultrasonography and pulsed Doppler spectrum analysis. Radiology 1985;155:777–81 [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald SW, Erickson SJ, Foley WD, Lipchik EO, Lawson TL. Color Doppler sonography in the evaluation of erectile dysfunction. Radiographics 1992;12:3–19 [DOI] [PubMed] [Google Scholar]
- 8.Connolly JA, Borirakchanyavat S, Lue TF. Ultrasound evaluation of the penis for assessment of impotence. J Clin Ultrasound 1996;4:481–6 [DOI] [PubMed] [Google Scholar]
- 9.Wilkins CJ, Sriprasad S, Sidhu PS. Colour Doppler ultrasound of the penis. Clin Radiol 2003;58:514–23 [DOI] [PubMed] [Google Scholar]
- 10.Patel U, Amin Z, Friedman E, Vale J, Kirby RW, Lees WR. Colour flow and spectral Doppler imaging after papaverine-induced penile erection in 220 impotent men: study of temporal patterns and the importance of repeated sampling, velocity asymmetry and vascular anomalies. Clin Radiol 1993;48:18–24 [DOI] [PubMed] [Google Scholar]
- 11.Gontero P, Sriprasad S, Wilkins CJ, Donaldson N, Muir GH, Sidhu PS. Phentolamine re-dosing during penile dynamic colour Doppler ultrasound: a practical method to abolish a false diagnosis of venous leakage in patients with erectile dysfunction. Br J Radiol 2004;77:922–6 [DOI] [PubMed] [Google Scholar]
- 12.Kim SH, Paick JS, Lee SE, Choi BI, Yeon KM, Han MC. Doppler sonography of deep cavernosal artery of the penis: variation of peak systolic velocity according to sampling location. J Ultrasound Med 1994;13:591–4 [DOI] [PubMed] [Google Scholar]
- 13.Montorsi F, Guazzoni G, Barbieri L, Galli L, Rigatti P, Pizzini G, et al. The effect of intracorporeal injection plus genital and audiovisual sexual stimulation versus second injection on penile color Doppler sonography parameters. J Urol 1996;155:536–40 [PubMed] [Google Scholar]
- 14.Katlowitz NM, Albano GJ, Morales P, Golimbu M. Potentiation of drug-induced erection with audiovisual sexual stimulation. Urology 1993;41:431–4 [DOI] [PubMed] [Google Scholar]
- 15.Donatucci CF, Lue TF. The combined intracavernous injection and stimulation test: diagnostic accuracy. J Urol 1992;148:61–2 [DOI] [PubMed] [Google Scholar]
- 16.Benson CB, Aruny JE, Vickers MA., Jr Correlation of duplex sonography with arteriography in patients with erectile dysfunction. AJR Am J Roentgenol 1993;160:71–3 [DOI] [PubMed] [Google Scholar]
- 17.Collins JP, Lewandowski BJ. Experience with intracorporeal injection of papaverine and duplex ultrasound scanning for assessment of arteriogenic impotence. Br J Urol 1987;59:84–8 [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald SW, Erickson SJ, Foley WD, Lipchik EO, Lawson TL. Color Doppler sonography in the evaluation of erectile dysfunction: patterns of temporal response to papaverine. AJR Am J Roentgenol 1991;157:331–6 [DOI] [PubMed] [Google Scholar]
- 19.Naroda T, Yamanaka M, Matsushita K, Kimura K, Kawanishi Y, Numata A, et al. Clinical studies for venogenic impotence with color Doppler ultrasonography—evaluation of resistance index of the cavernous artery. [In Japanese.] Nihon Hinyokika Gakkai Zasshi 1996;87:1231–5 [DOI] [PubMed] [Google Scholar]
- 20.Chilton CP, Castle WM, Westwood CA, Pryor JP. Factors associated in the aetiology of Peyronie's disease. Br J Urol 1982;54:748–50 [DOI] [PubMed] [Google Scholar]
- 21.Schwarzer U, Sommer F, Klotz T, Braun M, Reifenrath B, Engelmann U. The prevalence of Peyronie's disease: results of a large survey. BJU Int 2001;88:727–30 [DOI] [PubMed] [Google Scholar]
- 22.Golijanin D, Singer E, Davis R, Bhatt S, Seftel A, Dogra V. Doppler evaluation of erectile dysfunction. Int J Impot Res 2007;19:43–8 [DOI] [PubMed] [Google Scholar]
- 23.Kadioğlu A, Tefekli A, Erol H, Cayan S, Kandirali E. Color Doppler ultrasound assessment of penile vascular system in men with Peyronie's disease. Int J Impot Res 2000;12:263–7 [DOI] [PubMed] [Google Scholar]
- 24.Lopez JA, Jarow JP. Penile vascular evaluation of men with Peyronie's disease. J Urol 1993;149:53–5 [DOI] [PubMed] [Google Scholar]
- 25.Devine Jr CJ, Horton CE. Peyronie's disease. Clin Plast Surg 1988;15:405–9 [PubMed] [Google Scholar]
- 26.Weidner W, Schroeder-Printzen I, Weiske WH, Vosshenrich R. Sexual dysfunction in Peyronie's disease: an analysis of 222 patients without previous local plaque therapy. J Urol 1997;157:325–8 [DOI] [PubMed] [Google Scholar]
- 27.Montorsi F, Guazzoni G, Bergamaschi F, Consonni P, Rigatti P, Pizzini G, et al. Vascular abnormalities in Peyronie's disease: the role of color Doppler sonography. J Urol 1994;151:373–5 [DOI] [PubMed] [Google Scholar]
- 28.Brock G, Kadioglu A, Lue TF. Peyronie's disease: a modified treatment. Urology 1993;42:300–4 [DOI] [PubMed] [Google Scholar]
- 29.Kalash SS, Young Jr JD. Fracture of penis: controversy of surgical versus conservative treatment. Urology 1984;24:21–4 [DOI] [PubMed] [Google Scholar]
- 30.Gontero P, Sidhu PS, Muir GH. Penile fracture repair: assessment of early results and complications using color Doppler ultrasound. Int J Impot Res 2000;12:125–9 [DOI] [PubMed] [Google Scholar]
- 31.Mondaini N, Sriprasad S, Hopster D, Sidhu PS, Muir GH. Congenital herniation of cavernous tissue through the tunica albuginea with osseous metaplasia of the penis. Br J Urol Int 2002;89:971 [Google Scholar]
- 32.Kirkham AP, Illing RO, Minhas S, Minhas S, Allen C. MR imaging of nonmalignant penile lesions. Radiographics 2008;28:837–53 [DOI] [PubMed] [Google Scholar]
- 33.Uder M, Gohl D, Takahashi M, Derouet H, Defreyne L, Kramann B, et al. MRI of penile fracture: diagnosis and therapeutic follow-up. Eur Radiol 2002;12:113–20 [DOI] [PubMed] [Google Scholar]