Abstract
Ultrasound forms an important part of the assessment of the testicle. Nevertheless, there are a number of situations in which clinical and radiological assessment is unable to provide a definitive diagnosis of a testicular lump. In these situations, historically, either open biopsy or orchidectomy has been performed. Ultrasound-guided percutaneous testicular biopsy is an alternative, less invasive method of obtaining histological diagnosis. Here we describe the rationale, technique and potential uses of the technique.
Since its first description in the 1970s [1], ultrasound has been the imaging modality of choice for imaging of the scrotum. It is able to differentiate between solid and cystic lesions [2], and also able to differentiate intratesticular from paratesticular lesions [3]. With Doppler imaging, an assessment of lesion vascularity can be performed, further enhancing the utility of the technique [4]. However, although the sensitivity of ultrasound for the detection of testicular lesions is very high, and indeed approaches 100% in some series, the specificity is relatively poor, with a significant number of false-positive examinations reported [5].
The clinical management of ultrasound-detected testicular lesions has traditionally relied on assessment with clinical examination looking for a hard craggy mass, ultrasound examination and correlation with biochemical tumour markers. If the features suggest a malignancy, orchidectomy via an inguinal route [6] is performed. However, given the well-recognised lack of specificity of ultrasound, it is inevitable that on occasions orchidectomy is performed with subsequent benign histology.
An alternative approach to such lesions is to classify them into lesions (1) where orchidectomy is definitely indicated, (2) where it is undesirable and (3) where it should definitely not be performed. The first group of cases includes those in which the typical ultrasound features of a heterogeneous intratesticular mass with normal or slightly increased Doppler flow is present in conjunction with the classical examination findings and serological correlation. The second group includes cases in which there are mimics of testicular tumours such as an epidermoid. The third group of cases, in which orchidectomy should definitely not be performed, includes benign lesions such as focal atrophy, haematoma, infection, granulomatous orchitis, adrenal rests and Leydig cell hyperplasia, and malignant lesions such as myeloma and metastases. This third group could also include lymphoma, although in recent years there has been a trend towards primary orchidectomy [7].
Testicular biopsy: percutaneous vs open?
Given the diagnostic uncertainty present, there is an inherent attraction to obtaining histological diagnosis prior to surgery. This could be performed by either a percutaneous or an open technique. The advantages of percutaneous testicular biopsy are that it is minimally invasive, requires only local anaesthesia and can be performed on an outpatient basis. However, one of the theoretical disadvantages to percutaneous testicular biopsy has been the risk of tumour seeding due to presumed scrotal violation. The theoretical basis for this relates to the differential lymphatic supply of the testis, which drains via the para-aortic lymph nodes, and the scrotal skin, which drains to inguinal lymph nodes [8]. However, there is little convincing evidence in the literature that scrotal violation, whether due to previous scrotal surgery, open testicular biopsy or testicular aspiration, contributes to prognosis; indeed, a meta-analysis comparing inguinal with scrotal approaches to orchidectomy demonstrated no such difference in prognosis or overall survival rate [9].
The disadvantages of open testicular biopsy are obvious in that the procedure is invasive and requires an anaesthetic. If the lesion is impalpable there will also be a requirement for intra-operative ultrasound to localise the lesion. In recent years, a combined approach using a targeted testicular excision biopsy approach has been advocated in some centres. The testis is exposed through an inguinal incision, the cord is clamped, ultrasound is used to localise the lesion, dissection down to the needle performed and a sample sent for frozen section analysis. If the frozen section is positive then a full orchidectomy is performed [10]. This has the advantage of providing definitive treatment at the same time as biopsy; however, it also has the disadvantages mentioned above.
Percutaneous biopsy technique
The technique should be performed by an operator with experience of small part ultrasound-guided biopsy. No skin shaving is undertaken. Local anaesthetic is infiltrated into the scrotal skin, which is moderately sensitive, and into the tunica vaginalis, which is sensitive. There are no pain fibres in the testicular parenchyma, and no deeper infiltration is required. After localisation with ultrasound, a 16-gauge or 18-gauge core is obtained using a non-advancing, single-action spring-loaded biopsy core needle. One core is usually sufficient, although visual inspection of the core to assess the degree of fragmentation should be performed. The needle should be inserted into the testis at a distance from the lesion to enable greater manoeuvrability. The patient can assist the operator by immobilising their testis, enabling a single operator procedure. Tissue cores should be placed into a standard 10% buffered formalin solution and sent for histology. It is our experience that needle insertions in a cephalo-caudal direction result in considerably more discomfort and vagal reactions than directing the needle cephalad, possibly owing to traction on the spermatic cord.
A simple gauze dressing is applied and, after a period of 1 h bed rest, the patient is ready to be discharged. Complications are uncommon. In a series of cases performed at our institution, there was a single complication of minor haemorrhage, which was treated with elasticated underwear, and where subsequent histological analysis following orchidectomy 8 days later did not show any haemorrhage [11].
Case examples
Peritubular fibrosis
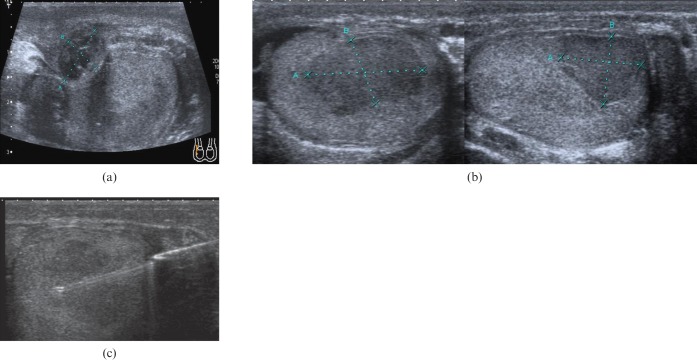
A 45-year-old patient presented with scrotal pain 2 weeks after a vasectomy reversal. Initial ultrasound (Figure 1a) showed a hypoechoic heterogeneous lesion along with the post-surgical change expected after recent surgery. Follow-up ultrasound at 3 months (Figure 1b) demonstrated a discrete hypoechoic intratesticular lesion. This was entirely impalpable. The ultrasound appearances were concerning for malignancy and a percutaneous biopsy (Figure 1c) was performed. Histologically, this demonstrated peritubular fibrosis with scattered inflammatory change but no neoplastic change, sparing the patient an orchidectomy.
Figure 1.
(a) Ultrasound showing a heterogeneous hypoechoic lesion 2 weeks after vasectomy reversal, considered a post-surgical haematoma. (b) Follow-up ultrasound at 3 months demonstrates a discrete low attenuation intratesticular lesion, which was concerning for malignancy. (c) Percutaneous needle biopsy of the lesion, which revealed peritubular fibrosis with scattered inflammatory change but no malignancy.
Coagulative necrosis
A 48-year-male with a history of previous undescended left testis treated with orchidopexy presented with right testicular pain but no palpable mass. Initial ultrasound showed bilateral hypoechoic lesions (Figure 2). Biopsy of the left testicle was performed, which demonstrated coagulative necrosis with no malignant features.
Figure 2.
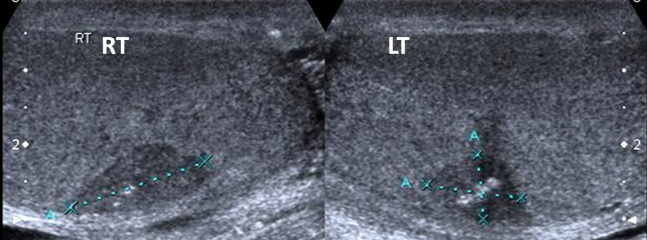
Bilateral hypoechoic lesions in a patient with a history of previous orchidopexy subsequently proven to be coagulative necrosis. LT, left; RT, right.
Lymphoma
A 50-year-old male with previous lymphoma thought to be in remission presented with bilateral testicular swelling. Ultrasound (Figure 3) demonstrated bilateral hypoechoic lesions, which were biopsied and showed diffuse B-cell lymphoma. Further staging CT demonstrated the disease was confined to the testes, and successful treatment with chemotherapy was performed.
Figure 3.
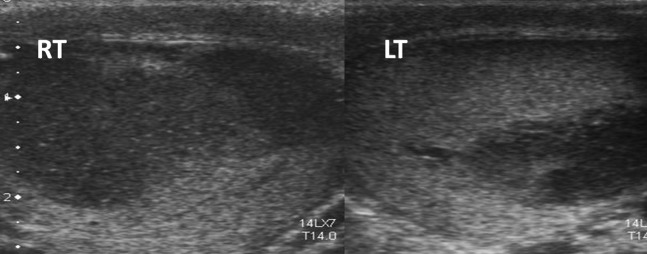
Bilateral testicular lesions in a 50-year-old male patient. Biopsy-proven lymphoma. LT, left; RT, right.
Haematoma
A 36-year-old initially presented with epididymo-orchitis, but no history of trauma. Ultrasound demonstrated a markedly hypoechoic intratesticular lesion with no detectable flow on Doppler examination (Figure 4). The lesion was biopsied and proved to be organised haematoma.
Figure 4.
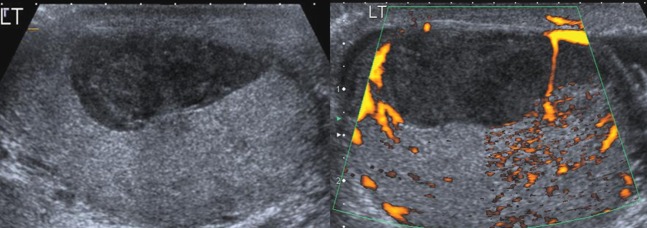
Ultrasound in a patient with a previous history of epididymo-orchitis demonstrated a hypoechoic lesion, which was biopsied and proven to be haematoma. LT, left.
Lymphoma recurrence
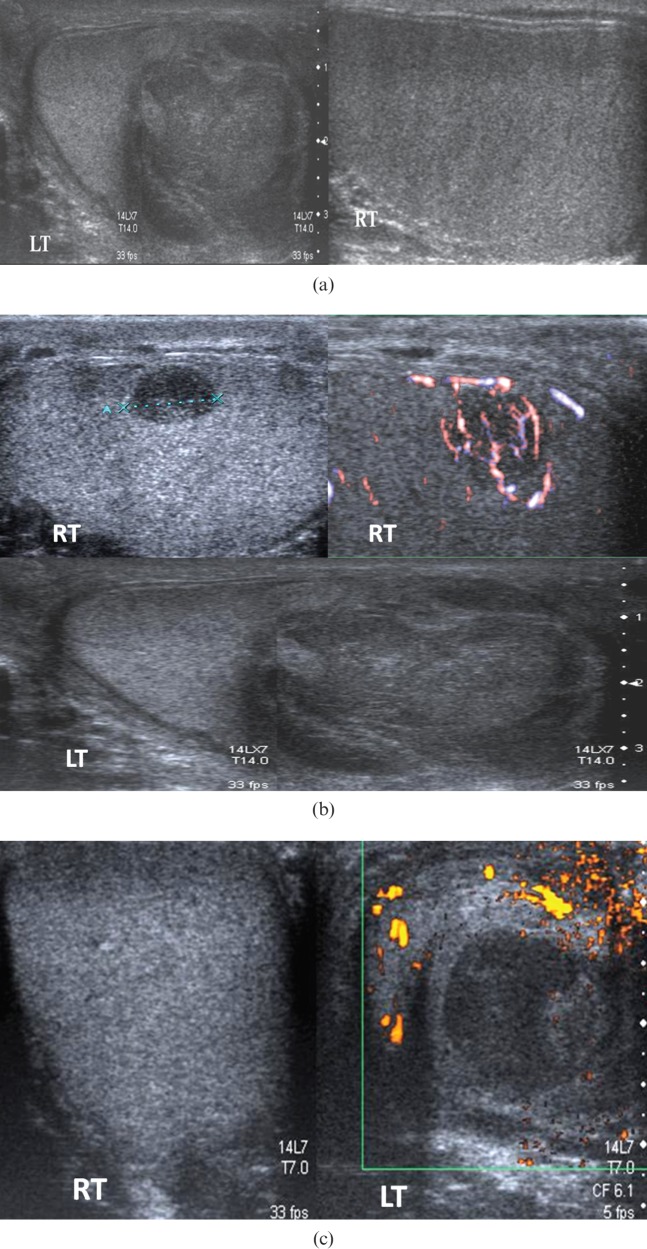
A 59-year-old male presented with left testicular symptoms, diagnosed clinically as epididymo-orchitis. At the time of presentation (Figure 5a) the right testis appeared normal and there was a large heterogeneous mass in the left testis, thought to be related to orchitis. Follow-up ultrasound 3 months later (Figure 5b) showed persistence of the left testicular lesion and a new right testicular lesion. The right testicular lesion was biopsied and a histological diagnosis of intratesticular lymphoma obtained, which was treated with chemotherapy. 6 months later a further scrotal ultrasound (Figure 5c) demonstrated a residual hypoechoic lesion in the left testis. A second contralateral biopsy was performed which showed haematoma and fibrous tissue, and no residual tumour or recurrence.
Figure 5.
(a) Initial ultrasound in a patient with clinical left epididymo-orchitis, demonstrating a normal right testis and changes on the left interpreted as infective in view of the clinical presentation. (b) Follow-up ultrasound 3 months later shows a residual lesion in the left testis with a new vascular hypoechoic right-sided lesion. Biopsy of the right testicular lesion revealed lymphoma. (c) Further follow-up ultrasound demonstrates a 2.5-cm residual avascular hypoechoic left-sided lesion. Biopsy revealed haematoma and fibrous tissue, but no malignancy. LT, left; RT, right.
Seminoma
A 42-year-old patient with a history of bilateral undescended testes as a child presented with right-sided scrotal pain. Initial ultrasound showed a right testicular hypoechoic impalpable lesion with some foci of calcification (Figure 6). The lesion was unchanged at follow-up ultrasound. Histology following a percutaneous biopsy demonstrated a seminoma, and orchidectomy was undertaken.
Figure 6.
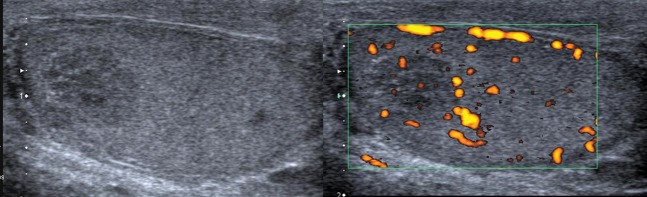
Hypoechoic lesion, hypovascular to surrounding testis in a patient with a history of undescended testis. Seminoma diagnosed at biopsy.
Conclusions
Percutaneous testicular biopsy is useful in situations where the ultrasound findings are equivocal, where there is a discrepancy between the clinical, biochemical and radiological findings, where orchidectomy would be undesirable, or in cases of atrophic testis.
It is now a routine procedure in the setting of indeterminate testicular lesions at our institute and frequently obviates the need for orchidectomy, as in the first five of the six cases described above.
Reports of the hazards of scrotal violation date from the pre-chemotherapeutic era. Our oncologists do not consider this technique to be contraindicated even should the lesion prove to be a malignant primary testicular tumour. It is a safe, reproducible and quick technique that is much less invasive than the alternative open testicular biopsy.
References
- 1.Sample WF, Gottesman JF, Skinner DG, Ehrlich RM. Grey scale ultrasound of the scrotum. Radiology 1978;127:225–8 [DOI] [PubMed] [Google Scholar]
- 2.Fournier GR, Laing FC, Jeffrey RB, McAninch JW. High resolution scrotal ultrasonography: a highly sensitive but non-specific diagnostic technique. J Urol 1985;134:490–3 [DOI] [PubMed] [Google Scholar]
- 3.Hamm B. Sonography of the testis and epididymus. Andrologia 1994;26:193–210 [DOI] [PubMed] [Google Scholar]
- 4.Dogra VS, Gottlieb , Oka M, Rubens DJ. Sonography of the scrotum. Radiology 2003;227:18–36 [DOI] [PubMed] [Google Scholar]
- 5.Coret A, Leibovitch I, Heyman Z, Goldwasser B, Itzchak Ultrasonographic evaluation and clinical correlation of intratesticular lesions: a series of 39 cases. Br J Urol 1995;76:216–19 [DOI] [PubMed] [Google Scholar]
- 6.Khan O, Protheroe A. Testis cancer. Postgrad Med J 2007;83:624–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koukourakis G, Kouloulias V. Lymphoma of the testis as primary location: tumour review. Clin Transl Oncol 2010;12:321–5 [DOI] [PubMed] [Google Scholar]
- 8.Corby HM, Lynch TH, Fitzpatrick JM, Smith JM. Inguinal lymph node metastases from a testicular tumour. Br J Urol 1996;77:923–4 [DOI] [PubMed] [Google Scholar]
- 9.Capelouto CC, Clark PE, Ransil BJ, Loughlin KR. A review of scrotal violation in testicular cancer: is adjuvant local therapy necessary? J Urol 1995;153:981–5 [PubMed] [Google Scholar]
- 10.Kirkham AP, Kumar P, Minhas S, Freeman AA, Ralph DJ, Muneer A, et al. Targeted testicular excision biopsy: when and how should we try to avoid radical orchidectomy? Clin Radiol 2009;64:1158–65 [DOI] [PubMed] [Google Scholar]
- 11.Soh E, Berman LH, Grant JW, Bullock N, Williams MV. Ultrasound-guided coreneedle biopsy of the testis for focal indeterminate intratesticular lesions. Eur Radiol 2008;18:2990–6 [DOI] [PubMed] [Google Scholar]