Abstract
Priapism is defined as a penile erection that persists for 4 h or longer and is unrelated to sexual activity. Its identification is important as lack of timely treatment (particularly of the low flow/ischaemic subgroup) can result in persisting erectile dysfunction as a consequence of irreversible corporal fibrosis. This review describes the physiology and anatomy of the normal erection, the aetiology and pathophysiology of the different types of priapism, and the role of the radiologist in the management of the condition. The treatment of iatrogenic priapism following intracavernosal injection of pharmacostimulant is discussed.
Priapism is defined as a penile erection that persists for 4 h or longer and is unrelated to sexual activity [1]. First described in medical literature in 1845 [2], the name is derived from Priapus, the ancient Greek god of fertility. Mythological tales describe the cursing of the unborn Priapus while in Aphrodite's womb, an act of vengeful spitefulness by the goddess Hera, which left the unfortunate child with enormous over-sized genitalia and an outcast from Mount Olympus. Although classically impotent, his image was adopted as a symbol of fertility.
The overall incidence of the condition is undetermined and probably unhelpful given the significantly higher incidence among certain groups. For example, the lifetime probability of a man with sickle cell disease (SCD) developing ischaemic priapism has been reported as 29–42% [3].
The identification of priapism is important as lack of timely treatment (particularly of the low-flow/ischaemic subgroup) can result in persisting erectile dysfunction as a consequence of irreversible corporal fibrosis [4].
Anatomy and physiology of normal penile erection
In order to understand the pathophysiology of priapism, an understanding of the normal penile anatomy and mechanism of penile erection is necessary.
The penis consists of three longitudinally oriented bodies, or corpora. The dorsolaterally located paired corpora cavernosa are surrounded by the thick echogenic tunica albuginea, and consist of multiple smooth muscle and endothelial-lined sinusoids, which are capable of significant volume expansion with consequential penile tumescence and erection. The single ventral corpus spongiosum is enveloped by a thinner layer of tunica albuginea and surrounds the penile urethra, extending distally to form the glans penis.
Although there is well-recognised variation in penile anatomy [5,6], in general the penis is supplied by the penile artery, which is a branch of the internal pudendal artery, which in turn arises from the anterior division of the internal iliac artery. The penile artery divides into three branches: the bulbar artery (to the corpus spongiosum), the dorsal artery and the cavernosal artery (which is the main blood supply to the erectile tissue of the corpus cavernosum).
In the flaccid penis, the high resting tone of the cavernosal arterioles and sinusoids results in low blood volume inflow and outflow. The onset of erection is heralded by smooth muscle relaxation and consequential significant increase in cavernosal artery flow. The enlarging sinusoids compress the exiting venules and emissary veins, thereby passively restricting venous outflow. This situation of high inflow with restricted outflow leads to an elevated intracorporal pressure, eventually causing reduced inflow when systolic pressure is exceeded.
The control of erection (both tumescence and detumesence) is a complex neurochemical balance that is governed by smooth muscle tone with input from neurotransmittors, hormones, vasoactive substances, signal transduction systems, and corporeal tissue cellular and molecular factors [7-9]. In the resting state, the sympathetic nervous system predominates. However, tumescence is parasympathetically driven with acetyl-choline-mediated generation of nitric oxide (NO). Utilising phosphodiesterase type 5 (PDE-5), this in turn causes generation of cyclic guanosine monophosphate (cGMP), which acts at a number of levels to cause smooth muscle relaxation [10].
Classification/aetiology
The most clinically relevant classification for priapism is into ischaemic (low flow/veno-occlusive) and non-ischaemic (high flow/arterial) categories, the two groups differing in their aetiologies, clinical presentation, treatment necessities and potential complications.
Ischaemic priapism
Ischaemic priapism represents over 95% of cases, and results from sinusoidal thrombosis and veno-occlusion with little or no cavernosal blood flow. It represents a urological emergency, necessitating rapid treatment to prevent corporal fibrosis and erectile dysfunction. Left untreated for greater than 24 h, severe cellular damage and penile necrosis may occur [11]. Aspiration of cavernosal blood reveals a hypoxic, acidic content. Usually only the corpora cavernosa are affected, with the corpus spongiosum (and therefore the glans) remaining soft. Tricorporal involvement in low-flow priapism has been reported in patients with SCD [12].
Causes of ischaemic priapism are myriad, but the main subcategories include haematological/thrombotic causes, drugs/pharmacological agents, intracorporal injection of pharmacostimulants, neurological causes and malignancy. SCD is the commonest aetiology in childhood, with pharmacological agents causing most cases in adults [13]. Nelson and Winter found SCD to be the primary aetiological factor in ischaemic priapism in 63% of paediatric and 23% of adult cases [14]. An observational study of 104 men attending a Kingston, Jamaica, sickle cell outpatient department showed that the prevalence of men with homozygous SCD was 42% [3]. Traditional thinking has long suggested a combination of increased blood viscosity and venous congestion causing tissue hypoxia [15]. More recently, attention has focused at the molecular level, with the dysfunctional action of molecular factors controlling smooth muscle physiology within the corpora cavernosa being proposed as a hypothesis for the pathophysiology of ischaemic priapism [16]. In particular, the importance of aberrant NO activity in the penis has been described [17]. NO is known to be a central regulatory molecule in penile erection and vascular haemostasis, and the SCD-associated haemolysis may reduce penile NO levels or induce aberrant NO activity [18-21].
Recreational drug causes include alcohol and cocaine abuse [22,23]. Other pharmaceutical causes include antipsychotics and antihypertensives [24,25].
Priapism as a consequence of spinal cord or cauda equina injury is a well-recognised cause of priapism [26,27]. The mechanism is believed most likely to be a consequence of disruption to the autonomic nervous system mechanisms that are involved in control of penile vascular tone [16].
Stuttering priapism
Stuttering (intermittent) priapism is a poorly understood variant of ischaemic priapism characterised by recurrent episodes of short-lived, self-limiting painful erections of typically less than 4 h duration. Historically described in patients with SCD, the erections are often nocturnal (or early morning) or related to sexual activity, with affected individuals suffering embarrassment, sleep deprivation and sexual anxiety [28]. Adeyoju et al studied sickle cell patients and reported a 35% history of priapism with the majority of these (72%) having a history of stuttering priapism [29]. The condition begins in later childhood (75% before the age of 20 in Adeyoju's series), with the attacks subsequently increasing in frequency and duration, often culminating in a classical episode of ischaemic priapism [30].
The exact mechanism remains unclear; however, recent understandings of the molecular mechanisms central to erectile function suggest that downregulation of penile PDE-5 is key, with consequential excess activity of the substrate cGMP, itself a second messenger in NO-mediated smooth muscle relaxation [31].
Non-ischaemic priapism
Non-ischaemic priapism was first described by Burt et al [32] in 1960. They described a case of priapism that developed in a man following traumatic coitus with the condition typically being a delayed complication of genital or perineal trauma with the development of arteriosinusoidal fistulas [33-35]. These bypass the usually restraining arteriolar vasoconstrictive tone and permit excess arterial cavernosal inflow (hence the traditional term “high flow”). Unlike the ischaemic variety, non-ischaemic priapism is classically not painful and the penis is usually non-rigid. The cavernosal blood is oxygenated and does not require emergency treatment.
Malignant priapism
Priapism as a consequence of non-haematological malignancy (so called “malignant priapism”) is a rare condition, resulting most commonly from penile metastases from primary bladder, prostatic, rectosigmoid and renal tumours [36]. 20–53% of metastases to the penis initially present with priapism [37]. The exact mechanism is unclear, with suggested mechanisms being extensive penile replacement by infiltrating tumour, venous obstruction and continuous stimulation of neuronal pathways [38].
The role of the radiologist
The radiologist has a central role in the management of priapism, both at the acute presentation and in the later assessment for potential sequelae (Table 1). Imaging modalities available for the assessment of priapism include a penile Doppler study (PDS), pelvic/penile angiography and MRI.
Table 1. The role of the radiologist.
| General | Ischaemic priapism | In non-ischaemic priapism |
| Characterise cavernosal artery flow dynamics using ultrasound Identify cavernosal infarction and fibrosis with MRI Determine underlying cause of priapism if not clinically apparent—CT to identify occult malignancy; MRI to detect penile metastases Treatment of high-flow priapism with compression or embolisation Follow-up following treatment with ultrasound |
Doppler ultrasound useful to: allow identification of cavernosal artery flow (no flow suggests probable cavernosal infarction) early treatment with penile implant may be appropriate identify variants of ischaemic priapism that still maintain high flow (often >1–2 m s–1) with high resistance (i.e. non-perfusing); early operation not advised owing to potential haemorrhagic difficulties MRI useful to: detect perfusion of corporal tissue; no perfusion suggests early prosthesis insertion detect rare causes of priapism, such as penile metastases |
Doppler ultrasound useful to: detect high-flow, low-resistance waveform localise arteriocavernosal fistula treat fistula with compressionInterventional radiology allows: angiography and embolisation of fistula |
A PDS is the key radiological tool in the assessment of a patient with priapism. In the acute setting, it serves as an adjunct to clinical assessment and cavernosal blood gas analysis to provide the essential diagnostic distinction between ischaemic and non-ischaemic priapism [1]. A study involves both standard greyscale assessment of the penis and Doppler assessment of cavernosal artery blood flow [39]. Unlike in the assessment of erectile dysfunction, penile injection of pharmacostimulant is not usually required. In the ischaemic subgroup, cavernosal blood flow typically will be absent, with a high-resistance, low-velocity trace. Diastolic flow will be low or absent with variable but not high arterial flow. The sinusoids will be engorged, demonstrating low (or mixed) echogenicity depending on the completeness of sinusoidal thrombosis. The sinusoids are typically not compressible, with probe pressure and flow in the dorsal vein either poor or unrecordable [39]. A fluid–fluid level may be seen in the corpora cavernosa owing to red cell sedimentation. A subgroup of ischaemic priapism is recognised where there remains high cavernosal artery flow (often >1–2 m s–1) but with high resistance (i.e. non-perfusing). The study also provides information as to the degree of resolution, either spontaneous or following treatment, and useful for follow-up assessment (Figure 1).
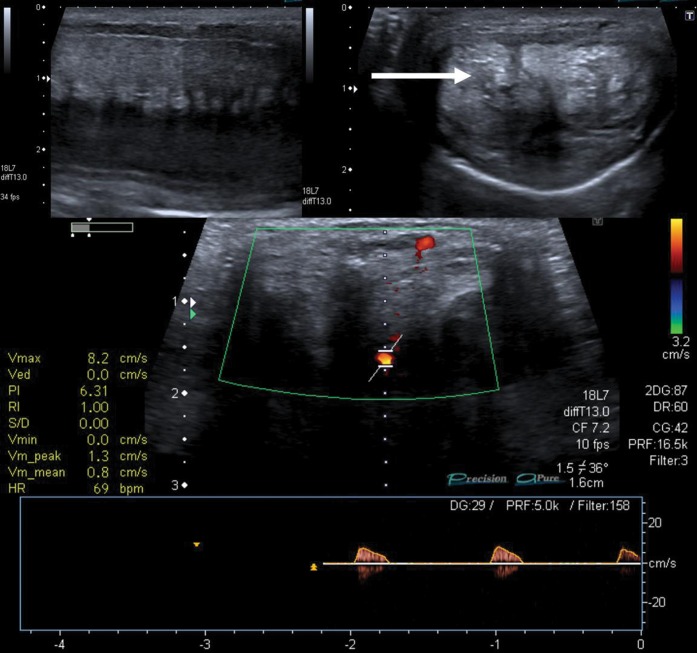
Figure 1.
Penile Doppler study in a patient with significant erectile dysfunction following an episode of prolonged ischaemic priapism. Upper images are greyscale longitudinal (left) and axial (right) images; note the echogenic, distorted cavernosa (arrow) reflecting significant corporal fibrosis. The lower image shows the Doppler assessment of the left cavernosal artery, revealing significantly reduced arterial flow (<10 cm s–1). There was minimal response to pharmacostimulant injection. The study demonstrated extensive cavernosal fibrosis interfering with sinusoidal relaxation and subsequent poor arterial inflow.
Conversely, non-ischaemic priapism demonstrates normal or elevated cavernosal artery velocities with high diastolic flow (i.e. low-resistance flow; Figure 2). The high venous outflow may cause prominence of draining veins [40], with rich flow identified within the dorsal vein. The sinusoids are transonic, being engorged with blood but without sinusoidal thrombosis and often compressible. The study has a sensitivity approaching 100% for the identification of post-traumatic arterio-cavernosal fistulas [34]. These often appear poorly defined in the acute setting, becoming well defined with chronicity [41]. Colour Doppler demonstrates the abnormal turbulent flow within the fistula (Figure 3).
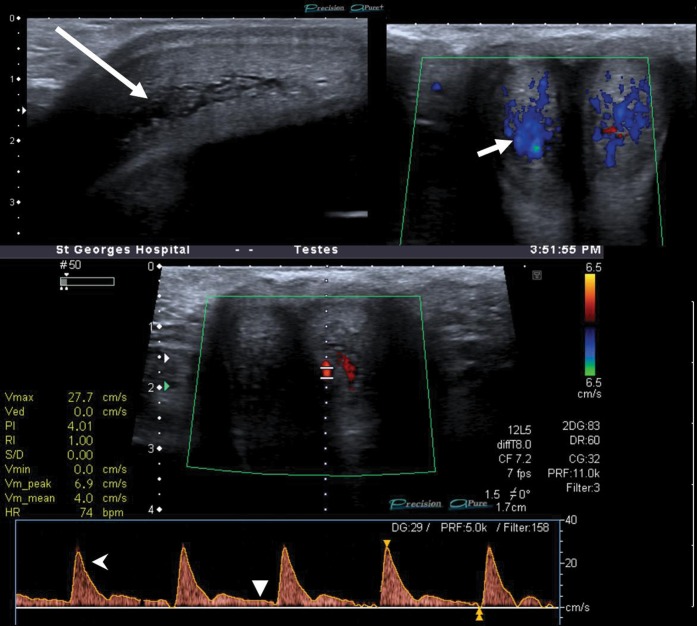
Figure 2.
Unstimulated penile Doppler study in a patient with non-ischaemic priapism. Longitudinal greyscale image (upper left) demonstrates centrifugal sinusoidal distension (long arrow).Utilising Doppler assessment (upper right image), sudden release of compressive pressure with the ultrasound probe allows rapid blood inflow to the (non-thrombosed) sinusoids (short arrow). Conversely, in ischaemic priapism the sinusoids are typically thrombosed and non-compressible, with no flow demonstrable. Duplex trace of left cavernosal artery (lower image) shows a high resting velocity of 30 cm s–1 (open arrowhead), with positive flow throughout the cycle (closed arrowhead), indicating a low-resistance circuit.
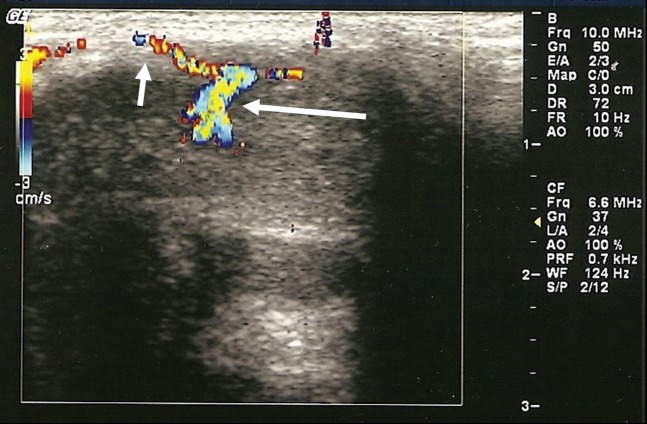
Figure 3.
An unstimulated penile Doppler study in a patient with high-flow priapism following perineal trauma. Note the turbulent arteriocavernosal fistula (long arrow) and the dorsal cavernosal artery (short arrow).
This diagnostic distinction between high- and low-flow priapism is essential as the subsequent treatment differs. The ischaemic type requires prompt aspiration to prevent cavernosal thrombosis (and later fibrosis). If refractory to treatment, surgical shunting may be necessary [42]. High-flow priapism necessitates transcatheter embolisation of either the internal pudendal or cavernosal artery (Figure 4). A PDS is useful in the follow-up of these patients to ensure successful resolution of the fistula and cavernosal artery recanalisation.
Figure 4.
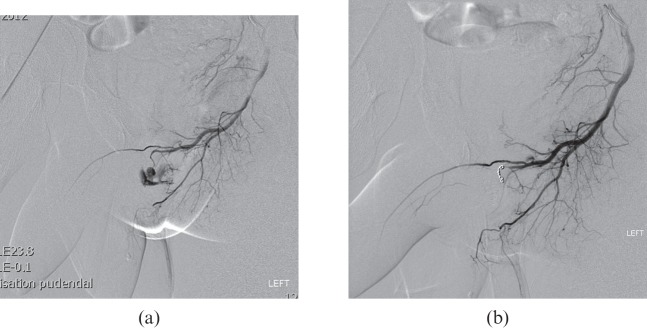
(a, b) 23-year-old patient who was kicked in the perineum and developed painless priapism 2 weeks later. Doppler ultrasound (not shown) showed high-velocity low-resistance flow within the left cavernosal artery and a sizable fistula within the left proximal corpus cavernosum. Left internal pudendal arteriogram confirmed a large fistula arising from the left cavernosal artery. This was embolised using two 2-mm microcoils. This resulted in complete occlusion of the fistula on both angiography and ultrasound, with detumescence of the penis. The patient retained normal erectile function.
The principal role of MRI in the management of priapism is the detection and quantification of cavernosal infarction in the low-flow group [43]. If there is significant infarcted material, evacuation of the corpora should be considered with insertion of a penile prosthesis [44] (Figures 5 and 6).
Figure 5.
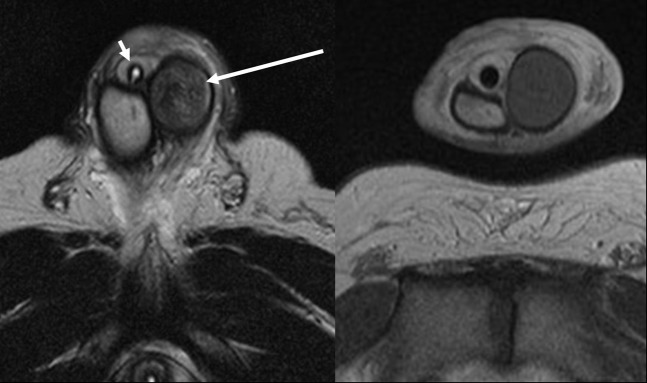
44-year-old patient with untreated prolonged ischaemic priapism. The axial T2 weighted sequence (left image) demonstrates low signal intensity within the distended and rounded thrombosed left cavernosum (long arrow). Compare with the normal homogeneous high-signal right cavernosum. The patient has a urinary catheter in situ (short arrow). The axial T1, post-contrast sequence (right image) shows no enhancement within the thrombosed left cavernosa, but normal enhancement within the right cavernosa. The study suggests little chance of recovery of the left cavernosa and potential early penile prosthesis insertion.
Figure 6.
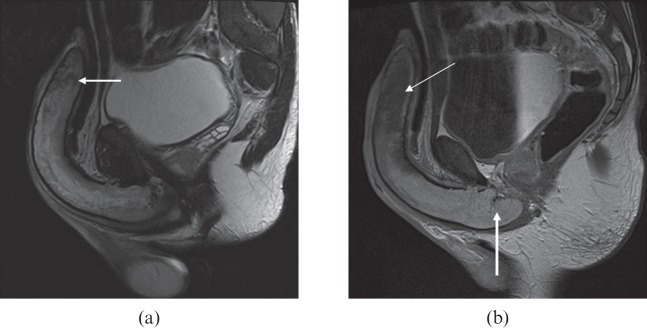
A 34-year-old patient with sickle cell disease who had a history of stuttering priapism. He presented with a 28-h history of mildly painful priapism. (a) T2 MRI showed heterogeneous signal throughout the corpus with more focal low T2 signal distally (arrow). (b) Delayed T1 post-contrast images showed lack of enhancement of the distal corpora (thin arrow), indicating distal fibrosis but normal proximal enhancement compatible with viable tissue (thick arrow).
Other potential uses include the identification of penile metastases that may be causing malignant priapism and the imaging of post-traumatic fistulas causing high-flow priapism, although a penile Doppler study and angiography remain far superior.
Partial cavernosal thrombosis is a rare cause of priapism and can be identified on MRI. The T1/T2 signal intensity depends on the age of the thrombus but typically demonstrates high T1 and low T2 signal relative to the surrounding unthrombosed cavernosa [45]. The appearance can be confirmed with intravenous contrast administration, as shown in Figure 6 [43].
A penile MRI protocol for the investigation of priapism has been described by Kirkham et al [43]. A study does not require the cavernosal injection of pharmacostimulant required for a non-priapism study. The penis should be secured with tape (and/or padding) to the anterior abdominal wall. Thin-section (4 mm or less) high-resolution images are necessary with a small field of view. A surface coil is necessary with spin-echo T2 weighted imaging and T1 in axials. Intravenous contrast is essential to demonstrate cavernosal perfusion and help predict areas of necrosis. In priapism a dynamic contrast-enhanced study is required with three-dimensional (3D) fat-suppressed gradient echo volumes with approximate 30 s acquisitions for four contrast-enhanced scans. This should be followed by axial or coronal contrast-enhanced spin-echo T1.
The most common cause of priapism in contemporary practice is that arising following intracavernosal injection of a pharmacostimulant. This may either be as part of a diagnostic investigation such as a PDS or as treatment of erectile dysfunction.
Inducing penile erection through intracavernosal injection of a pharmacostimulant has a number of recognised complications including penile pain, prolonged erections (5%), priapism and corporal fibrosis (2%) [46]. Alprostadil is the only drug approved for intracavernosal treatment of erectile dysfunction, being the most efficacious monotherapy for treatment using doses between 5 and 40 μg [47].
A radiologist who performs a PDS with penile injection of a pharmacostimulant should be aware of the potential complications and provide advice in the event of prolonged erection. Although evidence in the literature is scarce, general advice to try to alleviate a prolonged erection includes ejaculation, showering, exercise and compression with a bag of frozen peas, although this should not delay seeking medical attention if the erection lasts more than 4 h, with the aim being to avoid damaging cavernosal tissue. In this situation the management should include aspiration of corporal blood with a 19-G needle to reduce intracavernosal pressure. This technique is usually sufficient, with intracavernosal injection of phenylephrine reserved if rigidity returns [47]. The dose should start at 200 μg every 5 min, increasing to 500 μg if necessary [47].
References
- 1.Montague DK, Jarow J, Broderick GA, Dmochowski RR, Heaton JP, Lue TF, et al. American Urological Association guideline on the management of priapism. J Urol 2003;170:1318–24 [DOI] [PubMed] [Google Scholar]
- 2.Tripe J. Case of continued priapism. Lancet 1845;2:1274–6 [Google Scholar]
- 3.Emond AM, Holman R, Hayes RJ, Serjeant GR. Priapism and impotence in homozygous sickle cell disease. Arch Intern Med 1980;140:1434–7 [PubMed] [Google Scholar]
- 4.Pautler SE, Brock GB. Priapism. From Priapus to the present time. Urol Clin North Am 2001;28:391–403 [DOI] [PubMed] [Google Scholar]
- 5.Quam JP, King BF, James EM, Lewis RW, Brakke DM, Ilstrup DM, et al. Duplex and color Doppler sonographic evaluation of vasculogenic impotence. AJR Am J Roentgenol 1989;153:1141–7 [DOI] [PubMed] [Google Scholar]
- 6.Bähren W, Gall H, Scherb W, Stief C, Thon W. Arterial anatomy and arteriographic diagnosis of arteriogenic impotence. Cardiovasc Intervent Radiol 1988;11:195–210 [DOI] [PubMed] [Google Scholar]
- 7.Andersson KE, Stief CG. Neurotransmission and the contraction and relaxation of penile erectile tissues. World J Urol 1997;15:14–20 [DOI] [PubMed] [Google Scholar]
- 8.Lue TF. Erectile dysfunction. N Engl J Med 2000;342:1802–13 [DOI] [PubMed] [Google Scholar]
- 9.Christ GJ, Richards S, Winkler A. Integrative erectile biology: the role of signal transduction and cell-to-cell communication in coordinating corporal smooth muscle tone and penile erection. Int J Impot Res 1997;9:69–84 [DOI] [PubMed] [Google Scholar]
- 10.Yuan J, Desouza R, Westney OL, Wang R. Insights of priapism mechanism and rationale treatment for recurrent priapism. Asian J Androl 2008;10:88–101 [DOI] [PubMed] [Google Scholar]
- 11.Spycher MA, Hauri D. The ultrastructure of the erectile tissue in priapism. J Urol 1986;135:142–7 [DOI] [PubMed] [Google Scholar]
- 12.Sharpsteen JR, Jr, Powars D, Johnson C, Rogers ZR, Williams WD, Posch RJ. Multisystem damage associated with tricorporal priapism in sickle cell disease. Am J Med 1993;94:289–95 [DOI] [PubMed] [Google Scholar]
- 13.Cherian J, Rao AR, Thwaini A, Kapasi F, Shergill IS, Samman R. Medical and surgical management of priapism. Postgrad Med J 2006;82:89–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson 3rd JH, Winter CC. Priapism: evolution of management in 48 patients in a 22-year series. J Urol 1977;117:455–8 [DOI] [PubMed] [Google Scholar]
- 15.Hinman Jr F. Priapism: reasons for failure of therapy. J Urol 1960;83:420–8 [DOI] [PubMed] [Google Scholar]
- 16.Burnett AL. Pathophysiology of priapism: dysregulatory erection physiology thesis. J Urol 2003;170:26–34 [DOI] [PubMed] [Google Scholar]
- 17.Champion HC, Bivalacqua TJ, Takimoto E, Kass DA, Burnett AL. Phosphodiesterase-5. A dysregulation in penile erectile tissue is a mechanism of priapism. Proc Natl Acad Sci USA 2005;102:1661–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moon DG, Lee DS, Kim JJ. Altered contractile response of penis under hypoxia with metabolic acidosis. Int J Impot Res 1999;11:265–71 [DOI] [PubMed] [Google Scholar]
- 19.Brown GC. Nitric oxide and mitochondrial respiration. Biochim Biophys Acta 1999;1411:351–69 [DOI] [PubMed] [Google Scholar]
- 20.Lipton SA. Neuronal protection and destruction by NO. Cell Death Differ 1999;6:943–51 [DOI] [PubMed] [Google Scholar]
- 21.Nolan VG, Wyszynski DF, Farrer LA, Steinberg MH. Hemolysis-associated priapism in sickle cell disease. Blood 2005;106:3264–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pohl J, Pott B, Kleinhans G. Priapism: a three-phase concept of management according to aetiology and prognosis. Br J Urol 1986;58:113–18 [DOI] [PubMed] [Google Scholar]
- 23.Altman AL, Seftel AD, Brown SL, Hampel N. Cocaine associated priapism. J Urol 1999;161:1817–18 [PubMed] [Google Scholar]
- 24.Rubin SO. Priapism as a probable sequel to medication. Scand J Urol Nephrol 1968;2:81–5 [DOI] [PubMed] [Google Scholar]
- 25.Ankem MK, Ferlise VJ, Han KR, Gazi MA, Koppisch AR, Weiss RE. Risperidone-induced priapism. Scand J Urol Nephrol 2002;36:91–2 [DOI] [PubMed] [Google Scholar]
- 26.Munro D, Horne HW, Jr, Paull DP. The effect of injury to the spinal cord and cauda equina on the sexual potency of men. N Engl J Med 1948;239:903–11 [DOI] [PubMed] [Google Scholar]
- 27.Ravindran M. Cauda equina compression presenting as spontaneous priapism. J Neurol Neurosurg Psychiatry 1979;42:280–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chow K, Payne S. The pharmacological management of intermittent priapismic states. BJU Int 2008;102:1515–21 [DOI] [PubMed] [Google Scholar]
- 29.Adeyoju AB, Olujohungbe AB, Morris J, Yardumian A, Bareford D, Akenova A, et al. Priapism in sickle-cell disease; incidence, risk factors and complications—an international multicentre study. BJU Int 2002;90:898–902 [DOI] [PubMed] [Google Scholar]
- 30.Jesus LE, Dekermacher S. Priapism in children: review of pathophysiology and treatment. J Pediatr (Rio J) 2009;85:194–200 [DOI] [PubMed] [Google Scholar]
- 31.Olujohungbe AB, Adeyoju A, Yardumian A, Akinyanju O, Morris J, Westerdale N, et al. A prospective diary study of stuttering priapism in adolescents and young men with sickle cell anemia: report of an international randomized control trial–the priapism in sickle cell study. J Androl 2011;32:375–82 [DOI] [PubMed] [Google Scholar]
- 32.Burt FB, Schirmer HK, Scott WW. A new concept in the management of priapism. J Urol 1960;83:60–1 [DOI] [PubMed] [Google Scholar]
- 33.Ricciardi R, Jr, Bhatt GM, Cynamon J, Bakal CW, Melman A. Delayed high flow priapism: pathophysiology and management. J Urol 1993;149:119–21 [DOI] [PubMed] [Google Scholar]
- 34.Bastuba MD, Saenz de Tejada I, Dinlenc CZ, Sarazen A, Krane RJ, Goldstein I. Arterial priapism: diagnosis, treatment and long-term followup. J Urol 1994;151:1231–7 [DOI] [PubMed] [Google Scholar]
- 35.Brock G, Breza J, Lue TF, Tanagho EA. High flow priapism: a spectrum of disease. J Urol 1993;150:968–71 [DOI] [PubMed] [Google Scholar]
- 36.Powell BL, Craig JB, Muss HB. Secondary malignancies of the penis and epididymis: a case report and review of the literature. J Clin Oncol 1985;3:110–16 [DOI] [PubMed] [Google Scholar]
- 37.Chan PT, Bégin LR, Arnold D, Jacobson SA, Corcos J, Brock GB. Priapism secondary to penile metastasis: a report of two cases and a review of the literature. J Surg Oncol 1998;68:51–9 [DOI] [PubMed] [Google Scholar]
- 38.Wilson F, Staff WG. Malignant priapism: an unexpected response to local anaesthetic infiltration of the dorsal nerves of the penis. Br J Surg 1982;69:469. [DOI] [PubMed] [Google Scholar]
- 39.Halls J, Bydawell G, Patel U. Erectile dysfunction: the role of penile Doppler ultrasound in diagnosis. Abdom Imaging 2009;34:712–25 [DOI] [PubMed] [Google Scholar]
- 40.Wilkins CJ, Sriprasad S, Sidhu PS. Colour Doppler ultrasound of the penis. Clin Radiol 2003;58:514–23 [DOI] [PubMed] [Google Scholar]
- 41.Bertolotto M, Quaia E, Mucelli FP, Ciampalini S, Forgács B, Gattuccio I. Color Doppler imaging of posttraumatic priapism before and after selective embolization. Radiographics 2003;23:495–503 [DOI] [PubMed] [Google Scholar]
- 42.Carter RG, Thomas CE, Tomskey GC. Cavernospongiosum shunts in treatment of priapism. Urology 1976;7:292–5 [DOI] [PubMed] [Google Scholar]
- 43.Kirkham AP, Illing RO, Minhas S, Minhas S, Allen C. MR imaging of nonmalignant penile lesions. Radiographics 2008;28:837–53 [DOI] [PubMed] [Google Scholar]
- 44.Li CY, Agrawal V, Minhas S, Ralph DJ. The penile suspensory ligament: abnormalities and repair. BJU Int 2007;99:117–20 [DOI] [PubMed] [Google Scholar]
- 45.Pretorius ES, Siegelman ES, Ramchandani P, Banner MP. MR imaging of the penis. Radiographics 2001;21:S283–98; discussion S298–9 [DOI] [PubMed] [Google Scholar]
- 46.Hatzimouratidis K, Hatzichristou DG. A comparative review of the options for treatment of erectile dysfunction: which treatment for which patient? Drugs 2005;65:1621–50 [DOI] [PubMed] [Google Scholar]
- 47.Hatzimouratidis K, Amar E, Eardley I, Giuliano F, Hatzichristou D, Montorsi F, et al. Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur Urol 2010;57:804–14 [DOI] [PubMed] [Google Scholar]