Abstract
The aim of this review is to illustrate the potential of different and newer ultrasound techniques beyond conventional B-mode imaging, including colour Doppler ultrasound, contrast-enhanced ultrasound (CEUS) and tissue elastography, in the characterisation of both benign and malignant intratesticular lesions. Normally, testicular malignancies, either primary or secondary, demonstrate an increase in colour Doppler signal. However, there is a diversity of benign testicular lesions that may mimic testicular malignancies. The use of CEUS improves characterisation of testicular lesions, and confirms lack of vascularity in benign abnormalities such as epidermoid cysts, infarctions, abscesses and changes following trauma. Tissue elastography allows further evaluation of the cellular consistency of the abnormality. Familiarity with the appearances seen with these ultrasound techniques in both benign and malignant abnormalities should aid in improving confidence in arriving at the correct diagnosis.
Ultrasound is a sensitive and accurate technique for the evaluation of testicular abnormalities, and is widely accepted as the first-line imaging technique for many common and uncommon testicular diseases. Ultrasound is effectively the sole scrotal imaging technique that a patient will undergo prior to surgery. Traditionally, B-mode ultrasound is extremely sensitive in the detection of testicular masses, but does not provide histological diagnosis. Although most focal lesions will be malignant and require an orchidectomy, recognition of the benign entity may be challenging. When clearly implied, with all available ultrasound techniques, that the abnormality is a benign intratesticular process, testes-sparing management options become suitable for patient management. Currently, there are no ultrasound criteria that allow definitive differentiation of benign from malignant testicular lesions. Although not entirely diagnostic, ultrasound techniques such as colour Doppler ultrasound (CDUS), contrast-enhanced ultrasound (CEUS) and tissue elastography (TE), in addition to B-mode imaging, are available to provide a more detailed interrogation of focal testicular lesions.
Tumour or tumour-like pathology
Can colour Doppler ultrasound help?
CDUS is an important ultrasound technique for the evaluation of a focal indeterminate testicular lesion [1]. With rare exceptions, any solid intratesticular lesion with an increase in colour Doppler flow should be considered suspicious for malignancy. However, this is not without limitations, as small testicular tumours may appear avascular on the CDUS examination.
Can contrast-enhanced ultrasound help?
The use of CEUS improves characterisation of testicular lesions, with more detailed evaluation of intratesticular vascular flow [2]. More importantly, CEUS allows a conclusive demonstration of the lack of vascularity that is likely to be encountered in benign lesions [3]. Demonstration of an avascular abnormality, which is likely to be benign in nature and may resolve, would allow the option of “watchful waiting” with ultrasound, without subjecting the patient to unnecessary surgery [4].
Can tissue elastography help?
TE is an ultrasound measure of the stiffness of tissue. Given that most solid focal tumours differ in their consistency from the surrounding tissue, TE is a further technique that allows better differentiation between benign and malignant testicular lesions [5]. The “hard” lesions are more likely to be malignant, and a “soft” area may suggest benignity.
Applications of colour Doppler ultrasound, contrast-enhanced ultrasound and tissue elastography
The use of CDUS, CEUS and TE in the following testicular abnormalities illustrates the potential of these techniques in evaluating testicular lesions.
Testicular tumours
Testicular tumours contribute to approximately 1% of all malignancies in males. 90–95% of malignant intratesticular tumours are primary germ cell tumours. Germ cell tumours arise from spermatogenic cells and are almost uniformly malignant. Germ cell tumours are broadly divided into seminomatous and non-seminomatous types. Non-germ cell tumours represent the remainder of primary and secondary testicular tumours, and are made up of sex cord stromal tumours (Leydig or Sertoli cell tumours), lymphoma and metastasis.
Seminoma
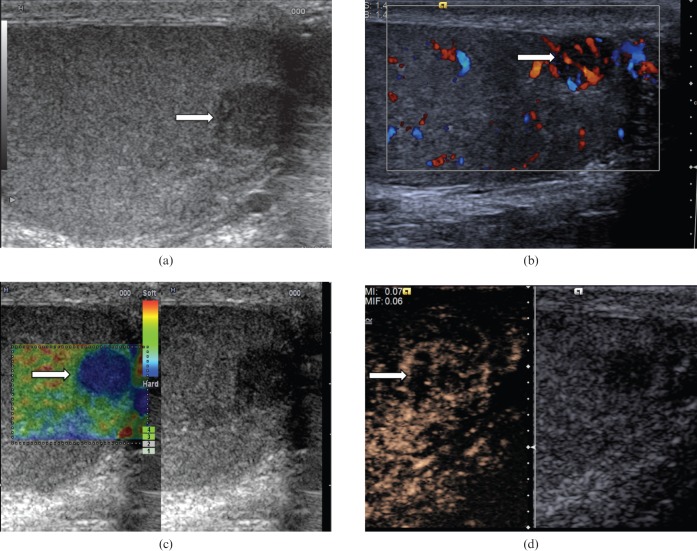
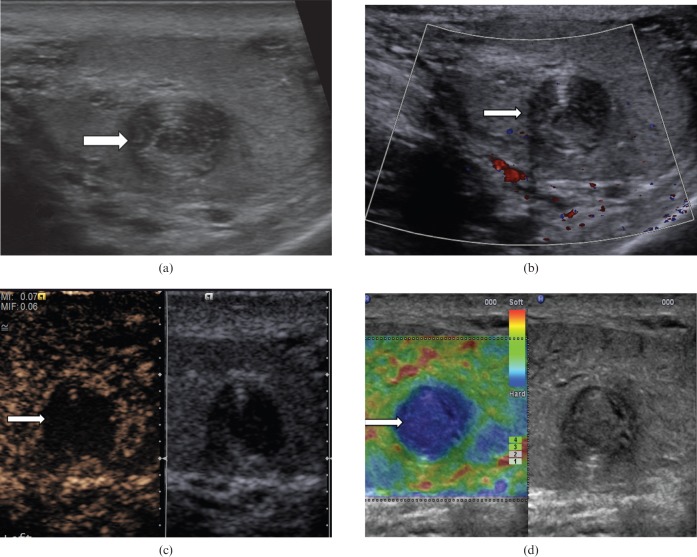
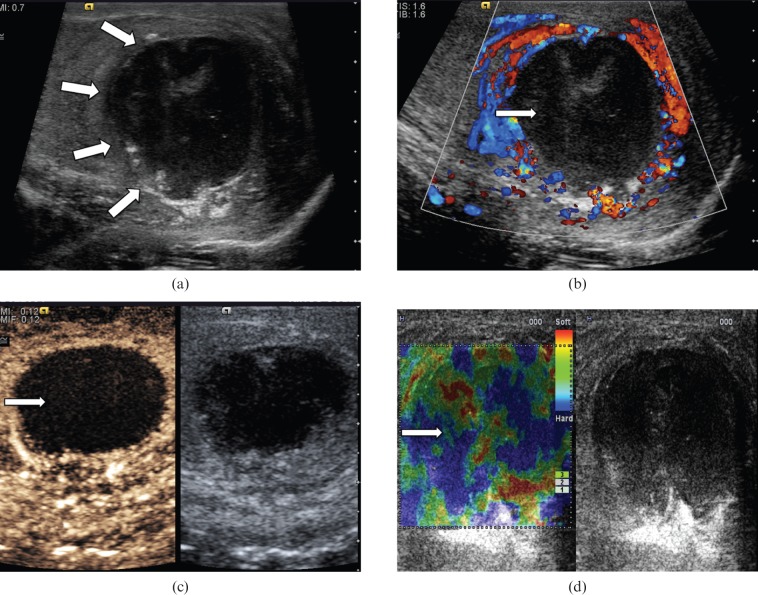
Seminoma (Figure 1) is the most common pure germ cell tumour. It accounts for 35–50% of all germ cell tumours. It occurs in an older population, in comparison with non-seminomatous tumours, with an average patient age of 40.5 years [6]. At histology, a seminoma is made of relatively uniform cells with clear cytoplasm and lymphoid infiltrate. On B-mode ultrasound, seminomas typically appear as a solid round homogeneous low-reflectivity mass without calcification inside the tumour mass, reflecting their uniform cellular nature. On CDUS there is demonstrable vascularity within the lesion. With CEUS, there is a rapid enhancement in the tumour (higher than the surrounding normal testicular parenchyma) and loss of the normal linear vascular pattern. Washout of the contrast within the lesion may be rapid, but with persistence of the abnormal “crossing” vessels within the lesion. On TE a hard lesion will be clearly demonstrated.
Figure 1.
Seminoma. (a) B-mode ultrasound demonstrates a small focal lesion (arrow) measuring 6 mm, with uniform low reflectivity. (b) Colour Doppler ultrasound demonstrates internal vascularity within the small tumour (arrow). (c) The lesion appears “hard” on tissue elastography (depicted by the blue area, arrow). (d) Contrast-enhanced ultrasound demonstrates early enhancement and rapid washout with loss of the normal vascular pattern.
Non-seminomatous germ cell tumours
Of the non-seminomatous germ cell tumours, mixed germ cell tumours (Figure 2) are much more common than any of the pure histological forms. Embryonal carcinoma (Figure 3) is the most common component, and is often combined with one or more components of teratoma, seminoma and yolk sac tumour. The imaging findings reflect the diversity of the components of these lesions. On B-mode images these tumours may be inhomogeneous, with areas of increased echogenicity, calcification and cyst formation. Increased vascularity may or may not be demonstrated, and therefore may be mistaken for a benign avascular abnormality, such as a segmental infarction of focal scarring. However, on CEUS, movement of individual microbubbles may be seen within the lesion in a haphazard pattern, which suggests that the abnormal vascularity favours a malignant diagnosis to the differential of an infarct. The key point is that the microbubble contrast is truly and exclusively intravascular, and any movement is within a vessel.
Figure 2.
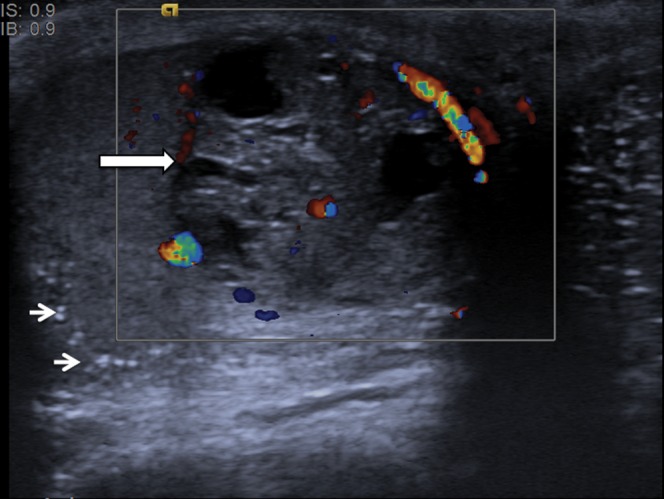
Mixed germ cell tumour. A focal lesion with heterogeneous reflectivity and cystic components (long arrow). Colour Doppler demonstrates distortion of the normal vascular pattern by the lesion. Note is also made of background testicular microlithiasis (short arrows).
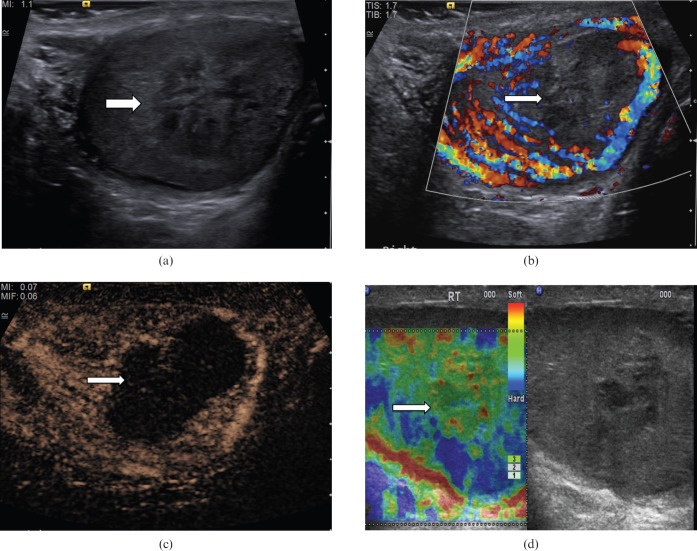
Figure 3.
Embryonal cell tumour. (a) B-mode ultrasound demonstrates a focal lesion with a slightly heterogeneous reflectivity (arrow). (b) Colour Doppler ultrasound demonstrates loss of normal parenchymal vascular pattern, replaced by an abnormal vascularity (the “criss-cross” vascular pattern; arrow). Colour Doppler flow is demonstrated in the large vessels only. (c) Tissue elastography demonstrates a “blue” lesion, therefore clearly a “hard” lesion (arrow). (d) On contrast-enhanced ultrasound, particulate movement of contrast (arrows) is seen throughout the lesion in a haphazard pattern, confirming the vascularity is present within all components of the lesion.
Sex cord stromal tumours
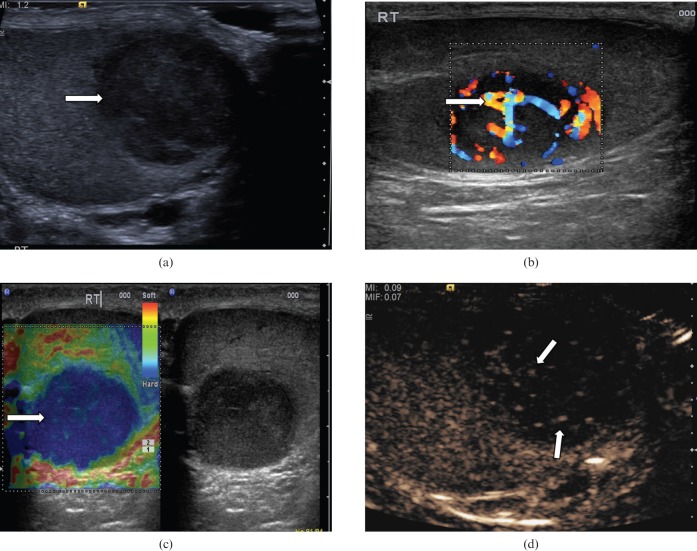
Non-germ cell primary tumours of the testis derive from the sex cords (Sertoli cells) and stroma (Leydig cells; Figure 4). These tumours are interstitial cell tumours, and 90% of non-germ cell tumours are benign. These tumours are usually small in size, and most are discovered incidentally. These are most common in patients aged between 20 and 50 years, and display hormonal activity in 30%. On B-mode ultrasound, these lesions appear as well-circumscribed homogeneous hypoechoic lesions. Personal experience suggests that small stromal cell tumours invariably display early enhancement on CEUS, which seems to persist longer than the normal testicular parenchymal enhancement.
Figure 4.
Leydig cell tumour. (a) B-mode ultrasound demonstrates a small hypoechoic lesion (arrow). (b) Colour Doppler ultrasound demonstrates increased vascularity within the lesion (arrow). (c) Tissue elastography demonstrates a small distinct “hard” lesion (mixed blue/green area, arrow). (d) With contrast-enhanced ultrasound the lesion (arrow) demonstrates early enhancement, a characteristic that has the potential to differentiate these lesions from other malignant tumours.
Non-primary malignant tumours
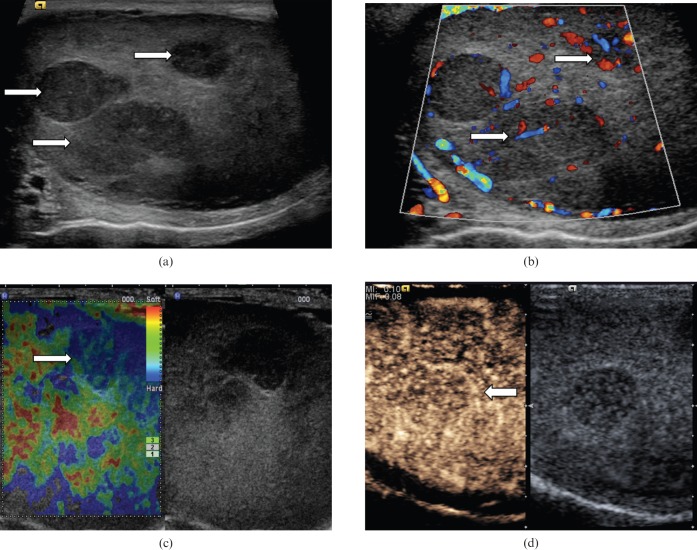
Non-primary tumours (Figure 5) such as lymphoma, leukaemia and metastasis can all manifest as an indeterminate testicular mass. Testicular lymphoma occurs in a much older population than those affected by primary germ cell tumours, and is the most common testicular neoplasm in males over 60 years of age. The epididymis and spermatic cord are commonly involved. Primary leukaemia of the testis is rare; secondary testicular involvement is more common. Sonographic findings in both lymphoma and leukaemia may be represented by focal or multifocal hypoechoic lesions, and may be indistinguishable from germ cell tumours. Correlation to relevant clinical history would be required in reaching correct diagnosis. Other metastases to the testes, which are rarely the presenting complaint, are most commonly seen in cases of widespread primary prostate and lung malignancies [7].
Figure 5.
Prostatic metastasis. (a) B-mode ultrasound demonstrates multifocal hypoechoeic lesions (arrows). (b) Colour Doppler ultrasound demonstrates internal vascularity within the lesions (arrows). (c) The lesions appear “hard” on elastography (blue/green area, arrow). (d) Enhancement is noted within the lesion, confirming internal vascularity and peripheral contrast enhancement (arrow).
Tumour-like pathologies
Epidermoid cysts
Epidermoid cysts (Figure 6) constitute approximately 1–2% of all resected testicular lesions [8]. Epidermoid cysts are composed of a variable quantity of keratinising, stratified epithelium, and in some a well-defined fibrous wall. They are one of the few intratesticular masses that demonstrate no malignant potential. Four ultrasound appearances have been described [9]:
Figure 6.
Epidermoid cyst. (a) B-mode ultrasound demonstrates a well-circumscribed, solid, mixed-reflectivity lesion with high-reflectivity “onion-skin” rims (arrow). (b) Colour Doppler signal is not observed within the lesion (arrow). (c) Contrast-enhanced ultrasound demonstrates a clear lack of enhancement within the lesion (arrow). (d) Tissue elastography demonstrates a well-demarcated “hard” lesion, blue in colour (arrow).
Type 1—classic “onion-ring” appearance with alternating hyperechoic and hypoechoic layers.
Type 2—densely calcified mass with an echogenic rim.
Type 3—cyst with a rim and either peripheral or central calcification.
Type 4—mixed pattern, heterogeneous and poorly defined.
Although the sonographic appearances of epidermoid cysts are characteristic, they are not pathognomonic. The combination of absence of colour Doppler flow, absence of vascular flow on CEUS and hardness on real-time elastography help further characterise these lesions [10]. Negative tumour markers with the above ultrasound findings increase diagnostic confidence. The treatment for epidermoid cyst is either enucleation or orchidectomy, which is usually performed when malignancy cannot be completely excluded.
Segmental testicular infarction
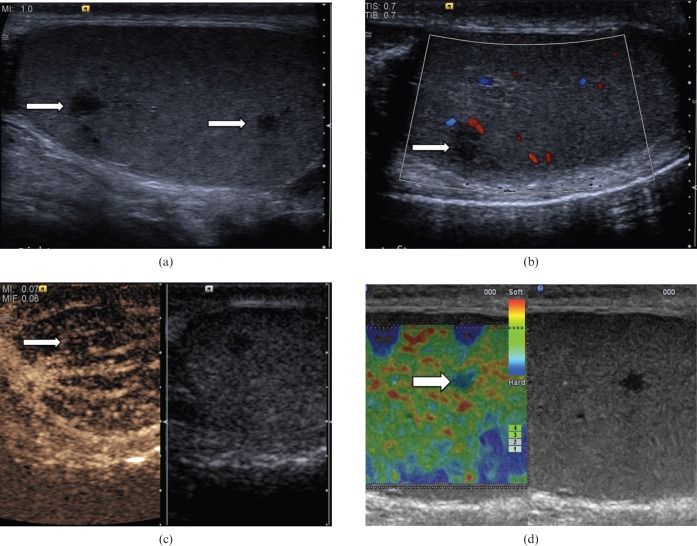
Segmental testicular infarction (Figure 7) is an infrequent finding in patients with acute testicular pain. Predisposing factors to segmental infarction include epididymo-orchitis, trauma, hypersensitivity angiitis, intimal fibroplasia of the testicular artery, previous surgery, polycythaemia and sickle cell disease [11]. Ultrasound examination demonstrates an area of mixed or low reflectivity, which may be wedge or round shaped [12]. There is poor or absent colour Doppler flow. CEUS will demonstrate any avascular testicular parenchyma. In the subacute stage, CEUS may show avascular lobules and in some cases perilesional rim enhancement [13]. Rim enhancement observed may be due to perilesional inflammatory changes around the infarcted areas or mass effect secondary to intralesional oedema in the infarcted area that displaces the surrounding testicular tissue and causes bundling of the perilesional parenchymal vessels. After 1 month or more, CEUS may depict reduced size of the lesion, with intralesional vascular “spots” in areas of infarction [13]. The ultrasound appearances, the absence of tumour markers and a change in the size or shape of the abnormality during follow-up will often establish the benign nature of the abnormality.
Figure 7.
Segmental testicular infarction. (a) There is a focal mixed-reflectivity area (arrow) in a patient who presented with testicular pain and clinical evidence of epididymo-orchitis. (b) There is no colour Doppler signal within the abnormality, suggesting absence of vascularity (arrow). (c) Contrast-enhanced ultrasound clearly depicts the infarcted areas with absence of enhancement (arrow). (d) Tissue elastography demonstrates that the abnormality is “soft” (red/green on colour scale, arrow) and no focal “hard” lesion is visualised, in contrast to the cases of focal testicular tumours.
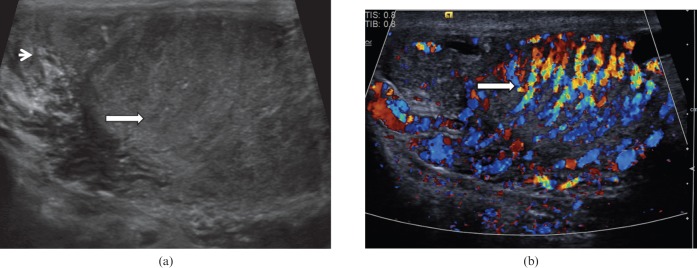
Orchitis
Primary orchitis (Figure 8) is rare without associated epididymo-orchitis, but may be caused by human immunodeficiency virus or mumps virus. The process may be seen as diffuse or focal. Orchitis may manifest as multiple hypoechoic abnormalities within the testicular parenchyma, with septal accentuation with foci of low reflectivity conforming to the lobular anatomy [14]. As the condition progresses, areas of venous infarction occur with associated haemorrhage, giving rise to areas of mixed or increased reflectivity. Increased blood flow to the epididymis and testis at CDUS and CEUS examination is a well-established criterion for the diagnosis of epididymo-orchitis. After treatment and healing, changes may resolve completely, or often there is loss of volume of the testis with fibrosis giving a heterogeneous pattern on ultrasound. The great variability in ultrasound appearances can cause diagnostic confusion, but awareness of the changes and progression may allow a more confident diagnosis to be made in the appropriate clinical setting.
Figure 8.
Orchitis. (a) Longitudinal ultrasound of the testis demonstrates patchy heterogeneous reflectivity within the testis (long arrow) and enlargement of the epididymis (short arrow). (b) There is marked increase in vascularity within the testis on colour Doppler ultrasound (arrow).
Venous infarction
Venous infarction (Figure 9) of the testis may occur in cases of severe epididymo-orchitis where local swelling occludes the venous drainage of portions of the testis or the entire testis. Venous infarction may also occur in patients with hypercoagulable states. On ultrasound the testis is of low or mixed echo reflectivity. There is an absence of colour Doppler flow and contrast enhancement. The diagnosis should also be suspected when reversal of intratesticular arterial flow in diastole is observed with an associated focal abnormality [15]. CEUS demonstrates clear demarcation of avascular areas to allow for appropriate clinical management [16].
Figure 9.
Venous infarction of the testis. (a) A focal testicular abnormality with mixed reflectivity (arrows) is noted on B-mode ultrasound. (b) No colour Doppler signal is seen in the focal testicular abnormality (arrow). (c) Following the administration of microbubble contrast, contrast flow is present in the normal testicular parenchymal, and clearly absent from the infarcted portion of the testis (arrow). (d) Tissue elastography demonstrates no focal “hard” lesions, and the area of abnormality appears “soft” (green on colour scale, arrow).
Intratesticular haematoma
A history of trauma should raise the suspicion of the differential of an intratesticular haematoma (Figure 10). Acutely, the haematoma appears as patchy increased reflectivity. On follow-up it may appear as an area of low reflectivity, with reduction in size as the haematoma retracts. The most important differential diagnosis is malignancy, and therefore an accurate history, lack of vascularity on both CDUS and CEUS, absence of tumour markers and reduction in the size of the abnormality on sequential scans is indicative of a benign entity [17].
Figure 10.
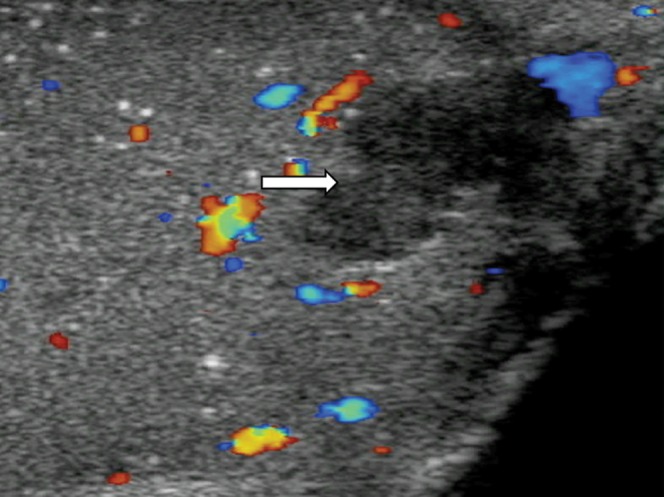
Intratesticular haematoma. A well-circumscribed (arrow) focal area of low reflectivity with internal echoes is noted in the testis of a patient involved in a motorcycle accident. The lesion demonstrates low reflectivity. Colour Doppler ultrasound confirms absence of vascularity, in keeping with the diagnosis of traumatic intratesticular haematoma. At 4 weeks there was reduction in size of the lesion. Incidental microlithiasis is present.
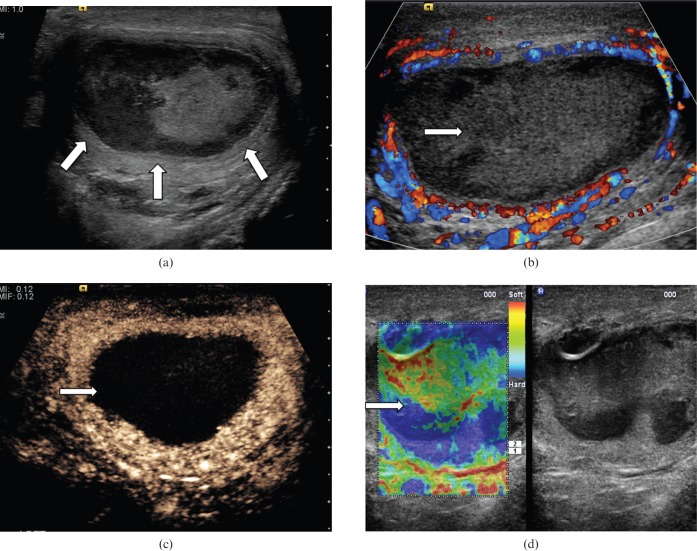
Intratesticular abscess
Intratesticular abscesses (Figure 11) are unusual and are associated with severe epididymo-orchitis [18]. They may also arise secondary to mumps, trauma or infarction. The ultrasound appearances are of a lesion of low reflectivity with irregular borders. Hypervascular rims may be visible surrounding a testicular abscess on CEUS and CDUS but no internal vascularity is present. The abnormality observed in testicular abscess does not conform to lobular distribution, which may help to differentiate this from a segmental infarction.
Figure 11.
Testicular abscess. (a) On B-mode ultrasound a focal lesion with low internal echoes (arrows) is seen in a patient with history of resolving epididymo-orchitis. (b) On colour Doppler ultrasound there is increased vascularity at the periphery of the lesion but none within the lesion (arrow). (c) Contrast-enhanced ultrasound image demonstrating increased absence of vascularity in the abscess (arrow) with some rim enhancement. (d) Tissue elastography demonstrates a heterogeneous pattern of firmness but no focal “hard” lesion is demonstrated (arrow).
Rete testis
The rete testis (Figure 12) is a system of numerous seminiferous tubules located at the mediastinum testis, which drain to the epididymal head. On ultrasound the rete testis has a spectrum of appearances ranging from a faintly visible ill-defined area of decreased reflectivity to a coarse tubular appearance with finger-like projection into the parenchyma [19]. No vascular flow is demonstrated within rete testis. They may resemble a hypoechoic mass when viewed in cross-section. As long as ultrasound appearances remain typical with no soft tissue component or abnormal colour Doppler signal or enhancement on CEUS, no further investigation is usually required. Although this is a benign entity, it may be of significance in a patient suffering from azoospermia as this implies there is obstruction of the ipsilateral spermatic ducts.
Figure 12.
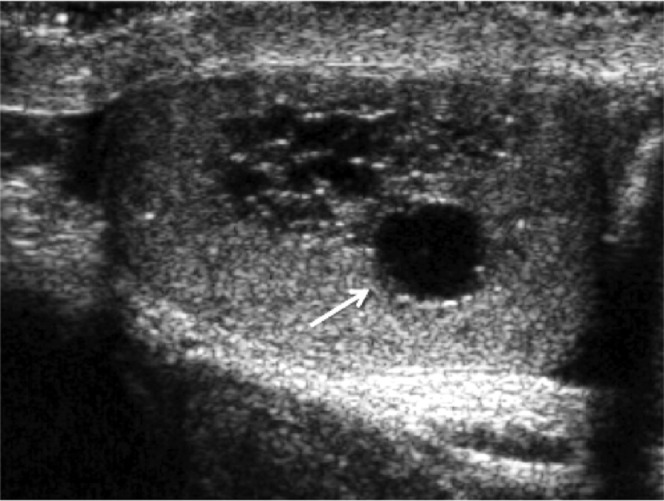
Rete testis. Localised area of tubular ectasia of the rete testis, with a further testicular cyst (arrow). No soft tissue component or internal colour Doppler signal (not shown) is demonstrated to suggest the presence of an underlying tumour.
Testicular sarcoidosis
Involvement of the genital system by sarcoidosis is rare (Figure 13). It more commonly affects the epididymis but can also involve the testis. On ultrasound the lesions of sarcoidosis are typically multiple, small, bilateral low-reflectivity masses. Differentiation from malignancy may be difficult, and clinical evidence of sarcoidosis elsewhere is required for diagnosis to be made more confidently [18]. If there are no associated symptoms or features, then ultimately tissue biopsy for pathological evaluation may be required.
Figure 13.
Testicular sarcoidosis. (a) B-mode ultrasound demonstrates multiple low-reflectivity focal testicular lesions (arrows) in a patient with a recent clinical diagnosis of sarcoidosis. (b) On the colour Doppler study the focal lesions do not clearly demonstrate vascular flow, but it is difficult to be certain because of the size of the lesions. (c) Contrast-enhanced ultrasound clearly confirms some vascularity within the lesion (arrow). (d) Tissue elastography demonstrates a moderate degree of “hardness” of these lesions (blue on colour scale, arrow).
Post-trauma testicular devascularisation
Devascularisation of the testis may occur following significant traumatic injury to the scrotum (Figure 14). On B-mode images the testis may appear heterogeneous and may not assume the normal testicular configuration. An associated haematocele may be present. On CDUS little vascular flow will be appreciated within the devascularised segment. The extent of the abnormality, however, is best appreciated with CEUS, where there may be a sharply demarcated non-enhancing area in contrast to the normally vascularised testicular tissue.
Figure 14.
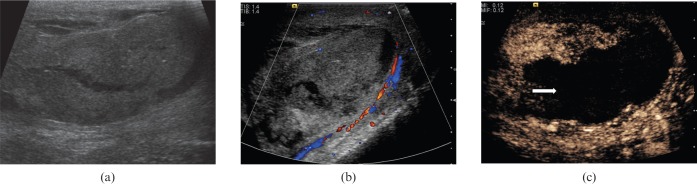
Post-traumatic testicular devascularisation. (a) On B-mode images demonstrates the testis appears very heterogeneous and appears to be “shattered”. (b) There is no clear evidence of colour Doppler flow to the affected testis. (c) Following administration of microbubble contrast, the testis is seen to be predominantly devascularised. The abnormality is much better demarcated on contrast-enhanced ultrasound (arrow).
Simple testicular cyst with debris
Simple cysts are detected incidentally and usually occur in males over 40 years of age, with a size range from 2 mm to 2 cm in diameter (Figure 15). The cysts are usually solitary, and may be associated with spermatoceles [20]. On B-mode ultrasound, a simple cyst would appear an anechoic centre surrounded by a thin wall, with a degree of posterior acoustic enhancement. Colour Doppler ultrasound—or, more precisely, CEUS—could exclude presence of complex features such as internal vascularity or enhancement.
Figure 15.
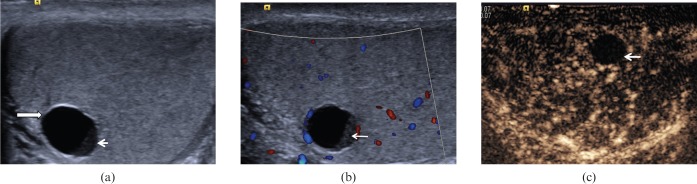
Cyst with debris. (a) A 6-mm anechoic lesion (long arrow) is noted in the testicle with a thin clear wall demonstrated. A “fluid-debris” level is noted (short arrow). (b) No internal colour Doppler signal is demonstrated within the debris present in the lower aspect of the cyst (arrow). (c) On contrast-enhanced ultrasound, there is clear absence of enhancement in the debris present in the lower aspect of the cyst (arrow), and a cystic tumour is unlikely.
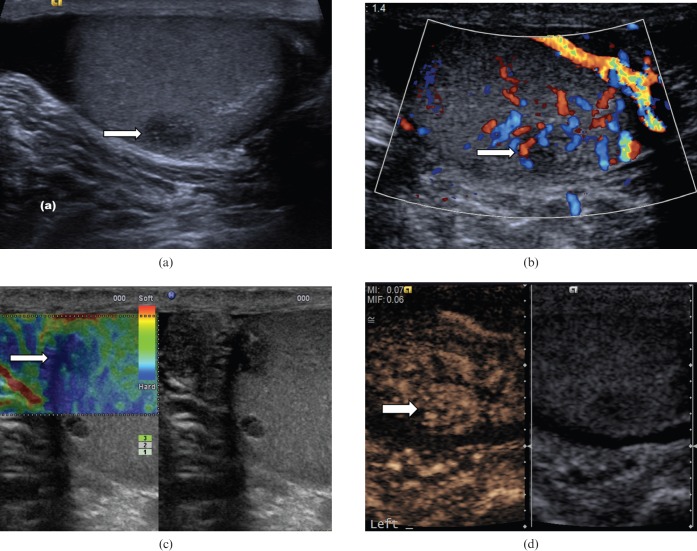
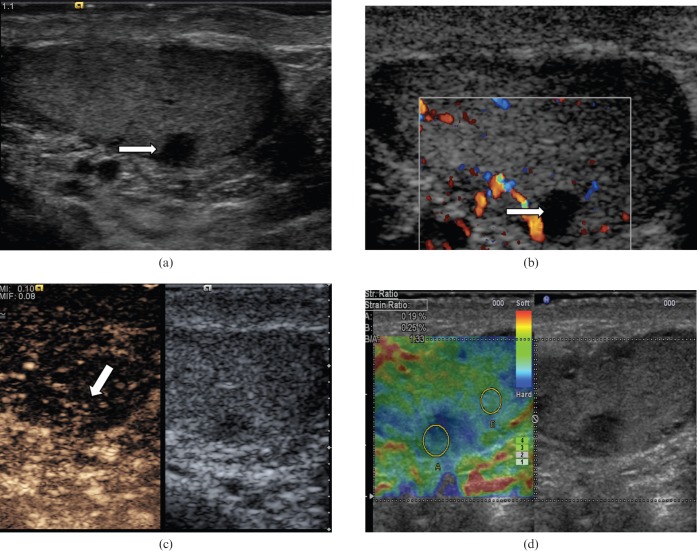
Post-biopsy scar
Scar tissue following testicular biopsy or partial orchidectomy may appear as a low-reflectivity lesion that corresponds to the site of surgery (Figure 16). There may be some enhancement following CEUS and on TE the scar tissue may appear slightly harder than the surrounding normal tissue. Clear understanding of the site where biopsy was performed, and close ultrasound surveillance at 1- to 3-month intervals to monitor progression, would allow increased confidence in the diagnosis and prevent unnecessary further intervention.
Figure 16.
Post-biopsy scar. (a) On B-mode image there is a 3.1×4.6-mm low-reflectivity lesion (arrow) in the mid-aspect of the testicle, which corresponds to the site of a previous testicular biopsy. (b) No internal colour Doppler vascular signal is demonstrated (arrow). (c) There is some subtle enhancement (arrow) on the contrast-enhanced ultrasound examination. (d) Tissue elastography suggests the area is slightly “harder” than the adjacent normal parenchyma (blue area, strain ratio of 1.33). Follow-up ultrasound 1 year later demonstrated no interval changes in appearances of the lesion.
Adenomatoid lesion
Extratesticular lesions, although almost always benign, may cause a diagnostic challenge clinically and significant patient anxiety. An adenomatoid tumour (Figure 17) is the second most common extratesticular tumour (cysts are the most common), followed by a lipoma. The ultrasound appearance of an adenomatoid tumour consists of a hyperechoic rounded tumour most commonly at the epidydimal tail. Following CEUS the focal epididymal lesion demonstrates enhancement and early washout of microbubble contrast.
Figure 17.
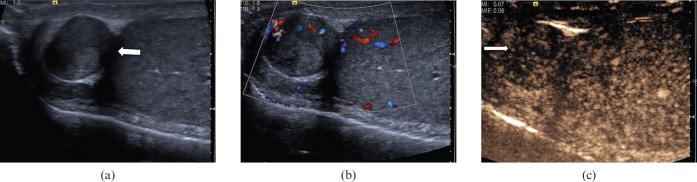
Extratesticular adenomatoid lesion. (a) A heterogeneous lesion (arrow) measuring 12 mm in diameter is noted within the right epididymal head. (b) Colour Doppler signal is demonstrated within the lesion. (c) Following contrast administration the focal epididymal lesion demonstrates enhancement and early washout (arrow).
Conclusion
The use of high-frequency B-mode ultrasound, CDUS, CEUS and TE help to establish the correct diagnosis of a variety of conditions involving testes. CEUS is a useful adjunct to the CDUS examination to clearly identify enhancement and, perhaps more importantly, non-vascularised abnormalities. The use of TE allows further characterisation of focal testicular lesions by differentiating consistency of a focal lesion from the surrounding normal testicular tissue. Although no ultrasound appearances are entirely diagnostic, features demonstrated with these technologies provide better characterisation of intratesticular lesions. With increasing experience, ultrasound evaluation of testicular pathology may allow a tailored follow-up plan, or targeted ultrasound-guided excision biopsy when deemed appropriate, thus potentially reducing the number of unnecessary orchidectomies.
References
- 1.Horstman WG, Melson GL, Middleton WD, Andriole GL. Testicular tumors: findings with color Doppler US. Radiology 1992;185:733–7 [DOI] [PubMed] [Google Scholar]
- 2.Lock G, Schmidt C, Helmich F, Stolle E, Dieckmann KP. Early experience with contrast-enhanced ultrasound in the diagnosis of testicular masses: a feasibility study. Urology 2011;77:1049–53 [DOI] [PubMed] [Google Scholar]
- 3.Hedayati V, Sellars ME, Sharma DM, Sidhu PS. Contrast-enhanced ultrasound in testicular trauma: role in directing debridement and aiding organ salvage. Br J Radiol 2012;85:e65–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah A, Lung PF, Clarke JL, Sellars ME, Sidhu PS. New ultrasound techniques for imaging of the indeterminate testicular lesion may avoid surgery completely. Clin Radiol 2010;65:496–8 [DOI] [PubMed] [Google Scholar]
- 5.Goddi A, Sacchi A, Magistretti G, Almolla J, Salvador M. Real-time tissue elastography for testicular lesion assessment. Eur Radiol 2012;22:721–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodward PJ, Sohaey R, O'Donoghue MJ, Green DE. Tumors and tumor-like lesions of the testis: radiologic–pathologic correlation. Radiographics 2002;22:189–216 [DOI] [PubMed] [Google Scholar]
- 7.Ulbright TM, Amin MB, Young RH. Tumors of the testis, adnexa, spermatic cord, and scrotum. Atlas of tumor pathology. Washington, DC: Armed Forces Institute of Pathology; 1999 [Google Scholar]
- 8.Price EB. Epidermoid cysts of the testis: a clinical and pathologic analysis of 69 cases from the testicular tumour registry. J Urology 1969;102:708–13 [DOI] [PubMed] [Google Scholar]
- 9.Atchley JTM, Dewbury KC. Ultrasound appearances of testicular epidermoid cysts. Clin Radiol 2000;55:493–502 [DOI] [PubMed] [Google Scholar]
- 10.Patel K, Sellars ME, Clarke JL, Sidhu PS. Sonographic features of testicular epidermoid cysts on contrast enhanced ultrasound and real time tissue elastography. J Ultrasound Med 2012;31:115–22 [DOI] [PubMed] [Google Scholar]
- 11.Fernández-Pérez GC, Tardáguila FM, Velasco M, Rivas C, Dos Santos J, Cambronero J, et al. Radiologic findings of segmental testicular infarction. Am J Roentgenol 2005;184:1587–93 [DOI] [PubMed] [Google Scholar]
- 12.Bilagi P, Sriprasad S, Clarke JL, Sellars ME, Muir GH, Sidhu PS. Clinical and ultrasound features of segmental testicular infarction: six-year experience from a single centre. Eur Radiol 2007;17:2810–18 [DOI] [PubMed] [Google Scholar]
- 13.Bertolotto M, Derchi LE, Sidhu PS, Serafini G, Valentino M, Grenier N, et al. Acute segmental testicular infarction at contrast-enhanced ultrasound: early features and changes during follow-up. AJR Am J Roentgenol 2011;196:834–41 [DOI] [PubMed] [Google Scholar]
- 14.Cook JL, Dewbury K. The changes seen on high-resolution ultrasound in orchitis. Clin Radiol 2000;55:13–18 [DOI] [PubMed] [Google Scholar]
- 15.Sanders LM, Haber S, Dembner A, Aquino A. Significance of reversal of diastolic flow in the acute scrotum. J Ultrasound Med 1994;13:137–9 [DOI] [PubMed] [Google Scholar]
- 16.Lung PFC, Jaffer OS, Sellars ME, Sriprasad S, Kooiman GG, Sidhu PS. Contrast enhanced ultrasound (CEUS) in the evaluation of focal testicular complications secondary to epididymitis. AJR Am J Roentgenol 2012;199:W345–54 [DOI] [PubMed] [Google Scholar]
- 17.Purushothaman H, Sellars ME, Clarke JL, Sidhu PS. Intratesticular haematoma: differentiation from tumour on clinical history and ultrasound appearances in two cases. Br J Radiol 2007;80:e184–7 [DOI] [PubMed] [Google Scholar]
- 18.Stewart VR, Sidhu PS. The testis: the unusual, the rare and the bizarre. Clin Radiol 2007;62:289–302 [DOI] [PubMed] [Google Scholar]
- 19.Sellars ME, Sidhu PS. Pictorial review: ultrasound appearances of the rete testis. Euro J Ultrasound 2001;14:115–20 [DOI] [PubMed] [Google Scholar]
- 20.Dogra VS, Gottlieb R, Rubens DJ, Liao L. Benign intratesticular cystic lesions: US features. Radiographics 2001;21:S273–81 [DOI] [PubMed] [Google Scholar]