Abstract
Context:
Animal and molecular studies have shown that cocaine exerts a neuroprotective effect against cerebral ischemia.
Aims:
To determine if the presence of cocaine metabolites on admission following traumatic brain injury (TBI) is associated with better outcomes.
Settings and Design:
Level-1 trauma center, retrospective cohort.
Materials and Methods:
After obtaining Institutional Review Board (IRB) approval, the trauma registry was searched from 2006 to 2009 for all patients aged 15-55 years with blunt head trauma and non-head AIS <3. Exclusion criteria were pre-existing brain pathology and death within 30 min of admission. The primary outcome was in-hospital mortality; secondary outcomes were hospital length of stay (LOS), and Glasgow Outcome Score (GOS).
Statistical Analysis:
Logistic regression was used to determine the independent effect of cocaine on mortality. Hospital LOS was compared with multiple linear regression.
Results:
A total of 741 patients met criteria and had drug screens. The screened versus unscreened groups were similar. Cocaine positive patients were predominantly African-American (46% vs. 21%, P < 0.0001), older (40 years vs. 30 years, P < 0.0001), and had ethanol present more often (50.7% vs. 37.8%, P = 0.01). There were no differences in mortality (cocaine-positive 1.4% vs. cocaine-negative 2.7%, P = 0.6) on both univariate and multivariate analysis.
Conclusions:
Positive cocaine screening was not associated with mortality in TBI. An effect may not have been detected because of the low mortality rate. LOS is affected by many factors unrelated to the injury and may not be a good surrogate for recovery. Similarly, GOS may be too coarse a measure to identify a benefit.
Keywords: Amphetamine, cocaine, traumatic brain injury
INTRODUCTION
Traumatic brain injury (TBI) is a major public health problem in the United States; more than 1.4 million people suffer from TBI annually and 50,000 die of it.[1] Positive alcohol levels on admission are associated with decreased in-hospital mortality in patients with TBI.[2,3,4] The effects of other drugs of abuse on outcome from TBI is unknown. It is estimated that there are more than 1.5 million cocaine users in the United States and more than 150,000 annual ED visits were related to cocaine intoxication.[5] The relationship between positive cocaine metabolite levels and outcomes from TBI remains unknown.
The effects of cocaine administration following induced TBI have been studied in animals. Cocaine is a sympathomimetic, which increases cardiac output, theoretically resulting in increased cerebral blood flow. It has been shown that the administration of cocaine following fluid percussion brain injury in rats reduced posttraumatic body weight losses and decreased the duration of suppression of postural and non-postural neurological reflexes.[6] Cocaine also increases cortical blood flow (CBF) more than saline control in rats.[7] In a large animal study using swine, it was found that although cocaine-intoxicated animals subjected to TBI showed no significant difference in the primary outcome measures of cerebral perfusion pressure (CBF), a trend toward lower intracranial pressure was noted.[8]
Molecular studies using cell cultures have demonstrated cocaine's neuroprotective effects in vitro. Cocaine induces the expression of cocaine- and amphetamine-regulated transcript (CART), an endogenous neuropeptide, which protects against cerebral ischemia through extracellular signal-regulated kinase (ERK) activation, which in turn mediates cellular growth and survival through regulation of transcription and translation.[9] There is evidence that CART may be neuroprotective in cerebral ischemia by increasing the phosphorylation of ERK1 and ERK2.[10]
Based on the animal studies showing cocaine's possible neuroprotective effects and molecular groundwork demonstrating cocaine's active role in cerebral ischemia, we hypothesized that positive cocaine metabolites upon admission following TBI is associated with lower in-hospital mortality and better outcomes.
MATERIALS AND METHODS
Subjects
After obtaining Institutional Review Board (IRB) approval, the trauma registry at Hurley Medical Center was searched for all patients aged 15-55 years admitted with a head abbreviated injury scale (AIS) score >0 and no other AIS score ≤ 2. Hurley Medical Center, located in Flint, Michigan, is a 443-bed teaching hospital and the region's only level-1 trauma center. Exclusion criteria were penetrating head trauma, burns, significant co-morbidities (APACHE II definitions), previous craniotomy, underlying neurological disorder, prior TBI, and death within 30 min of admission.
Data
Patient data were extracted from charts and compiled in a computerized database (Microsoft Access, Microsoft Corporation, Redmond WA). The patient variables collected included age, race, gender, co-morbidities, negative toxicology screen, positive toxicology screen(s) other than cocaine (amphetamine, barbiturate, benzodiazepine, heroin, opiate, phencyclidine (PCP), tetrahydrocannabinol (THC)), blood alcohol level (BAL), Glasgow Coma Scale (GCS), head AIS, injury severity score (ISS), initial respiratory rate, initial blood pressure, initial heart rate, computed tomography (CT) findings, and treatment. Head CT findings were used to calculate the Rotterdam score, a CT-based outcome predictor.[11] The trauma injury severity score (TRISS) and probability of survival were calculated from these data. The Glasgow Outcome Score (GOS) was determined from the patients’ rehabilitation evaluations and discharge status. The outcome data collected included in-hospital mortality, ventilator days, and hospital length of stay (LOS).
Statistical analysis
The primary outcome was mortality; secondary outcomes were GOS and hospital LOS. Descriptive statistics were used to describe the patient population under study. Variables are expressed as medians and inter-quartile ranges or proportions with 95% confidence intervals (modified Wald). The chi-squared test was used to assess the effect of cocaine on mortality. A P < 0.05 was considered significant. Univariate analysis was conducted with chi-squared test, Student's t, Wilcoxon rank-sum, or Spearman's rank correlation, as appropriate, and variables were entered in a backward fashion into the regression equation, if P < 0.10 and, removed, if P > 0.15. Logistic regression was to determine the independent effect of cocaine on mortality and good GOS. Good GOS was 4 or 5, which is complete recovery or moderate disability, but ability to live independently. Multiple linear regression was used to determine the independent effect of cocaine on hospital LOS. Deaths were censored from the GOS and LOS analysis. All calculations were done with MedCalc (MedCalc Software Mariakerke, Belgium).
RESULTS
The registry search yielded 1,004 patients who met the inclusion criteria, and 71 patients were excluded. Of the remaining 933 patients, 741 (79%) had drug screens and constituted the study group. Of the drug screened patients, 138 (18.6%) were positive for the presence of cocaine metabolites [Figure 1].
Figure 1.
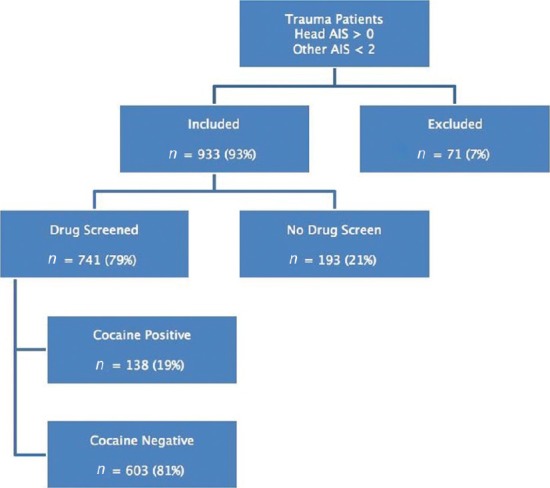
Selection of patients in this study
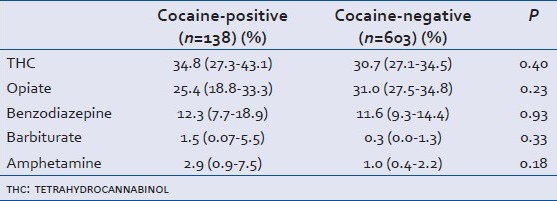
The drugs screened versus unscreened groups were similar, except for the distribution of head AIS scores where there was a trend toward less severe injuries in the unscreened group [Table 1]. There were significant differences in the characteristics of cocaine-positive versus cocaine-negative patients. Cocaine-positive patients were predominantly African-American (46% vs. 21%, P < 0.0001), older (40 years vs. 30 years, P < 0.0001), and more likely to have ethanol detected (50.7% vs. 37.8%, P = 0.01). Characteristics of the cocaine-positive versus cocaine-negative groups are shown in Table 2. There was no association between the presence of cocaine metabolites and use of any other substance of abuse [Table 3].
Table 1.
Characteristics of all patients meeting inclusion and exclusion criteria
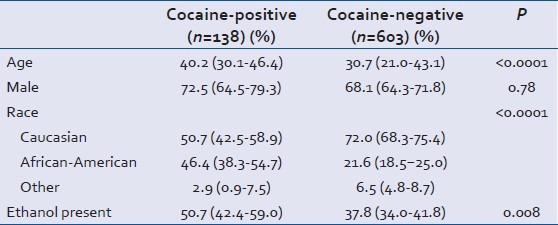
Table 2.
Characteristics of cocaine-positive and cocaine-negative subjects
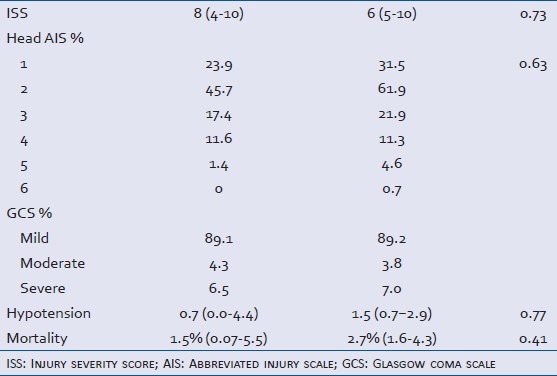
Table 3.
Association between cocaine use and other drugs of abuse
The overall mortality rate for the study group was 2.4%. On univariate analysis, there were no significant differences in mortality between cocaine-positive and cocaine-negative patients (1.4% vs. 2.7%, P = 0.6). In univariate analysis where we compared racial groups, which was dichotomized into African-American and non-African-American, race did not have an effect on the GOS (P = 0.26) and mortality (P = 0.088). Therefore, race was not included in the regression model. Craniotomy had no effect on survival in our cohort and was therefore not included in our regression analysis. Presence of benzodiazepines, GCS, ISS, head AIS, mechanical ventilation, hypotension, Rotterdam score, and TRISS were all associated with mortality. GCS was dichotomized as to > 10 and ≤ 10, based on ROC curve analysis (data not shown), and the Rotterdam score was dichotomized as 1 or 2 (epidural hematoma only or no abnormality) and >2 for logistic regression in order to minimize the number of dummy variables and maintain a favorable event/variable ratio. Only TRISS and the dichotomized Rotterdam score remained significant. When the independent effect of cocaine use on mortality was tested with TRISS and Rotterdam score 1 or 2 as covariates, the presence of cocaine metabolites was not significantly associated with increased mortality (OR 0.33 (0.042-2.7), P = 0.30). To investigate whether positive ethanol level would influence mortality in the setting of positive cocaine testing, we included positive ethanol level as a variable in our final logistic regression, along with other significant variables (Rotterdam score and TRISS). With positive ethanol level included as a variable, the OR of the presence of cocaine metabolites in associated with increased mortality becomes 0.36 (0.046-3.6) with P = 0.64.
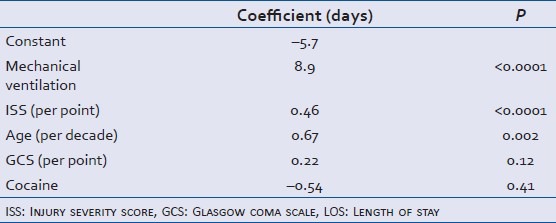
Presence of benzodiazepines, GCS, head AIS, mechanical ventilation, and Rotterdam score were significantly related to a good GOS among survivors. After risk-adjustment with TRISS, Rotterdam score of 1 or 2 (OR = 2.9 (0.8-10.5), P = 0.10), head AIS >2 (OR = 0.19 (0.04-0.80), P = 0.024), and mechanical ventilation (OR = 0.04 (0.01-0.15), P < 0.0001) remained independent predictors of a good GOS. The odd ratios less < 1 indicate that the presence of these factors was negatively associated with a good GOS. The presence of cocaine metabolites had a positive, but non-significant effect on the likelihood of a good GOS (OR = 2.5 (0.53-11.8), P = 0.24). Age, GCS, ISS, head AIS, mechanical ventilation, and Rotterdam score were significantly associated with hospital LOS among survivors. The independent predictors of LOS are show in Table 4. The presence of cocaine metabolites had a non-significant reduction in hospital LOS of 0.54 days (P = 0.41).
Table 4.
Independent predictor of LOS among survivors
DISCUSSION
In our study, we identified significant differences between individuals who tested positive for cocaine metabolites upon admission for TBI versus those who tested negative. The proportion of African-Americans who tested positive for cocaine was twice that of those testing negative. This is disproportionate to the demographics of the study location, as according to the U.S. Census Bureau, the population of the metropolitan area is 78.9% Caucasian and 14.2% African-American.[12] This is in agreement with the current trend of substance abuse stratified by race in the United States with African-American testing positive more often than other racial/ethnic groups for alcohol, cocaine, and opiates.[13] Those that tested positive for cocaine in our study were also more likely to have ethanol detected in their toxicology screens. This agrees with the finding reported by Staines et al. that the self-reported prevalence of cocaine-use among alcohol-dependent patients was as high as 56%.[14] Interestingly, cocaine use was not associated with the presence of any other drug of abuse and may reflect a particular substance abuse pattern in this study population.
Cocaine is a common drug of abuse in the United States and may be the most common drug of abuse leading to an emergency department visit in an inner-city setting.[15] The prevalence of cocaine abuse has been increasing among trauma patients.[16,17] Cocaine is a pleotropic and psychotropic drug that has various effects on multiple organ systems. Its most pronounced effect is vasoconstriction. The drug's vasoconstrictive properties has been reported to worsen respiratory function, increase the risk of renal failure, gastrointestinal necrosis, thrombosis of large arteries, muscle ischemia progressing to compartment syndrome, stroke, and myocardial infarction.[16,18,19,20,21] However, in vitro and animal studies have demonstrated protective effects of cocaine on the neurological system.[6,7,8] In this study, we attempted to focus on the effects of positive cocaine metabolite levels on patients with TBI without other major injuries. We found that the overall mortality of patients admitted for TBI was low and there was no significant association between the presence of cocaine metabolites and mortality. There was a non-significant trend toward a better GOS among survivors. No effect may have been found because the presence of cocaine metabolites does not indicate current intoxication; if there is a protective effect of cocaine, it may only be effective if cocaine is present at the time of injury.
Overall, the mortality of patients who were cocaine-positive was low. Our mortality rate (2.4%) was relatively low compared to that in another study[22] that focused on non-stratified trauma patients (6.5%). It should, however, be noted that, in that study, only 14.9% of their population had severe head injuries and their study did not isolate the effects of TBI from that of polytrauma.[22] Their study found that cocaine-positive patients had significantly higher odds of developing pneumonia as a complication, an outcome which we did not study.[22] Another study that investigated the effects of cocaine on TBI patients who underwent surgery during the first 24 hours after admission found that the rate of cardiac complications was lower for those who were cocaine-positive.[23] The results from the above studies suggest that cocaine may have significant clinical effects on certain, but not all, organ systems. The effects of cocaine on the neurological system are less clear and may be hard to discern with the low mortality rate in our patient population. Furthermore, the effects of cocaine may only be evident in patients with moderate head injury, a sub-population that we were unable to study. Patients with mild head injury will do well anyway, and patients with severe TBI may not benefit from any pharmacological intervention.
Hospital LOS was not different between the two groups, a finding which agrees with the results reported by Hadjizacharia et al.[22] The LOS of our patients was influenced by many other non-clinical factors such as insurance issues, lack of social support, and difficulties in discharge planning.
One of our secondary endpoints was the effect of cocaine-abuse on neurological outcome following TBI. We found a non-significant association between positive cocaine metabolite screens and a good GOS. In general, it is thought that patients with a history of substance abuse after TBI tend to have higher mortality rates, poorer neuropsychological outcome, and greater likelihood of repeat injuries and late deterioration.[24] They were reported to also score lower on the Full Scale IQ and Verbal IQ indices of the Wechsler Adult Intelligence Scale-Revised and the Verbal Memory, General Memory, Attention-Concentration, and Delayed Recall indices of the Wechsler Memory Scale-Revised.[25] A previous study by Jong et al. focused specifically on cocaine-users in TBI and found no group differences in Disability Rating Scale and the Functional Independence Measure and its subsets, but showed that the cocaine group scored lower on the Rey Auditory Verbal Learning Test.[26] We did not have close neuropsychological follow-up of our patients, and GOS is too coarse a measure to detect any difference. This measure also suffers from inter-observer variation ranging from 17% to 40% in clinical practice;[27,28] this finding warrants further investigation.
Many studies focusing on drugs of abuse often rely on conventional toxicology screening and are thus limited by its testing characteristics. The toxicology screen was not designed to quantify the amount of metabolites present or to identify current intoxication. The most common clinical assay for the detection of cocaine, specifically its metabolite benzoylecgonine, is the enzyme multiplied immunoassay technique.[29] False positives caused by other drugs are very rare in immunoassays for cocaine.[30] The sensitivity and specificity of cocaine at the cutoff of 300 ng/ml are 86% and 99%, respectively.[31] Although cocaine immunoassays generally have high sensitivities and specificities, it does not differentiate a positive result from chronic cocaine use from a positive result obtained from acute cocaine use.[32] In general, the hydroxy metabolites of cocaine, specifically benzoylecgonine, cannot be detected in the urine beyond 2 days, regardless of the route of administration.[31,33] It is possible that, if cocaine is ingested not long before the screening is performed, there is a chance that cocaine might not have been metabolized enough to be detected by the conventional assay, as the minimal benzoylecgonine concentration for detection must be at least 300 ng/ml.[34] However, since the average time to reach peak plasma concentration is between 0.04 hours (intravenous and smoked routes) and 0.62 hours (intranasal route), we thought that this scenario was unlikely.[35] It would be reasonable to assume on average that the cocaine-positive patients in our study population had abused cocaine within 48 hours of screening. For some patients in our study population who had drugs other than cocaine detected, false positives are possible due to the presence of other biochemically similar drugs. These include amphetamine, benzodiazepines, opiates, cannabinoids, tricyclic antidepressants, and phencyclidine.[34] However, these drugs were not the focus of the current study, and, after statistical consideration, their positive results were not found to significantly impact our results.
There were other limitations to this study. Our study was retrospective and was thus limited by the quality of data in the medical record. Retrospective power calculation indicated a study population of nearly 8,000 patients with the same characteristics as our study population would be need for the mortality difference to reach significance. Nonetheless, we believe that the current study is representative of the catchment area of a level-1 trauma center and provides a good depiction of cocaine-users and their outcomes from TBIs.
In conclusion, we found that the overall mortality rate from TBI was low. Mortality was non-significantly lower in the cocaine-positive group. A neuroprotective effect, if present, may not have been detected because of the overall low mortality rate, which also precluded meaningful subgroup analysis. LOS is affected by many factors unrelated to the injury and may not be a good surrogate for recovery. Similarly, GOS may be too coarse a measure to identify a benefit. This study did not find an association of positive cocaine levels with in-hospital mortality.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Langlois JA, Sattin RW. Traumatic brain injury in the United States: Research and programs of the Centers for Disease Control and Prevention (CDC) J Head Trauma Rehabil. 2005;20:187–8. doi: 10.1097/00001199-200505000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Shandro JR, Rivara FP, Wang J, Jurkovich GJ, Nathens AB, MacKenzie EJ. Alcohol and the risk of mortality in patients with traumatic brain injury. J Trauma. 2009;66:1584–90. doi: 10.1097/TA.0b013e318182af96. [DOI] [PubMed] [Google Scholar]
- 3.Salim A, Ley EJ, Cryer HG, Margulies DR, Ramicone E, Tillou A. Positive serum ethanol level and mortality in moderate to severe traumatic brain injury. Arch Surg. 2009;67:865–71. doi: 10.1001/archsurg.2009.158. [DOI] [PubMed] [Google Scholar]
- 4.Salim A, Teixeira P, Ley EJ, DuBose J, Inaba K, Margulies DR. Serum ethanol levels: Predictor of survival after severe traumatic brain injury. J Trauma. 2009;67:697–703. doi: 10.1097/TA.0b013e3181b5dcf2. [DOI] [PubMed] [Google Scholar]
- 5.Leshner AI. Rockville: National Institute on Drug Abuse; 1999. National Institute on Drug Abuse Research Report: Cocaine abuse and addiction. [Google Scholar]
- 6.Muir JK, Lyeth BG, Hamm RJ, Ellis EF. The effect of acute cocaine or lidocaine on behavioral function following fluid percussion brain injury in rats. J Neurotrauma. 1995;12:87–97. doi: 10.1089/neu.1995.12.87. [DOI] [PubMed] [Google Scholar]
- 7.Muir JK, Ellis EF. Acute cocaine administration alters posttraumatic blood pressure and cerebral blood flow in rats. Am J Physiol. 1995;268:H68–73. doi: 10.1152/ajpheart.1995.268.1.H68. [DOI] [PubMed] [Google Scholar]
- 8.McBeth BD, Stern SA, Wang X, Mertz M, Zink BJ. Effects of cocaine in an experimental model of traumatic brain injury. Acad Emerg Med. 2005;12:483–90. doi: 10.1197/j.aem.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 9.Jia J, Chen X, Zhu W, Luo Y, Hua Z, Xu Y. CART protects brain from damage through ERK activation in ischemic stroke. Neuropeptides. 2008;42:653–61. doi: 10.1016/j.npep.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Xu Y, Zhang W, Klaus J, Young J, Koerner I, Sheldahl LC, et al. Role of cocaine and amphetamine-regulated transcript in estradiol-mediated neuroprotection. Proc Natl Acad Sci U S A. 2006;103:14489–94. doi: 10.1073/pnas.0602932103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: A comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005;57:1173–82. doi: 10.1227/01.neu.0000186013.63046.6b. [DOI] [PubMed] [Google Scholar]
- 12.United States Bureau of the Census, County Population Estimates by Demographic Characteristics-Age, Sex, Race and Hispanic Origin; updated annually for states and counties 2010 Census of Population and Housing for places; updated every 10 years. [Last accessed on 2011 Dec 16, Last accesed on 2011 Dec 16]. http://factfinder2.census.gov . Available from: http://www.census.gov/popest/counties/asrh/
- 13.Karch DL, Barker L, Strine TW. Race/ethnicity, substance abuse, and mental illness among suicide victims in 13 US states: 2004 data from the National Violent Death Reporting System. Inj Prev. 2006;12:ii22–7. doi: 10.1136/ip.2006.013557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staines GL, Magura S, Foote J, Deluca A, Kosanke N. Polysubstance use among alcoholics. J Addict Dis. 2001;20:53–69. doi: 10.1300/j069v20n04_06. [DOI] [PubMed] [Google Scholar]
- 15.Leikin JB, Morris RW, Warren M, Erickson T. Trends in a decade of drug abuse presentation to an inner city ED. Am J Emerg Med. 2001;19:37–9. doi: 10.1053/ajem.2001.19986. [DOI] [PubMed] [Google Scholar]
- 16.Lucas CE. The impact of street drugs on trauma care. J Trauma. 2005;59(3 Suppl):S57–60. doi: 10.1097/01.ta.0000176044.21200.b0. [DOI] [PubMed] [Google Scholar]
- 17.United States Department of Health and Human Services; Substance Abuse and Mental Health Services Administration (SAMHSA): Drug Abuse Warning Network (DAWN) [Last accessed on 2011 Dec 16]. Available from: http://www.dawninfo.samhsa.gov/files/DAWN-ED-2005-Web.pdf .
- 18.Derlet R, Albertson T. Cocaine intoxication. West J Med. 1989;151:65. [PMC free article] [PubMed] [Google Scholar]
- 19.Shanti C, Lucas CE. Cocaine and the critical care challenge. Crit Care Med. 2003;31:1851–9. doi: 10.1097/01.CCM.0000063258.68159.71. [DOI] [PubMed] [Google Scholar]
- 20.Pitts WR, Lange RA, Cigarroa JE, Hillis LD. Cocaine-induced myocardial ischemia and infarction: Pathophysiology, recognition, and management. Prog Cardiovasc Dis. 1997;40:65–76. doi: 10.1016/s0033-0620(97)80023-0. [DOI] [PubMed] [Google Scholar]
- 21.Singhal PC, Rubin RB, Peters A, Santiago A, Neugarten J. Rhabdomyolysis and acute renal failure associated with cocaine abuse. J Toxicol Clin Toxicol. 1990;28:321–30. doi: 10.3109/15563659008994433. [DOI] [PubMed] [Google Scholar]
- 22.Hadjizacharia P, Green DJ, Plurad D, Chan LS, Law J, Inaba K, et al. Cocaine use in trauma: Effect on injuries and outcomes. J Trauma. 2009;66:491–4. doi: 10.1097/TA.0b013e3181622b9b. [DOI] [PubMed] [Google Scholar]
- 23.Ryb GE, Cooper C. Outcomes of cocaine-positive trauma patients undergoing surgery on the first day after admission. J Trauma. 2008;65:809–12. doi: 10.1097/TA.0b013e318187803f. [DOI] [PubMed] [Google Scholar]
- 24.Corrigan JD. Substance abuse as a mediating factor in outcome from traumatic brain injury. Arch Phys Med Rehabil. 1995;76:302–9. doi: 10.1016/s0003-9993(95)80654-7. [DOI] [PubMed] [Google Scholar]
- 25.Kelly MP, Johnson CT, Knoller N, Drubach DA, Winslow MM. Substance abuse, traumatic brain injury and neuropsychological outcome. Brain Inj. 1997;11:391–402. doi: 10.1080/026990597123386. [DOI] [PubMed] [Google Scholar]
- 26.Jong CN, Zafonte RD, Millis SR, Yavuzer G. The effect of cocaine on traumatic brain injury outcome: A preliminary evaluation. Brain Inj. 1999;13:1017–23. doi: 10.1080/026990599121025. [DOI] [PubMed] [Google Scholar]
- 27.Marmarou A. New York: Wiley; 2001. Head Trauma: Basic, preclinical, clinical direction. [Google Scholar]
- 28.Wilson JT, Slieker FJ, Legrand V, Murray G, Stocchetti N, Maas AI. Observer variation in the assessment of outcome in traumatic brain injury: Experience from a multicenter, international randomized clinical trial. Neurosurgery. 2007;61:123–8. doi: 10.1227/01.neu.0000279732.21145.9e. [DOI] [PubMed] [Google Scholar]
- 29.Baker JE, Jenkins AJ. Screening for cocaine metabolite fails to detect an intoxication. Am J Forensic Med Pathol. 2008;29:141–4. doi: 10.1097/PAF.0b013e318174e7ab. [DOI] [PubMed] [Google Scholar]
- 30.Eskridge KD, Guthrie SK. Clinical issues associated with urine testing of substances of abuse. Pharmacotherapy. 1997;17:497–510. [PubMed] [Google Scholar]
- 31.Cone EJ, Sampson-Cone AH, Darwin WD, Huestis MA, Oyler JM. Urine testing for cocaine abuse: Metabolic and excretion patterns following different routes of administration and methods for detection of false-negative results. J Anal Toxicol. 2003;27:386–401. doi: 10.1093/jat/27.7.386. [DOI] [PubMed] [Google Scholar]
- 32.Vearrier D, Curtis JA, Greenberg MI. Biological testing for drugs of abuse. EXS. 2010;100:489–517. doi: 10.1007/978-3-7643-8338-1_14. [DOI] [PubMed] [Google Scholar]
- 33.Huestis MA, Darwin WD, Shimomura E, Lalani SA, Trinidad DV, Jenkins AJ, et al. Cocaine and metabolites urinary excretion after controlled smoked administration. J Anal Toxicol. 2007;31:462–8. doi: 10.1093/jat/31.8.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moeller KE, Lee KC, Kissack JC. Urine drug screening: Practical guide for clinicians. Mayo Clin Proc. 2008;83:66–76. doi: 10.4065/83.1.66. [DOI] [PubMed] [Google Scholar]
- 35.Cone EJ. Pharmacokinetics and pharmacodynamics of cocaine. J Anal Toxicol. 1995;19:459–78. doi: 10.1093/jat/19.6.459. [DOI] [PubMed] [Google Scholar]


