Abstract
Introduction:
Allopurinol acts protectively in the ischemia reperfusion injury of the small intestine. The aim of this experimental study is to define the ideal time of administration of allopurinol, in experimental models of ischemia/reperfusion.
Materials and Methods:
We used 46 rabbits that were divided into four groups. Group A was the control. In Group B allopurinol was administered 10 min before ischemia and in Group C 2 min before reperfusion. In Group D, allopurinol was administered before ischemia and before reperfusion in half doses. Blood samples were collected at three different moments: (t1) prior to ischemia, (t2) prior to reperfusion, and (t3) after the end of the reperfusion, in order to determine superoxide dismutase (SOD) and neopterin values. Specimens of the intestine were obtained for histological analysis and determination of malondialdehyde (MDA).
Results:
In Group A, mucosal lesions were more extensive compared to those of the other three groups. Similarly, MDA, SOD and neopterin values were significantly higher. On the contrary, Group D showed the mildest mucosal lesions, as well as the lowest MDA, SOD and neopterin values. Finally, the lesions and the above mentioned values were bigger in Group C than in Group D.
Conclusions:
The administration of allopurinol attenuates the production and damage effect of free oxygen radicals during ischemia reperfusion of the small intestine, thus protecting the intestinal mucosa. Its maximum beneficial action is achieved when administered both before ischemia and before reperfusion of the small intestine.
Keywords: Allopurinol, allopurinol's timing, ischemia reperfusion injury, small intestine ischemia
INTRODUCTION
Ischemia is defined as the significant reduction of blood flow and the insufficiency of oxygen and nutrients’ provision to the various tissues and organs. Reperfusion is essential for the restoration of the energy needs of the ischemic cells and the removal of toxic products. Nevertheless, it has been proved that reperfusion of ischemic tissues induces damages that frequently exceed the original ischemic insult. This is called ischemia reperfusion injury (IRI).[1] Oxidative damage due to IRI is thought to play an important role.[2]
Allopurinol, an analogue of hypoxanthine, is a specific potent inhibitor and substrate for xanthine oxidase,[3] but there are several reports suggesting that the beneficial action of allopurinol in models of reperfusion injury may be related to its direct radical-scavenging effect.[4,5] However, much disagreement still exists about the mode of action of allopurinol in protecting against IRI as well as the time that allopurinol has to be administered.
The aim of this experimental study is to identify the action of allopurinol as well as to define the ideal time of its administration, in experimental models of ischemia/reperfusion (I/R).
MATERIALS AND METHODS
Forty-six male New Zealand adult rabbits (3 kg body weight) were anesthetized subcutaneously with ketamine/xylazine (35/5 mg per 1 kg body weight). The small intestine was exteriorized by midline laparotomy and the superior mesenteric artery (SMA) was ligated. After 50 min of ischemia, the SMA was allowed to be reperfused for another 50 min. Rabbits with intestinal I/R were divided into four experimental groups (A, B, C, D), each one with five rabbits. Rabbits of Group A did not receive any allopurinol (control group). In Group B allopurinol (30 mg/kg) was intravenously administered 10 min prior to ischemia, while in Group C 2 min prior to reperfusion. Finally, in Group D allopurinol was intravenously administered 10 min prior to ischemia (15 mg/kg) and 2 min prior to reperfusion (15 mg/kg).
Blood samples were collected via a jugular catheter at three different moments: (t1) 15 min prior to ischemia, (t2) 5 min prior to reperfusion, and (t3) after the end of the reperfusion. Specimens of the intestine were obtained for all animals after the end of the reperfusion phase for histological analysis and determination of malondialdehyde (MDA). Phasmatophotometric analysis was used for determining superoxide dismutase (SOD) and neopterin values.
Mucosal MDA concentration, which is one of the end products of fatty acid peroxidation, has been used in our study as a marker of necrotic lesions in the intestinal mucosa after ischemia and reperfusion. For the measurement of MDA levels, the tissue samples were homogenized in a Tris-buffer solution on ice and centrifuged at 4°C at 6000 rpm for 15 min. The supernatant was collected and used to measure free MDA with the use of an MDA-534 spectrophotometric assay kit.
In normal perfusion, superoxide dismutase (SOD) can be identified only inside the cell. The presence of SOD in the blood indicates the massive production of free radicals. In order to measure SOD blood levels, we used the spectrophotometric assay of RANDOX Company with the RANDSOD-SD kit after centrifuging 0.5 ml blood samples for 10 min at 3000 rpm.
Neopterin is produced and released by the activated polymorphonuclear cells. Detection of neopterin in the serum is another indicator of free oxygen radicals’ production. For the measurement of neopterin serum levels we used the ELISA method, along with six standard values of neopterin concentration (0, 1, 2, 4, 12, 37 and 111 ng/ml) by the World Health Organization (WHO) in order to make the reference curve.
For the histopathologic evaluation, tissue specimens were embedded in paraffin and cut into 5 μm sections. Slides were stained with hematoxylin–eosin and examined under a light microscope. During histological evaluation the following parameters were taken into consideration: Epithelial cell necrosis, lamina propria inflammation, mucosal hyperemia and intestinal villi length. The histological analysis was then performed by using the five-grade classification of Parks, Bulkley and Granger:[6] Grade 0 = Normal intestinal mucus, Grade 1 = Localized inflammatory lesions, Grade 2 = Necrotic lesions in the apex of the villus, Grade 3 = Necrotic lesions in the apex and the upper third of the body of the villus, and Grade 4 = Total damage and necrosis of the villus.
For the statistical analysis, all data were expressed as the mean standard error of the mean (SEM). One-way analysis of variance (ANOVA) and Student's t test with Bonferroni modification for small samples were used to evaluate differences of the means between groups. A P value of 0.05 was considered significant.
RESULTS
MDA
The results of the measurement of tissue MDA were 422.2 nmol/g protein (SD 7.22 nmol/g protein) for Group A, 208.4 nmol/g protein (SD 7.9 nmol/g protein) for Group B, 124.2 nmol/g protein (SD 5.4 nmol/g protein) for Group C and 24.0 nmol/g protein (SD 2.3 nmol/g protein) for Group D. Comparison of MDA values showed a statistically significant difference (P < 0.001) between groups A and B, A and C, as well as A and D. Comparison of the mean differences showed higher deviation between groups A and D than those between groups A and B, and groups A and C. Similarly, statistically significant differences (P < 0.001) were found after comparison between groups B and C, B and D, and also C and D.
SOD
The mean values as well as the standard deviations for SOD for the four groups at the three different moments are presented in Table 1. The ANOVA one-way inter-group analysis gave statistically significant increased values (P < 0.001) for groups A and B between t1 and t2, t1 and t3; and t2 and t3. For Group C statistically significant differences (P < 0.01) existed between t1 and t2; and t2 and t3. On the other hand the difference was not significant between t1 and t3. Finally, for Group D statistically significant difference occurred between t1 and t2 (P < 0.05), while for the other moments the differences were not significant. Alternatively, concerning the ANOVA one-way intra-group analysis, at t1 no statistically significant difference occurred. At t2 statistically significant differences (P < 0.05) were observed between groups A and B, A and D, B and C; and C and D. No differences were observed between groups A and C, and between B and D. Finally, at t3 statistically important differences were not observed only when comparing groups C and D. The comparisons of all other groups were statistically important (P < 0.05).
Table 1.
SOD values for each group at each moment (values are expressed in U/ml)

Neopterin
The mean values as well as the standard deviations for neopterin for the four groups at the three different moments are presented in Table 2. The ANOVA one-way inter-group analysis gave statistically significant increased values (P < 0.001) for groups A and B between t1 and t3 and between t2 and t3. For Group C statistically significant differences (P < 0.001) existed between t1 and t2; and t2 and t3. On the other hand the difference was not significant between t1 and t3. Finally, for Group D a statistically significant difference occurred between t1 and t2 (P < 0.05), while for the other moments the differences were not significant. Alternatively, concerning the ANOVA one-way intra-group analysis, at t1 no statistically significant difference occurred. At t2 statistically significant differences (P < 0.05) were observed between groups A and B, A and D, B and C; and C and D. No differences were observed between groups A and C; and between B and D. Finally, at t3 statistically important differences were not observed only when comparing groups C and D. The comparisons of all other groups were statistically important (P < 0.05).
Table 2.
Neopterin values for each group at each moment (values are expressed in ng/mg of protein)

Histological analysis
High-grade ischemic changes were observed for groups A and B (no allopurinol and allopurinol prior to ischemia, respectively). Moderate ischemic changes were observed for Group C, while mild changes were observed for Group D [Table 3]. There seems to be a correlation between the histological alterations and SOD or neopterin concentrations [Figures 1-4].
Table 3.
Histological alterations for each group classified after Parks

Figure 1.
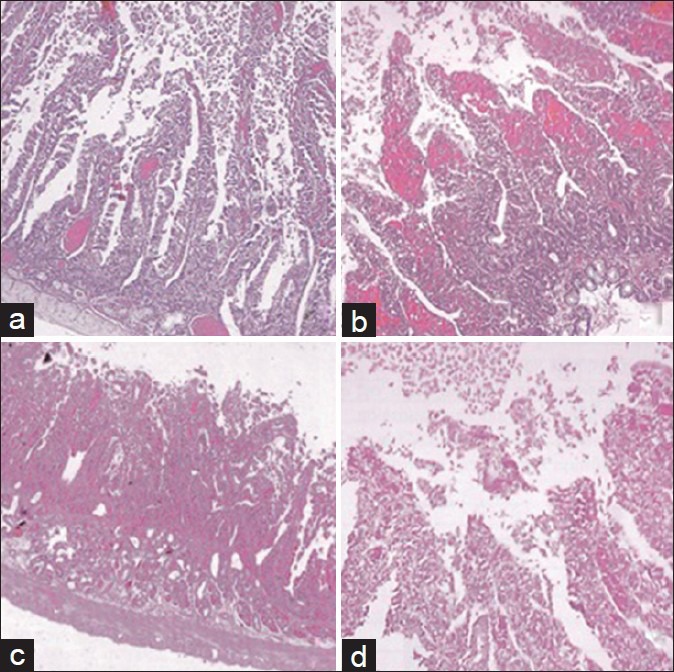
Histological alterations of intestinal mucosa: (a) Grade 1: Localized inflammatory lesions (H and E, ×25), (b) Grade 2: Necrotic lesions in the apex of the villus (H and E, ×25), (c) Grade 3: Necrotic lesions in the apex and the upper third of the body of the villus (H and E, ×10), (d) Grade 4: Total damage and necrosis of the villus (H and E, ×25)
Figure 4.
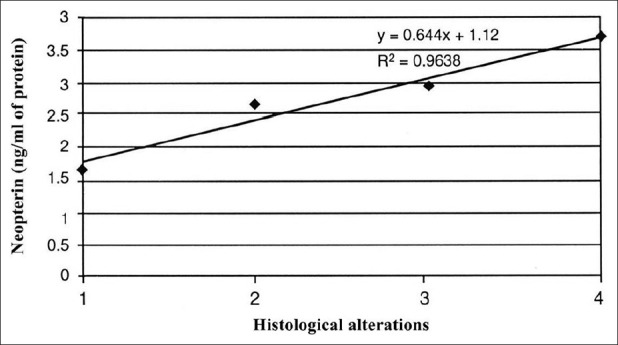
Correlation between neopterin and histological alterations
Figure 2.

Correlation between malondialdehyde and histological alterations
Figure 3.

Correlation between superoxide dismutase and histological alterations
DISCUSSION
Ischemia–reperfusion injury (IRI) of the intestine is an important factor associated with a high morbidity and mortality in both surgical and trauma patients.[7] It is of importance in situations such as the interruption of blood flow to the gut as in abdominal aortic aneurysm surgery, cardiopulmonary bypass, strangulated hernias, neonatal necrotizing enterocolitis, and intestinal transplantation.[8] IRI of the intestine also occurs in septic and hypovolemic shock.[9,10]
Whenever a tissue is subjected to ischemia, a chain reaction begins that may eventually lead to cellular dysfunction and necrosis. Although there has not been found any particular process in the ischemia-induced damage of the tissue, most studies show that the consumption of energy reserves, along with the accumulation of toxic metabolites contribute to cell death.[1] Paradoxically, restoration of blood flow to the ischemic tissue initiates a cascade of events that may lead to additional cell injury known as reperfusion injury.[11] Parks and Granger showed that the ischemia-induced damage to intestinal mucosa is significantly smaller than the one that follows reperfusion.[6] This phenomenon has been confirmed in our experimental study. The histopathologic examination of the small-intestine specimens in Group A showed more extensive necrotic lesions in the mucosa after 50 min of reperfusion than those resulting after 50 min of ischemia.
Among the internal organs, the intestine is probably the most sensitive to IRI.[12] The intestine is composed of labile cells that are easily injured by episodes of ischemia. Subsequent reperfusion of the intestine results in further damage to the mucosa. It has been shown that the enterocytes that are located at the tips of the villi are more sensitive to the effect of ischemia.[13] One explanation of this process is that when the restoration of blood flow to the ischemic tissue occurs, a local inflammatory response begins. This inflammatory state is enhanced by the production of various cytokines, the excessive expression of adhesion molecules, and the generation of bioreactive oxygen radicals.[14] These radicals promote increases in mitochondrial membrane permeability.[15] The damage is magnified by a variety of events, such as release of iron storage, damage of the microvasculature of I/R organs, complement activation and neutrophil infiltration at the site of injury.[16]
The intact gut mucosa serves as a barrier between the non-sterile lumen and the sterile peritoneal cavity and other tissues. The lesions induced by intestinal I/R are characterized by massive loss of epithelial cells from the enteric villi, especially in the apical part, epithelial necrosis with typical cell apoptosis, as well as fragmentation of the basal membrane.[17] These lesions represent the fragmentation of the mucosal enteric barrier, giving bacteria a way out of the intestinal lumen to the organism, and resulting, eventually, in the systematic inflammatory response syndrome (SIRS).[17,18]
The release of free oxygen radicals during ischemia and mainly reperfusion of the intestine has proved to be the main cause that damages the intestinal mucosal barrier and increases bacterial translocation.[6] Two possible disease mechanisms are suggested to explain the generation of oxygen radicals after ischemia and reperfusion. The enzyme xanthine oxidase (XO) is believed to be the primary source of these reactive oxygen metabolites.[6,12,19] The second potential source of oxygen radicals are activated polymorphonuclear leucocytes (neutrophils) which accumulate after reperfusion in the capillaries and venules of the tissue.[20]
As far as the first mechanism is concerned, the mucosa of the small intestine has the biggest concentration of xanthine dehydrogenase than any other tissue.[6] This enzyme is converted to XO which, during ischemia reperfusion, fills the intestinal mucosa with free oxygen radicals, leading to damage of the tissues’ biomolecules (nucleic acids, fatty acids, polymorphonuclear cells, enzymes and receptors).[12] Peroxidation of the membrane's lipids leads to lysis of the cytoplasmic membrane and cell death. The end products of fatty acid peroxidation are cytotoxic aldehydes. One of them, malondialdehyde (MDA), is minimally toxic and can be easily measured. This is the main reason why it can be used as the measure of peroxidation of fatty acids, and consequently, of the production of free oxygen radicals in the small intestine.[21] Mucosal MDA concentration has also been used in our study as a marker of necrotic lesions in the intestinal mucosa after ischemia and reperfusion. Analysis of the results has shown a positive correlation between MDA concentration and histopathologic lesions in the intestinal mucosa.
In case of IRI, the enzyme superoxide dismutase (SOD) catalyses the conversion of superoxide anion to oxygen and hydrogen superoxide, which releases the cytotoxic free oxygen radicals. In normal perfusion, SOD can be identified only inside the cell. The presence of SOD in the blood indicates the massive production of free radicals.[22] In our study, the grade and extend of pathologic lesions in the intestinal mucosa was proportional to the concentration of SOD in the animal's blood.
As previously mentioned, another potential source of oxygen radical generation during reperfusion of ischemic tissues is activated neutrophils.[20] It has been shown that during ischemia and after reperfusion neutrophils accumulate in the capillaries and venules of the damaged tissue. If activated, neutrophils secrete various enzymes such as myeloperoxidase, elastase, neutral and acid proteases, as well as prostaglandins and leucotrienes, which can induce severe tissue damage.[23] Neopterin seems to act as a mediator of the immune system and is produced and released by the activated polymorphonuclear cells. Detection of neopterin in the serum is another indicator of free oxygen radicals’ production. In our study, the measurement of neopterin concentration has shown that it is related to the severity of the lesions found in the intestinal mucosa.
Various studies have explored the possible benefit of the administration of substances that can reduce the production of free oxygen radicals during I/R of tissues. Allopurinol and its major metabolite oxypurinol are structural analogues of the purine bases hypoxanthine and xanthine, and they competitively bind to XO. Doing this they inhibit the conversion of hypoxanthine to xanthine and further to uric acid, and they prevent the generation of superoxide and other superoxide-derived oxygen radicals.[24] Moreover, allopurinol is also claimed to be an effective scavenger of free oxygen radicals.[25] The protective role of allopurinol against the post-ischemia injury, in tissues that lack XO, such as the intestinal mucosa, indicate that it inhibits the activation of neutrophils as well.[26] In conclusion, allopurinol has a triple mode of action in the IRI of the small intestine: (a) XO inhibition, (b) oxygen radical scavenging and (c) attenuation of neutrophils’ infiltration and activation. The action of allopurinol has been experimentally explored by Karwinski et al., in a normothermic liver and intestinal I/R rat model.[27] By evaluating various biochemical parameters, such as high-energy phosphoric molecules (ATP, ADP, AMP), hypoxanthine and xanthine before and during ischemia and reperfusion, they reached the conclusion that allopurinol administration before ischemia[27] and before reperfusion[28] contributes significantly in the preservation of the integrity of the liver parenchyma and intestinal mucosa. The beneficial effect of allopurinol was ascertained in our study. The pathologic lesions after ischemia and reperfusion were more severe in Group A, in which allopurinol was not administered, than in groups B, C and D. It is also worth mentioning that the protective effects of allopurinol are observed only when the drug is given intraperitoneally, as found in other ischemia/reperfusion models of rat small intestine.[29]
The extent of the mucosal damage in IRI seems to depend on the time of administration of allopurinol. However, much disagreement still exists about the ideal administration time. In most studies, allopurinol was administered before ischemia or before reperfusion. In other cases, allopurinol was proved to be effective only with chronic administration of at least three days before the ischemic interval.[30,31] In our study, allopurinol was administered before ischemia, before reperfusion, and both before ischemia and before reperfusion. The results were, then, evaluated based on the histopathologic mucosa lesions, as well as the variations of MDA, SOD and neopterin values. As a result, we found that the administration of allopurinol both before ischemia (half dose) and before reperfusion (half dose) gives the best possible outcome in IRI. In this way, the maximum inhibitory action of allopurinol in the production of oxygen radicals is achieved, along with its maximum beneficial role in the protection of the intestinal mucosa by IRI.
CONCLUSION
Small-intestine ischemia triggers the production of free oxygen radicals, which becomes massive during reperfusion, causing extensive damage to the intestinal mucosa. The administration of xanthine-oxidase inhibitor allopurinol decreases the production and harmful effect of free oxygen radicals. The best time for administration, as shown in our study, is before ischemia and before reperfusion. This model of administration maximizes the beneficial effect of allopurinol on the intestinal mucosa.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Zimmerman BJ, Granger DN. Reperfusion injury. Surg Clin North Am. 1992;72:65–83. doi: 10.1016/s0039-6109(16)45628-8. [DOI] [PubMed] [Google Scholar]
- 2.Loft S, Larsen PN, Rasmussen A, Fischer-Nielsen A, Bondesen S, Kirkegaard P, et al. Oxidative DNA damage after transplantation of the liver and small intestine in pigs. Transplantation. 1995;59:16–20. doi: 10.1097/00007890-199501150-00004. [DOI] [PubMed] [Google Scholar]
- 3.Fields M, Lewis CG, Lure MD. Allopurinol, an inhibitor of xanthine oxidase, reduces uric acid levels and modifies the signs associated with copper deficiency in rats fed fructose. Free Radical Biol Med. 1996;20:595–600. doi: 10.1016/0891-5849(95)02056-x. [DOI] [PubMed] [Google Scholar]
- 4.Ricardo SD, Bertram JF, Ryan GB. Podocyte architecture in puromycin aminonycleoside-treated rats administered tungsten or allopurinol. Exp Nephrol. 1995;3:270–9. [PubMed] [Google Scholar]
- 5.Kurose I, Granger DN. Evidence implicating xanthine oxidase and neutrophils in reperfusion-induced microvascular dysfunction. Ann NY Acad Sci. 1994;723:158–79. [PubMed] [Google Scholar]
- 6.Parks DA, Bulkley GB, Granger DN, Hamilton SR, McCord JM. Ischemic injury in the cat small intestine: Role of superoxide radicals. Gastroenterology. 1982;82:9–15. [PubMed] [Google Scholar]
- 7.Koike K, Moore FA, Moore EE, Read RA, Carl VS, Banerjee A. Gut ischemia mediates lung injury by a xanthine oxidase dependent neutrophil mechanism. J Surg Res. 1993;54:469–73. doi: 10.1006/jsre.1993.1072. [DOI] [PubMed] [Google Scholar]
- 8.Collard CD, Gelman S. Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology. 2001;94:1133–8. doi: 10.1097/00000542-200106000-00030. [DOI] [PubMed] [Google Scholar]
- 9.Moore EE, Moore FA, Franciose RJ, Kim FJ, Biffl WL, Banerjee A. The postischemic gut serves as a priming bed for circulating neutrophils that provoke multiple organ failure. J Trauma. 1994;37:881–7. doi: 10.1097/00005373-199412000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Swank GM, Deitch EA. Role of the gut in multiple organ failure: Bacterial translocation and permeability changes. World J Surg. 1996;20:411–7. doi: 10.1007/s002689900065. [DOI] [PubMed] [Google Scholar]
- 11.Stallion A, Kou TD, Miller KA, Dahms BB, Dudgeon DL, Levine AD. IL-10 is not rotective in intestinal ischemia reperfusion injury. J Surg Res. 2002;105:145–52. doi: 10.1006/jsre.2002.6398. [DOI] [PubMed] [Google Scholar]
- 12.Granger DN, Höllwarth ME, Parks DA. Ischemia-reperfusion injury: Role of oxygen-derived free radicals. Acta Physiol Scand Suppl. 1986;548:47–63. [PubMed] [Google Scholar]
- 13.Kong SE, Blennerhassett LR, Heel KA, McCauley RD, Hall JC. Ischaemia-reperfusion injury to the intestine. Aust NZ J Surg. 1998;68:554–61. doi: 10.1111/j.1445-2197.1998.tb02099.x. [DOI] [PubMed] [Google Scholar]
- 14.Köksoy C, Kuzu MA, Ergün H, Demirpençe E, Zülfikaroglu B. Intestinal ischemia–reperfusion impairs vasomotor functions of pulmonary vascular bed. Ann Surg. 2000;231:105–11. doi: 10.1097/00000658-200001000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qian T, Nieminen AL, Herman B, Lemasters JJ. Mitochondrial permeability transition in pH-dependent reperfusion injury to rat hepatocytes. Am J Physiol. 1997;273:c1783–92. doi: 10.1152/ajpcell.1997.273.6.C1783. [DOI] [PubMed] [Google Scholar]
- 16.Carden DL, Granger DN. Pathophysiology of ischaemia reperfusion injury. J Pathol. 2000;190:255–66. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Hanglund U. Gut ischaemia. Gut. 1994;1:73–6. doi: 10.1136/gut.35.1_suppl.s73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCord J. Oxygen-derived free radicals in postischemic tissue injury. N Eng J Med. 1985;312:159–63. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 19.Schoenberg MH, Fredholm BB, Haglund U, Jung H, Sellin D, Younes M, et al. Studies on the oxygen radical mechanism involved in the small intestinal reperfusion damage. Acta Physiol Scand. 1985;124:581–9. doi: 10.1111/j.1748-1716.1985.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 20.Schmid-Schonbein GW. Capillary plugging by granulocytes and the no-reflow phenomenon in the microcirculation. FASEB J. 1987;46:2397–401. [PubMed] [Google Scholar]
- 21.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 22.Repine JE, Cheronis JC, Rodell TC, Linas SL, Patt A. Pulmonary oxygen toxicity and ischemia-reperfusion injury. A mechanism in common involving xanthine oxidase and neutrophils. Am Rev Respir Dis. 1987;136:483–5. doi: 10.1164/ajrccm/136.2.483. [DOI] [PubMed] [Google Scholar]
- 23.Babior BM. Oxygen-dependant microbial killing by phagocytes. N Engl J Med. 1978;298:659–68. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- 24.Ahmadinejad M, Rex M, Sutton RH, Pollitt CC, Cribb B. The effects of allopurinol on the ultrastructure of ischaemic and reperfused large intestine of sheep. Aust Vet J. 1996;74:135–9. doi: 10.1111/j.1751-0813.1996.tb14814.x. [DOI] [PubMed] [Google Scholar]
- 25.Moorhouse PC, Grootveld M, Halliwell B, Quinlan JG, Gutteridge JM. Allopurinol and oxypurinol are hydroxyl radical scavengers. FEBS Lett. 1987;213:23–8. doi: 10.1016/0014-5793(87)81458-8. [DOI] [PubMed] [Google Scholar]
- 26.Rice-Evans CA, Diplock AT. Current status of antioxidant therapy. Free Radic Biol Med. 1993;1(15):77–96. doi: 10.1016/0891-5849(93)90127-g. [DOI] [PubMed] [Google Scholar]
- 27.Karwinski W, Søreide O. Allopurinol improves scavenging ability of the liver after ischemia/reperfusion injury. Liver. 1997;17:139–43. doi: 10.1111/j.1600-0676.1997.tb00796.x. [DOI] [PubMed] [Google Scholar]
- 28.Nordström G, Seeman T, Hasselgren PO. Beneficial effect of allopurinol in liver ischemia. Surgery. 1985;97:679–84. [PubMed] [Google Scholar]
- 29.Ciz M, Cizova H, Lojek A, Kubala L, Papezikova I. Ischemia/Reperfusion Injury of Rat Small Intestine: The Effect of Allopurinol Dosage. Transplant Proc. 2001;33:2871–3. doi: 10.1016/s0041-1345(01)02223-0. [DOI] [PubMed] [Google Scholar]
- 30.Svensson LG, Von Ritter CM, Groeneveld HT, Rickards ES, Hunter SJ, Robinson MF, et al. Cross-clamping of the thoracic aorta: Influence of aortic shunts, laminectomy, papaverine, calcium channel blocker, allopurinol and superoxide dismutase on spinal cord blood flow and apraplegia in boboon. Ann Surg. 1986;204:38–47. doi: 10.1097/00000658-198607000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qayumi AK, Jamieson WR, Godin DV, Lam S, Ko KM, Germann E, et al. Response to allopurinol pretreatment in a swine model of heart-lung transplantation. J Invest Surg. 1990;3:331–40. doi: 10.3109/08941939009140359. [DOI] [PubMed] [Google Scholar]


