Abstract
Context:
Acid immersion of victim's body is one of the methods employed to subvert identification of the victim, and hence of the perpetrator. Being hardest and chemically the most stable tissue in the body, teeth can be an important forensic investigative medium in both living and nonliving populations. Teeth are also good reservoirs of both cellular and mitochondrial DNA; however, the quality and quantity of DNA obtained varies according to the environment the tooth has been subjected to. DNA extraction from acid-treated teeth has seldom been reported.
Aims:
The objectives of the present study were to assess the morphological changes along with DNA recovery from acid-immersed teeth.
Materials and Methods:
Concentrated hydrochloric acid, nitric acid, and sulfuric acid were employed for tooth decalcification. DNA was extracted on an hourly basis using phenol–chloroform method. Quantification of extracted DNA was done using a spectrophotometer.
Results:
Results showed that hydrochloric acid had more destructive capacity compared to other acids.
Conclusion:
Sufficient quantity of DNA was obtainable till the first 2 hours of acid immersion and there was an inverse proportional relation between mean absorbance ratio and quantity of obtained DNA on an hourly basis.
Keywords: Acid-immersed teeth, DNA, forensic odontology, forensic sciences, gel electrophoresis, inorganic acids, nanodrop spectrophotometer
Introduction
DNA analysis is an important method employed for forensic identification with a higher degree of certainty as compared to the other traditional methods. Teeth are a good source of DNA as they are lodged in the jaws, well protected by the soft tissues of the oral cavity, and their composition ensures a better resistance against extreme conditions of temperature, pH, water, and aging, and in many cases serve as the only tool available for victim identification.[1]
The practise of destroying the human body by immersing in acid or some other caustic substance in order to avoid any personal identification is drawing a great deal of importance in forensic sciences. The idea of such crime is to destroy any physical evidence of cause of death, time interval of death to body identification, and victim identification. In such cases, whole body will be scorched and very few identification marks are left. DNA extraction, from soft tissues from bodies immersed in acid, has proven to be difficult, if not impossible.[2] In such cases, teeth by virtue of their physical and chemical properties can prove the criminal's proverbial “Achilles heel.” Few studies exist on the extraction of DNA from hard tissues of the body after immersion in acids. This study was performed to assess the morphological changes of teeth at regular intervals of time upon immersion in inorganic acids till their complete dissolution and extraction of DNA followed by quantification from these teeth.
Materials and Methods
Study group consisted of 85 freshly extracted human teeth. The teeth were extracted either for orthodontic or surgical reasons. Only non-carious teeth with visibly sound morphology were selected. The teeth were cleaned of any blood clot, bone, and soft tissue debris, followed by surface decontamination procedure by immersion in 5% sodium hypochlorite for 15 min, washed with 96% ethanol, and rinsed in distilled water. The teeth were then taken for decalcification. Sixty teeth were immersed in three different inorganic acids [concentrated hydrochloric acid (HCl), sulfuric acid (H2SO4), and nitric acid (HNO3)] to assess morphological changes and 25 teeth were used for DNA extraction after acid immersion.
Assessment of morphological changes and dissolution time in acid
The 60 extracted teeth were divided into three groups of 20 each, which were immersed in three different concentrated acids, HCl, HNO3, and H2SO4, placed in different containers. Morphological changes and softness of teeth were then observed and tabulated at time intervals starting at 0.5 h followed by 1 h and subsequently on an hourly basis until there was complete dissolution or precipitation of the tooth.
DNA extraction
Based on the results obtained, 25 teeth were then immersed in concentrated hydrochloric acid and DNA extraction procedure was performed at a regular time interval of 1 h each. Conventional DNA extraction using phenol chloroform method was employed. Sample was collected by conventional endodontic approach in 1.5 ml microcentrifuge tube (Eppendorf) in phosphate-buffered saline (PBS) buffer. For digestion, 500 μl of DNA extraction TE buffer is used which contains: 1 M NaCl, 1 M Tris-HCl, pH 8.0, 0.5 ethylenediaminetetraacetic acid (EDTA,), 10% sodium dodecyl sulfate (SDS) was added and washed by centrifuging the microcentrifuge tube in 10,000 rpm at room temperature for 10 min. The supernatant was discarded and the pellet was resuspended in 100 μl of DNA extraction TE buffer containing 40 μl of protienase K (Merck, microtubes were briefly vortexed and were incubated at 60-65°C for 2 hrs. Protienase K was inactivated by heating at 85°C for 15 min and centrifuging it in 10,000 rpm at room temperature for 10 min. Supernatant was collected carefully and phenol:chloroform:isoamyl alcohol (Merck) was added in a ratio of 25:24:1 followed by centrifugation in 10,000 rpm at room temperature for 10 min. Upper phase was carefully transferred into a new microcentrifuge tube. The obtained DNA was precipitated by adding 2.5 volume parts of 100% chilled ethanol (Merck) and centrifuging in 10,000 rpm at room temperature for 10 min. The supernatant was discarded and DNA pellet formation was seen at the end of microcentrifuge tube. This pellet was washed with 75% ethanol and air dried at room temperature, resuspended in 100 μl TE buffer, and quantification was done.
DNA quantification
DNA was electrophoretically separated on an agarose 2% gel, and visualized on a UV screen after staining with ethidium bromide. DNA quantification was done by using Nanodrop Spectrophotometer (ND-1000, Thermo Fischer Scientific, Wilmington DE, USA), along with determining absorbance ratio at 260/280 nm for evaluating the quality of obtained viable DNA.
Results
Morphological changes and dissolution time in acid
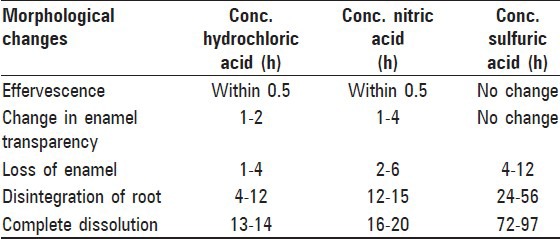
Morphological changes of teeth upon immersion in three organic acids were assessed at 0.5 h followed by 1 h and subsequently on an hourly basis until there was complete dissolution or precipitation of the tooth [Table 1].
Table 1.
Morphological changes observed in tooth samples on hourly basis in three different acids
Hydrochloric acid samples
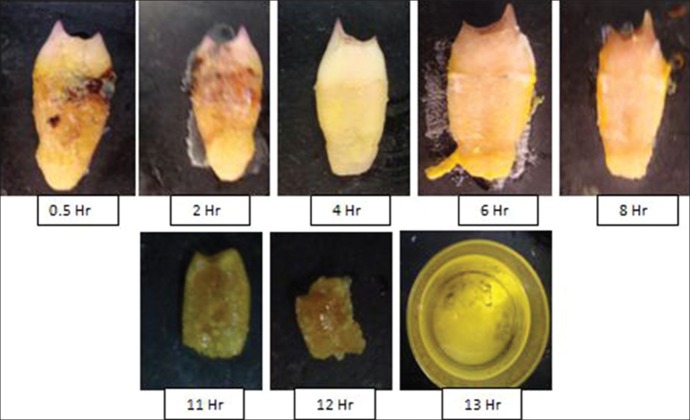
Effervescence in solution was noticed in 0.5 h, followed by mild softness with loss of enamel observed after 2 h. Disintegration of apical third, middle third, and cervical third took place in the next 4-10 h. Loss of structural details was noticed in 8 h and complete dissolution was seen in 13 h [Figure 1].
Figure 1.
Sequential morphological changes after immersion in concentrated hydrochloric acid
Nitric acid samples
The changes observed in 1 h were presence of effervescence in solution followed by yellow-colored deposits on tooth surface. Loss of enamel was observed in 2 h and softness of tooth increased gradually from 4 to 10 h. Disintegration of apical third, middle third, and cervical third was seen around 12, 14, and 15 h, respectively, and complete dissolution was seen in 18 h [Figure 2].
Figure 2.
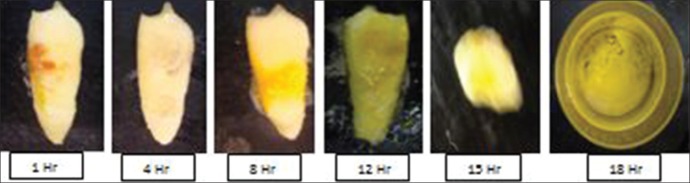
Sequential morphological changes after immersion in concentrated nitric acid
Sulfuric acid samples
No change was seen in the first 1 h, but increased color alteration was seen in 4 h. Mild structural alteration in root portion was observed in the next 10 h. White precipitate was observed around 18 h. Disintegration of apical third, middle third, and cervical third was seen around 24, 40, and 56 h, respectively, and complete dissolution was seen in 97 h [Figure 3].
Figure 3.
Sequential morphological changes after immersion in concentrated sulfuric acid
The average time taken for complete dissolution of tooth in hydrochloric acid was 13 h, in nitric acid was 18 h, and in sulfuric acid was 97 h. The P value between HCl and HNO3 (<0.0001), HCl and H2SO4 (<0.0001), and HNO3 and H2SO4 (<0.0001) was considered to be statistically significant.
Thus, based on the above observation of shorter time required for tooth dissolution, hydrochloric acid was considered for the second part of the study involving decalcification followed by DNA extraction.
DNA extraction and quantification
Based on the above findings, further experiment of DNA analysis for both quantity and quality was carried out on 25 tooth samples immersed in hydrochloric acid with five samples each in 1-5 h.
Results [Table 2] showed that the quantity of DNA extracted from 1-h sample varied from 167.9 to 210.3 ng/μl, with an average of 194.7 ng/μl and a mean absorbance ratio of 1.62. The quantity of DNA extracted showed a steady decline over the next 4 h. The 5-h samples showing a steep decrease in extracted DNA with a mean of 29.4 ng/μl. The difference between 4- and 5-h samples was extremely statistically significant (P < 0.0001).
Table 2.
Mean absorbance ratio and quantity of DNA in hourly samples
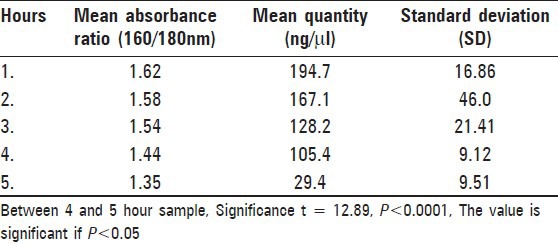
The mean absorbance ratio also showed similar change. The mean absorbance ratio also showed a decline from the 1-h sample onward and the ratio of the 5-h sample was 1.35.
Discussion
Practice of forensic sciences often involves person/victim identification. As methods in personnel identification have increased or improved, so have the attempts of perpetrators of crime in circumventing those methods. Destruction of evidence including dead bodies by the use of concentrated inorganic acids is one such attempt. This is not entirely a novel method and came out to the fore of public realm probably half a century ago. George John Haigh, “famously” nicknamed the acid bath murderer, had murdered five people in UK between 1944 and 1948 using concentrated sulfuric acid which took 2 days to destroy the body. The only remains left were gall stones and false teeth.[3]
In such cases of destruction of body by acids, soft tissues are destroyed faster than bone and tooth. Identification by morphological assessment of teeth can be hampered by dissolution of teeth or at least the surface traits of teeth. Use of advanced techniques like DNA analysis in such scenario has seldom been studied. Samples containing degraded DNA are an obstacle to the resolution of practical forensic cases that involve trace amounts of evidence and offer limited or no opportunities for meaningful analysis or conclusion.[4] Hence, this study was undertaken to assess the effect of acid dissolution on DNA extraction.
Commonly used acids in such crimes are hydrochloric acid, nitric acid, and sulfuric acid because of their ease of availability, cost effectiveness, and faster destruction. In the present study, these acids were employed and their ability to destruct teeth was compared.
Among the three acids, complete dissolution of teeth was faster in concentrated hydrochloric acid and was the slowest in sulfuric acid. This is in agreement with the previous studies done by Mazza et al.[2] and Kiran et al.[5] The time taken for dissolution varied in these studies as the acids were of varying concentrations. Morphological changes in the teeth can help the forensic investigator to deduce the acid used and the time elapsed since immersion of the body in the acid until it can be further corroborated with biochemical investigations. Recognizable morphological appearance of teeth persisted for 8 h in HCl, for 12 h in HNO3, and for 25 h in H2SO4. However, enamel changes were noticed very early leading to complete loss of enamel within the first 4 h. Any identification by morphological assessment of teeth is thus lost within the first 4 h. The time taken for dissolution of tooth with hydrochloric acid in vitro was 13 h. In actual crime settings, this may be prolonged as teeth are well protected within the jaws and the soft tissues (cheeks and tongue).
Pulp and the hard tissues (dentin and cementum) are the sources of DNA from teeth. The corrosive action of inorganic acids affects the macromolecules of the teeth, including DNA. The low quantity or impure DNA in forensic samples often results in partial or unsuccessful short tandem repeat (STRs) profiles. Studies have been done on fresh water and salt water drowning cases, where genetic material recovery was possible in only 37.5% of teeth, demonstrating that the water interfered directly in DNA preservation.[6] Mechanism by which water affects DNA recovery is not known; however, some components such as microbial growth and humidity are able to degrade genetic material[7] or inhibit the Taq DNA polymerase enzyme (humic acid). Effect of short- and long-term storage of teeth was studied wherein higher concentration of DNA was found in freshly extracted teeth than in long-term stored teeth (2, 5, and 10 years), which was found to be statistically significant.[4]
Our study showed marked reduction in the quantity of DNA obtained in correlation to the increase in duration of acid immersion. The first 4 h after immersion of teeth in acids provide sufficient quantity of DNA. In the fifth hour samples, the quantity of DNA extracted reduced dramatically. This was also true for the quality of DNA extracted. Absorbance ratio of the obtained DNA can be used as an indicator of the quality of DNA, with the range of 1.6-1.8 nm for pure DNA. Pure quality of DNA was obtained in 1-h sample and there was progressive decrease in terms of quality of the obtained DNA in later hours, which signifies the presence of impurities in the form of RNA or protein content into it. Mechanisms such as depurination have been suggested for DNA degradation by acids. Absorbance ratio, though not entirely precise in assessing the molecular changes involved in DNA degradation, is widely used as an indicator of purity of DNA. DNA typing post quantisation is more precise in assessing the extent of DNA damage.
The quality and quantity of DNA obtained from teeth depend on several external factors such as storage temperature, degree of humidity, time between death and examination, and on individual factors such as type of teeth, pathological conditions, dental treatments, and pulp weight.[8] An important factor that may lead to DNA non-amplification is the quality of the forensic biological sample. The insignificant amount of biological material to DNA extraction may result in absence of the target sequence in the fraction used for the reaction or the same can be degraded, not allowing DNA amplification by polymerase chain reaction (PCR).[9] Our study indicates that teeth could possibly show inconsistent results for DNA analysis after acid treatment for more than 5 h. The results, however, should be seen in perspective of different tooth types used in the study, age of teeth, and absence of jaw and soft tissue protection for the teeth.
Further investigations on the effects of inorganic acids on DNA, analysis of the total residue solution and not only pulpal tissue, study of different DNA extraction protocols, and DNA typing of acid-treated teeth need to be carried out to improve the efficacy of DNA analysis in such scenarios.
Acknowledgments
The authors thank Mr. Pramod Sarikar, Laboratory Technician, Biotechnology, Madhya Pradesh Council of Science and Technology, Bhopal, for his guidance and assistance in DNA analysis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Linjen I, Willems G. DNA research in forensic dentistry. Methods Find Exp Clin Pharmacol. 2001;23:1–7. doi: 10.1358/mf.2001.23.9.662139. [DOI] [PubMed] [Google Scholar]
- 2.Mazza A, Meralatig, Savio C, Fassina G. Observation of dental structure when placed in contact with acids. J Forensic Sci. 2005;50:406–10. [PubMed] [Google Scholar]
- 3.John George Haigh. From Wikipedia, the free encyclopedia. http://en.wikipedia.org/ wiki/John_ George_Haigh/
- 4.Leticia R, Luis JM, Ester M, Stella M. Study of short- and long- term storage of teeth and its influence on DNA. J Forensic Sci. 2009;54:1411–3. doi: 10.1111/j.1556-4029.2009.01159.x. [DOI] [PubMed] [Google Scholar]
- 5.Kiran J, Nidhi G, Mujib A, Vikram SA. Effect of acid on teeth and its relevance in postmortem identification. J Forensic Dent Sci. 2009;1:93–8. [Google Scholar]
- 6.Jamilly de OM, Amanda da CN, Evelyn KA, Mário HH, Regina Maria BC, Rogério Nogueira de O. Freshwater and salt-water influence in human identification by analysis of DNA: An epidemiologic and laboratory study. Braz J Oral Sci. 2009;8:71–5. [Google Scholar]
- 7.Bender K, Farfán MJ, Schneider PM. Preparation of degraded human DNA under controlled conditions. Forensic Sci Int. 2004;139:135–40. doi: 10.1016/j.forsciint.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 8.De Leo D, Turrina S, Marigo M. Effects of individual dental factors on genomic DNA analysis. Am J Forensic Med Pathol. 2000;21:411–5. doi: 10.1097/00000433-200012000-00023. [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee KK, Biswas R. Short tandem repeat (STRs) and sex specific Amelogenin analysis of blood samples from neurosurgical female transfused patients. J Clin Forensic Med. 2005;12:10–3. doi: 10.1016/j.jcfm.2004.06.003. [DOI] [PubMed] [Google Scholar]