Abstract
Social isolation (SI) rearing, a model of early life stress, results in profound behavioral alterations, including increased anxiety-like behavior, impaired sensorimotor gating and increased self-administration of addictive substances. These changes are accompanied by alterations in mesolimbic dopamine function, such as increased dopamine and metabolite tissue content, increased dopamine responses to cues and psychostimulants, and increased dopamine neuron burst firing. Using voltammetric techniques, we examined the effects of SI rearing on dopamine transporter activity, vesicular release and dopamine D2-type autoreceptor activity in the nucleus accumbens core. Long–Evans rats were housed in group (GH; 4/cage) or SI (1/cage) conditions from weaning into early adulthood [postnatal day (PD) 28–77]. After this initial housing period, rats were assessed on the elevated plus-maze for an anxiety-like phenotype, and then slice voltammetry experiments were performed. To study the enduring effects of SI rearing on anxiety-like behavior and dopamine terminal function, another cohort of similarly reared rats was isolated for an additional 4 months (until PD 174) and then tested. Our findings demonstrate that SI rearing results in lasting increases in anxiety-like behavior, dopamine release and dopamine transporter activity, but not D2 activity. Interestingly, GH-reared rats that were isolated as adults did not develop the anxiety-like behavior or dopamine changes seen in SI-reared rats. Together, our data suggest that early life stress results in an anxiety-like phenotype, with lasting increases in dopamine terminal function.
Keywords: fast-scan cyclic voltammetry, release, social, stress, uptake
Introduction
Early life stress (Heidbreder et al., 2000) increases vulnerability to a variety of affective mental health disorders, including anxiety, schizophrenia and substance abuse (for review, see Scheller-Gilkey et al., 2004; Nugent et al., 2011). Social isolation (SI) rearing is commonly used as an animal model of early life stress, where post-weanling animals are raised in single-housed conditions (Lapiz et al., 2003; Fone & Porkess, 2008). SI produces several behavioral outcomes similar to those observed in humans with early life stress. For example, SI rats display anxiety/depressive-like behaviors, including decreased time spent on open arms of the elevated plus-maze (EPM; Da Silva et al., 1996), decreased mobility during the Porsolt forced swim test and decreased social interaction (Kokare et al., 2010). Consistent with reports of increased rates of schizophrenia in people with early life stress (Scheller-Gilkey et al., 2004), SI rats demonstrate schizophrenia-like impairments, including reduced pre-pulse inhibition (PPI) of the acoustic startle reflex, indicating sensorimotor gating deficits, and disrupted latent inhibition in associative learning (Shao et al., 2009; Han et al., 2012). Lastly, similar to the increased risk for drug addiction observed in people with early life stress (Enoch, 2012), SI animals display increased self-administration of drugs of abuse (Schenk et al., 1987; Bozarth et al., 1989; Yajie et al., 2005; McCool & Chappell, 2009; but see Phillips et al., 1994).
Many SI rearing-induced behavioral alterations are long-lasting and may be permanent. For instance, resocialization of SI-reared rats fails to ameliorate anxiety-like behaviors and PPI deficits (Einon & Morgan, 1977; Wright et al., 1991; but see Kokare et al., 2010). Additionally, PPI impairments are not present in adult isolated group-reared rats (McCool & Chappell, 2009), suggesting that there is a critical period for developing impaired PPI (Liu et al., 2011; but see Varty et al., 1999).
SI-induced increases in drug self-administration, hyperlocomotor activity, impaired PPI, and latent inhibition may be related to changes in dopamine signaling, as each of these behavioral assays can be modulated by dopamine activity (Domeney & Feldon, 1998; Pierce & Kumaresan, 2006; Sora et al., 2009; Weiner & Arad, 2009). SI rearing produces robust alterations in mesolimbic dopamine systems (for review, see Lukkes et al., 2009), including increased ventral tegmental area dopamine neuron phasic bursting activity (Fabricius et al., 2010), nucleus accumbens (NAc) tissue dopamine levels (Miura et al., 2002), dopamine turnover (Hall et al., 1998b; Heidbreder et al., 2000), and dopamine responses to cocaine, amphetamine and footshock stress (Jones et al., 1992; Fulford & Marsden, 1998; Hall et al, 1998b; Howes et al., 2000). Together, these various neurobiological studies suggest that SI animals have overall increased NAc dopaminergic activity. However, while many different mechanisms may contribute to dopamine increases, the specific dopamine changes in SI animals remain unknown. NAc dopamine is primarily regulated by a balance of release and uptake, and knowing whether release or uptake is affected will help define the mechanisms involved for the observed increased dopamine activity and drug responsivity. For example, previous studies have shown that higher dopamine transporter levels are associated with increased sensitivity to stimulants (Chen & Reith, 2007), and that increased dopamine uptake rates are associated with augmented stimulant-induced hyperlocomotion (Salahpour et al., 2008). In short, understanding specific neurochemical changes that occur after early life stress may help us better understand the observed behavioral impairments. Therefore, to further explore the effects of SI rearing on NAc dopamine transmission, using voltammetric methods, we examined dopamine release and uptake kinetics, as well as dopamine D2-type autoreceptor activity, in brain slices of SI and group-housed (GH) rats.
Materials and methods
Animal housing
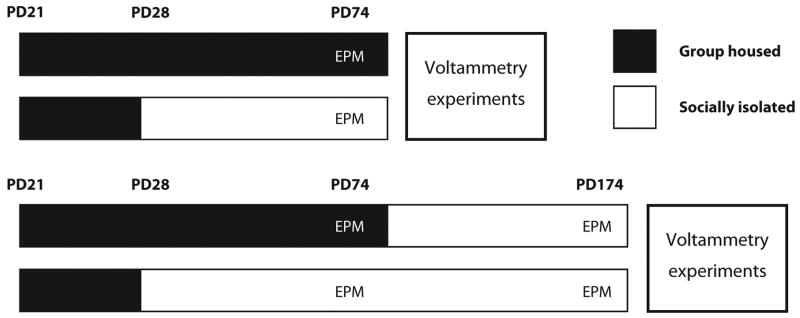
Previous studies have reported robust sex differences in measures related to the present studies, including greater NAc dopamine release, uptake and tissue levels in females than males (Walker et al., 2000; Duchesne et al., 2009), and increased anxiety-like behavior in males, although the behavioral differences are inconsistent (for review, see Simpson & Kelly, 2012). In the current study, male rats were chosen to avoid hormonal fluctuations in females and complex sex interactions, and to compare with previous SI studies examining dopamine parameters and anxiety-like behavior in males. Male Long–Evans rats (Harlan Laboratories, Indianapolis, IN, USA) were procured on postnatal day (PD) 21, and housed for 1 week under standard conditions (4 rats/cage, food/water ad libitum, 12/12 h light/dark). On PD 28, rats were randomly assigned to two groups: SI (1 rat/cage; 20 × 27-cm cages; Allentown, Allentown, NJ, USA); and GH (4 rats/cage; 33 × 60-cm cages; Ancare, Bellmore, NY, USA) for 6 weeks (Fig. 1). Experimental protocols adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Wake Forest University Institutional Animal Care and Use Committee.
Fig. 1.
Schematic model of housing paradigm and experimental timeline. Rats were obtained on postnatal day (PD) 21 and placed in group-housed (GH) conditions until PD 28. Two groups maintained GH, while another two groups were socially isolated (SI) for the remainder of the study. At PD 74 all rats were tested on the elevated plus-maze (EPM). A set of SI and GH rats were killed, and slice voltammetry experiments were performed to examine release, uptake and autoreceptor activity (PD = 84 ± 7). The second set of SI and GH rats were isolated for four additional months (PD = 77–174), and examined again on the EPM and in similar voltammetry experiments to the previous group (PD = 181 ± 7).
Young adult rats
Preceding the end of the initial housing period (PD 77), SI and GH rats were tested for anxiety-like behavior on the EPM (PD 74). Afterward, in vitro voltammetry experiments were performed. To reduce isolation stressor effects in GH-reared rats for voltammetry experiments, GH animals were killed in pairs, so that each cage of four rats was examined across 2 days (2 rats/day).
Adult rats
Adult GH- and SI-reared rats followed similar housing procedures to young adult rats until PD 77. At this time GH and SI rats were single-housed for about 4 months, and were involved in additional behavioral experiments that were unrelated to this study (Chappell et al., 2012). At the end of this period (PD 174), adult rats were reexamined in the EPM, and dopamine function was characterized using in vitro voltammetric experiments. Two adult animals were removed from the study due to technical difficulties during brain slice voltammetric experiments.
EPM
Anxiety-like behavior was assessed using a standard EPM (Med Associates, St Albans, VT, USA). The maze consisted of four radial arms (10.2 × 50.8 cm) elevated 72.4 cm above the floor. Two opposing arms were enclosed by black polypropylene walls (40.6 cm high), and the other two arms were open and illuminated by incandescent light (approximately 40 lux). Infrared sensors were positioned at the opening of each arm to score an animal's entry and/or exit from each arm, and data acquisition was performed using a personal computer interfaced with control units and programmed with MED-PC (Med Associates). Subjects were placed at the central junction, facing an open arm, and activity measures were recorded for 5 min. Decreases in open-arm time, and increases in closed arm time, were used as a measure of anxiety-like behavior, and closed-arm entries were used as a measure of locomotor activity.
In vitro slice preparation
Rats were killed, and their brains rapidly removed and prepared as previously described (John & Jones, 2007). Coronal slices (400 μm) of the striatum were maintained at 32 °C in oxygen-perfused (95% O2–5% CO2) artificial cerebrospinal fluid, which consisted of (in mm): NaCl, 126; NaHCO3, 25; D-glucose, 11; KCl, 2.5; CaCl2, 2.4; MgCl2, 1.2; NaH2PO4, 1.2; L-ascorbic acid, 0.4; pH adjusted to 7.4. A capillary glass-based carbon-fiber electrode was positioned approximately 75 μm below the surface of the slice in the NAc core. Dopamine release was evoked every 5 min by a 4-ms, one-pulse stimulation (monophasic, 350 μA)from a bipolar stimulating electrode (Plastics One, Roanoke, VA, USA) placed 100–200 μm from the carbon-fiber electrode.
Fast-scan cyclic voltammetry
Fast-scan cyclic voltammetry recordings were performed and analysed using recently developed in-house software (Demon Voltammetry and Analysis; Yorgason et al., 2011). The electrode potential was linearly scanned as a triangular waveform from −0.4 to 1.2 V and back to −0.4 V (Ag vs. AgCl) using a scan rate of 400 V/s. Cyclic voltammograms were recorded at the carbon-fiber electrode every 100 ms by means of a potentiostat (Dagan, Minneapolis, MN, USA). Once the stimulated dopamine response was stable for three successive collections, baseline measurements were taken and evaluated using a Michaelis–Menten-based kinetic model (Wightman et al., 1988; Yorgason et al., 2011). Michaelis–Menten-based changes in release and uptake were obtained by setting baseline apparent affinity (Km) values to 0.16 μm, and establishing a baseline maximal uptake rate (Vmax) and stimulated dopamine release ([DAp]) individually for each subject (Wightman et al., 1988; Wightman & Zimmerman, 1990; Wu et al., 2001). Extracellular concentrations of dopamine were assessed by comparing the current at the peak oxidation potential for dopamine with electrode calibrations of known concentrations of dopamine (1–3 μm).
For dopamine D2 autoreceptor studies, the selective D2-type receptor agonist (–)-quinpirole hydrochloride (Sigma-Aldrich, St Louis, MO, USA) was used to induce autoreceptor activation. Quinpirole-induced decreases in electrically stimulated dopamine release were compared with pre-drug values (each animal served as its own control) to obtain a percent change in stimulated dopamine release. The dose–response curve was then plotted as log concentration (M) of quinpirole vs. percent of control dopamine response, and the data were fit using a non-linear regression curve fit (sigmoidal dose–response curve) to determine EC50 concentrations.
Statistical analyses
Behavioral measures on the EPM were analysed using unpaired t-tests or the Mann–Whitney Rank Sum U-test when variance differences were detected. Anxiety-like behavior was assessed using open- and closed-arms times, and closed-arm entries were used as a measure of general locomotor activity (Holmes & Rodgers, 1998).
All dopamine release voltammetric assessments are reported as μm concentration, or as percentage of baseline. To examine whether dopamine uptake and release measures differ across housing conditions in young and adult rats, a two-way omnibus analysis of variance (anova) was performed with age (adult vs. young) and housing (SI vs. GH) as the between-subject variables, followed by Tukey's post hoc tests on statistically significant effects. Correlation analyses examining relationships between EPM behavior and dopamine function were performed using Pearson's correlation. For correlation analysis, all data were collapsed across housing and age groups. Autoreceptor sensitivity comparisons were performed using a three-way mixed-measures anova or analysis of covariance (ancova), with age and housing as the between-subject variables, drug concentration as the within-subject variable, and baseline stimulated release as the covariant. ancova was performed using the Delaney and Maxwell method (Delaney & Maxwell, 1981) to prevent the covariate from changing the main effect of the repeated measures. Statistical analyses were performed using spss 20 (IBM, New York, NY, USA) and Sigmaplot (Systat Software, San Jose, CA, USA).
Results
EPM
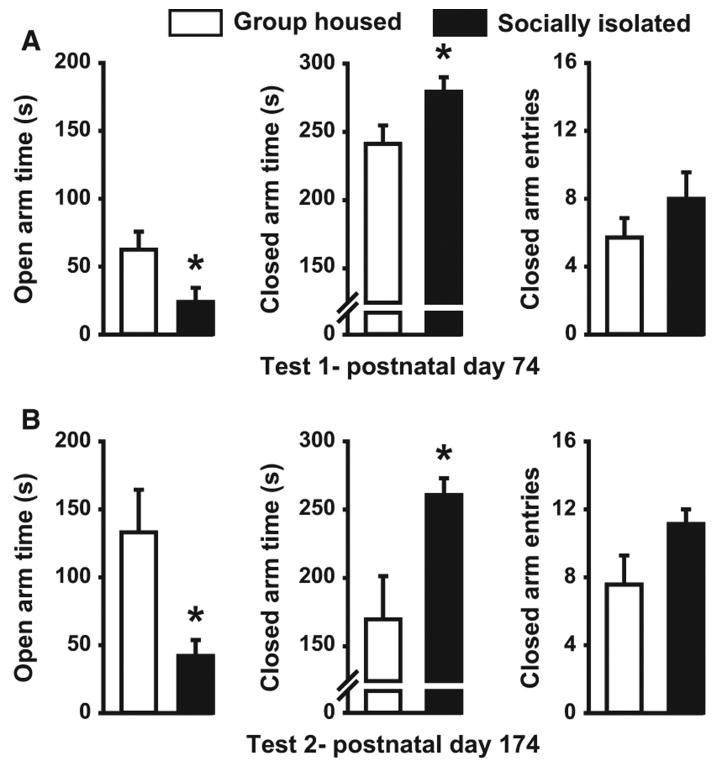
We first assessed the effect of juvenile SI on anxiety-like behavior using the EPM on PD 74 (Fig. 2). SI rats (n = 7) spent significantly less time in the open arms of the EPM than GH (n = 7) animals (t12 = 2.29, P < 0.04), and more time in the closed arms (t12 = 2.231, P < 0.05), suggesting increased anxiety-like behavior in these rats. In contrast, no differences in closed-arm entries, a measure of general locomotor activity, were noted (t12 = 1.18, P = 0.26). To determine the extent to which anxiety-like behavior endured into adulthood, we repeated the EPM assay 4 months later in the same animals (PD 174). Despite the fact that both cohorts had been housed singly during this 4-month period, SI-reared rats still displayed decreased open-arm time (U = 7, P < 0.03) and increased closed-arm time (U = 7, P < 0.04) relative to the GH-reared subjects. Again, no group differences in closed-arm entries were observed on this second test of anxiety-like behavior (t12 = 1.86, P = 0.09).
Fig. 2.
Effect of isolation rearing on anxiety-like behavior on the EPM. Bar graph illustrating the effect of adolescent rearing condition on mean (± SEM) open- and closed-arm time, and number of closed-arm entries in the EPM at two separate time points, PD 74 (A) and PD 174 (B) in group-housed (GH n = 7) and socially isolated (SI n = 7) rats. *P < 0.05.
Stimulated dopamine release and uptake
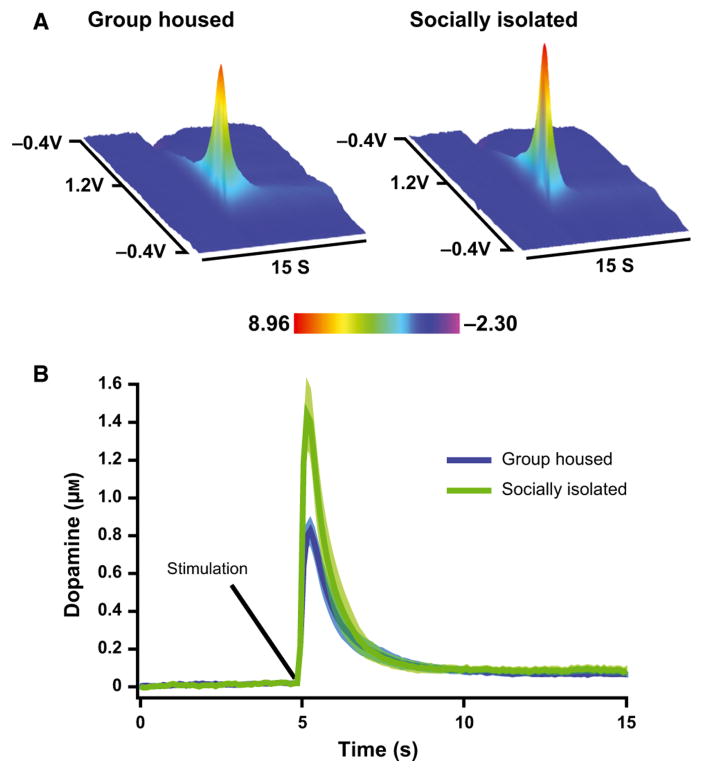
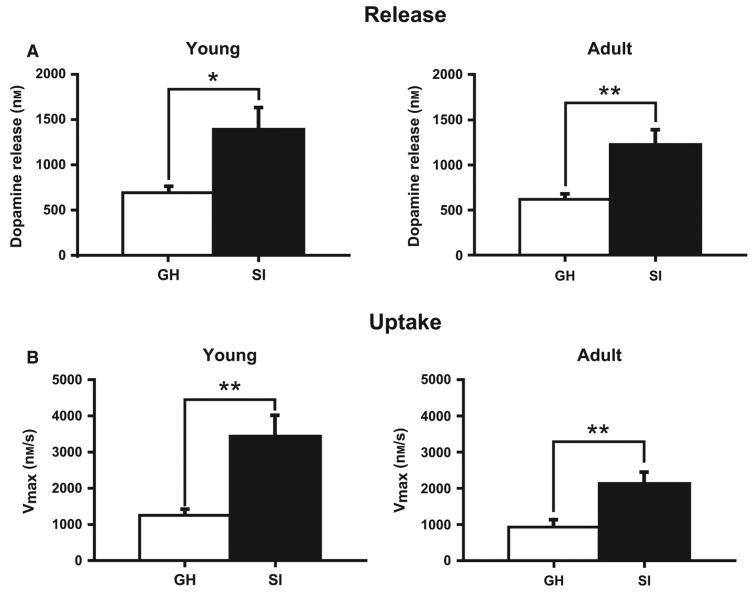
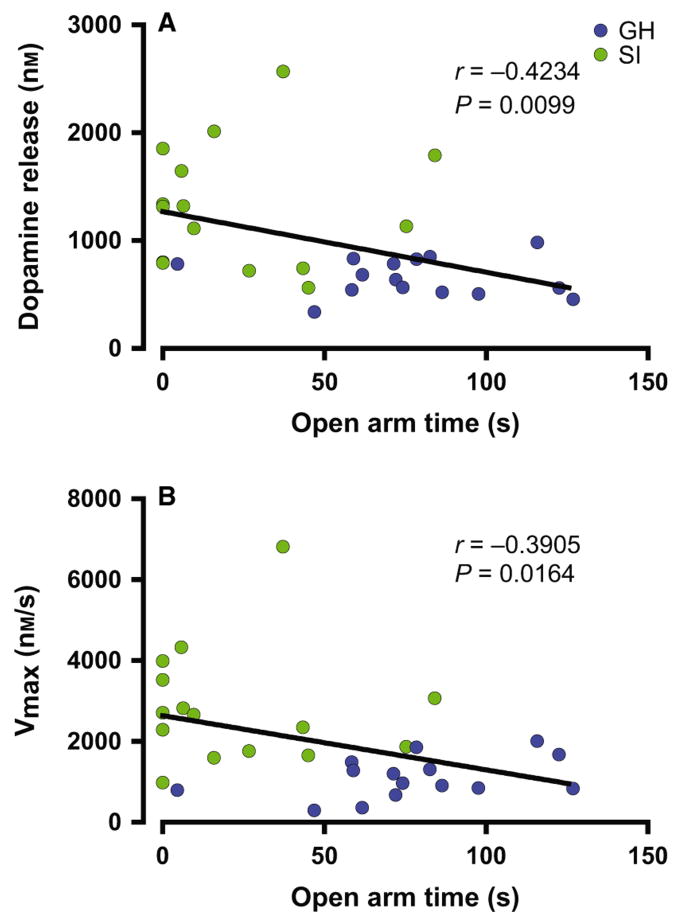
To examine the enduring effects of SI rearing on dopamine neurochemistry, electrically evoked dopamine release was measured in the NAc core of young (PD = 84 ± 7; GH n = 8; SI n = 8) and adult (PD = 181 ± 7; GH n = 7; SI n = 7) GH and SI animals (Fig. 3). The averaged dopamine traces display clear differences, with increased overall amplitude in dopamine signals from SI animals (t28 = 3.273, P = 0.0028). As shown in Fig. 4, two-way anova indicated that there was a significant effect of SI on μm DA release (Housing, F1,26 = 11.78, P < 0.002), but no effect of age (F1,26 = 0.370, P = 0.548) or housing × age interaction (F1,26 = 0.374, P = 0.546). For Vmax, anova indicated a significant effect of housing (F1,26 = 5.034, P < 0.001) and age (Vmax Age, F1,26 = 5.034, P = 0.034), but no housing × age interaction (F1,26 = 1.837, P = 0.187). Further analysis of these dopamine signals using Tukey's post hoc tests revealed that dopamine release and uptake Vmax are both increased in SI rats regardless of age (Fig. 4B; [DAp]: Young, q1,16 = 3.91, P < 0.05; Adult, q1,14 = 5.23, P < 0.01; Vmax: Young, q1,16 = 5.21, P < 0.01; Adult, q1,14 = 4.79, P < 0.01), suggesting that observed dopamine changes are long-lasting, occurring both in young and adult SI rats. To examine relationships between anxiety-like behavior and dopamine measures, open-arm time from previous EPM experiments in young (PD 84 ± 7) and adult (PD 181 ± 7) rats was compared with dopamine release and Vmax values using correlation analysis. As shown in Fig. 5, these analyses revealed that changes in EPM behavior are highly correlated with dopamine signaling ([DAp], r30 = −0.423, P = 0.0099; Vmax, r30 = −0.3905, P = 0.0164).
Fig. 3.
NAc core stimulated dopamine overflow in socially isolated (SI; young n = 8; adult n = 7) and group-housed (GH; young n = 8; adult n = 7) rats. (A) Averaged background subtracted voltammetric color plots for SI and GH rats, with applied potential (y-axis) plotted against time (x-axis) and current (z-axis). (B) Mean (± SEM) concentration vs. time traces for GH- and SI-reared rats. Solid line is mean data, whereas the lighter outline represents the SEM.
Fig. 4.
Effect of isolation rearing on stimulated dopamine release and uptake rate within the NAc. (A) Bar graph of mean (± SEM) stimulated dopamine release in socially isolated (SI) and group-housed (GH) rats at young (left; PD = 84 ± 7; GH n = 8; SI n = 8) and adult (right; PD = 181 ± 7; GH n = 7; SI n = 7) time points. (B) Bar graph of mean (± SEM) dopamine uptake rates (Vmax) in SI and GH rats at young (left; PD = 84 ± 7; GH n = 8; SI n = 8) and adult (right; PD = 181 ± 7; GH n = 7; SI n = 7) time points. *P < 0.05, **P < 0.01.
Fig. 5.
Relationship between dopamine function and anxiety-like behavior. NAc dopamine release (A) and rate of uptake (Vmax; B) from voltammetric studies were significantly correlated with open-arm time on the EPM in data combined from young (PD 74) and adult (PD 174) group-housed (GH; blue) and socially isolated (SI; green) reared rats n = 30. Release P = 0.0099; Uptake P = 0.0164.
Dopamine autoreceptor activity
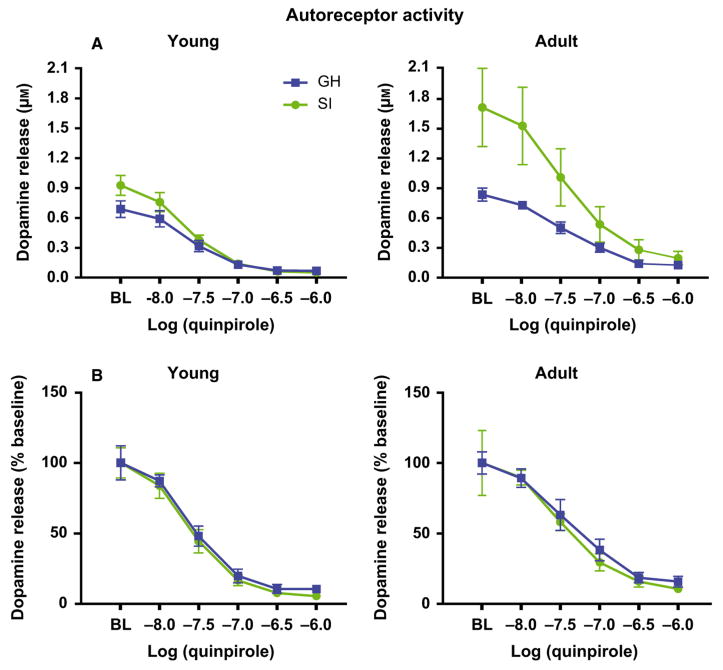
Previous studies indicate that NAc dopamine release and uptake are both regulated by dopamine D2-type autoreceptor activity (Benoit-Marand et al., 2001; Kramer et al., 2011). Therefore, in another set of voltammetry experiments in young (PD = 84 ± 7; GH n = 5; SI n = 5) and adult (PD = 181 ± 7; GH n = 4; SI n = 4) rats, we tested whether presynaptic autoreceptor activity was different in SI and GH animals. Increasing concentrations of the D2-type receptor agonist, quinpirole, were bath-applied to NAc core brain slices, and electrically stimulated dopamine release was measured (Fig. 6; Mateo et al., 2005; Maina & Mathews, 2010). Given that the variations observed in baseline dopamine release between SI and GH rats could potentially affect autoreceptor activity, we conducted a three-way ancova with housing and age as the between-subject variables, quinpirole concentration as the repeated-measures variable, and baseline levels of dopamine release as the covariate. As demonstrated previously (Mateo et al., 2005), quinpirole reduced stimulated dopamine release in a concentration-dependent manner (Fig. 6A; Quinpirole, F4,52 = 284.6, P < 0.001). Although ancova revealed that the baseline covariate significantly interacted with the effects of quinpirole (Baseline × Quinpirole, F4,52 = 33.034, P < 0.001), there were no effects of age or housing on quinpirole-induced reductions in dopamine release (Age, F1,13 = 2.00, P = 0.18; Housing, F1,13 = 0.436, P = 0.521; Age × Housing, F1,13 = 0.106, P = 0.749). A similar set of findings was observed when we conducted a three-way anova on the effects of quinpirole with dopamine release expressed as a percent baseline. In these analyses, quinpirole significantly reduced dopamine release, but again there were no significant effects of age or housing (Fig. 6B; quinpirole, F4,56 = 373.836, P < 0.001; Age, F1,14 = 4.16, P = 0.061; Housing, F1,14 = 0.031, P = 0.863; Age × Housing, F1,14 = 0.031, P = 0.863). When taken together, these results suggest that autoreceptor activity is the same regardless of housing condition or age.
Fig. 6.
NAc dopamine D2 autoreceptor activity in group-housed (GH) and socially isolated (SI) reared rats. Electrically stimulated dopamine overflow was measured while bath-applying increasing concentrations of the D2-type dopamine receptor agonist quinpirole (10 nm–1 μm). (A) Mean μm dopamine release peak amplitude and (B) percent baseline stimulated dopamine in young (left; PD = 84 ± 7; GH n = 5; SI n = 5) and adult (right; PD = 181 ± 7; GH n = 4; SI n = 4) SI and GH rats. Quinpirole significantly reduced dopamine signals to the same extent in GH- and SI-reared rats for both young and adult cohorts.
Discussion
In the current study, we tested the long-term effects of SI rearing on anxiety-like behavior and on NAc dopamine terminal function. Using the EPM assay in young and adult animals, we demonstrated that SI rearing decreases time spent on the open arms, consistent with greater anxiety-like behavior. Additionally, voltammetric studies in these animals provided evidence that juvenile SI rearing increases dopamine terminal release and reuptake rates. Additional groups of SI and GH animals were isolation housed at PD 77 and tested 4 months later (PD 181). At the later time point, GH rats had lower levels of anxiety-like behavior and decreased dopamine activity compared with their SI counterparts, despite several months of adult social isolation. This suggests that the protective effects of GH rearing on dopamine transmission and anxiety-like behavior are long lasting. Alternatively, SI housing may only have these effects when performed during a critical period of development. Future studies specifically examining critical periods' effects on dopamine and anxiety-like behavior may provide insight into this possibility.
As mentioned earlier, early life stress is associated with increased risk for developing disorders of anxiety, schizophrenia and substance abuse (for review, see Scheller-Gilkey et al., 2004; Nugent et al., 2011). However, it is important to note that long-lasting changes in dopamine function and behavior may occur after only mild stress in early life, which may fulfill a behaviorally protective role. For example, increases in dopamine function may serve to increase awareness to environmental cues related to natural rewards. Also, increased anxiety-like behavior may be appropriate for animals raised in more threatening environments, where it may be beneficial to spend more time in enclosed areas.
Enduring behavioral changes after SI rearing
As mentioned earlier, SI rearing has been shown to produce behavioral phenotypes that are similar to the sequelae observed in people who have experienced severe early life stress (Fone & Porkess, 2008; Lukkes et al., 2009). A myriad studies have shown increases in anxiety-like behavior after SI rearing using both the EPM and open-field assays (Wright et al., 1991; Da Silva et al., 1996;Hall et al., 1998a; McCool & Chappell, 2009), increased aggression (Wongwitdecha & Marsden, 1996), impulsivity/reactivity to novelty (Gentsch et al., 1988; Hall et al., 1997), impaired sensorimotor gating (Powell et al., 2003) and attenuated memory function (Hellemans et al., 2004; Bianchi et al., 2006). Some of these behavioral alterations are permanent, whereas others can be reversed through resocialization and enrichment. For example, SI rearing-induced locomotor hyperactivity, hypoalgesia and increased latency for emergence into a novel environment are all reversible through resocialization (Einon & Morgan, 1977; Gentsch et al., 1988; Cilia et al., 2001; Liu et al., 2011). In contrast, PPI impairment, increased novel object reactivity, decreased open-arm time on the EPM and increased anxiety-like vocalizations are not reversed through resocialization (Einon & Morgan, 1977; Wright et al., 1991; Bassi et al., 2007; Liu et al., 2011).
GH rearing also produces enduring behaviors. In addition to showing that resocialization does not reverse SI-induced reductions in EPM open-arm time, Wright et al. (1991) also examined the effects of 30-day isolation in adulthood on GH-reared animals in the EPM assay, and found that GH rearing blocks the anxiogenic-like effect of single housing on EPM behavior. We have confirmed these previous findings, demonstrating that GH rearing protects against isolation-induced impairments in EPM behavior. Additionally, we have extended these studies by showing that the protective effects of GH rearing on EPM behavior endure for at least 4 months of isolation, further demonstrating the long-term nature of these behavioral alterations. Because the present results are documented in older animals than in the previous study (PD 174 vs. PD 81), this confirms that SI-induced anxiety-like effects are very long lasting. In contrast to these results, Wallace et al. (2009) demonstrated that adult isolation increases anxiety-like behavior on the EPM. However, there are several important differences between these studies, including strain, age and length of isolation, and early life housing conditions, which may affect EPM behavior. Our present results suggest that GH rearing makes animals less susceptible to the stress associated with single-housing conditions.
Enduring dopamine terminal changes after SI rearing
We showed that SI rearing produces robust increases in electrically evoked dopamine release in the NAc. Similar to our findings, other experimental paradigms have also shown results consistent with greater dopamine release. For instance, electrophysiology and tissue content studies have revealed that SI rearing increases the number of ventral tegmental area dopamine neurons that burst fire, and levels of dopamine and its metabolites in the NAc (Fabricius et al., 2010; Han et al., 2011). Also, CDCrel-1, a presynaptic septin protein that inhibits dopamine release through interactions with the SNARE-protein, syntaxin (Beites et al., 1999), is downregulated in the striatum of SI rats, suggesting that NAc dopamine release should be increased after SI (Barr et al., 2004). Lastly, psychostimulants such as cocaine and amphetamine have a greater increasing effect on extracellular dopamine levels in SI animals (Hall et al., 1998b; Howes et al., 2000). Together, these previous studies have given a clear picture of increased dopamine activity after early life stress. In addition to validating these previous studies, by using voltammetric methods to study dopamine function in an early life stress model, we have demonstrated that increases in dopaminergic activity are not due to increased cell firing alone (Fabricius et al., 2010), but also due to increased dopamine terminal activity.
We have documented increased dopamine transporter function in isolates. These results may be surprising as extracellular dopamine levels are regulated by transporter activity (Salahpour et al., 2008; Owesson-White et al., 2012), and microdialysis studies measuring basal dopamine levels in SI and GH cohorts have shown either no difference (Wilkinson et al., 1994; Fulford & Marsden, 1998; Howes et al., 2000) or increases in SI rats (Hall et al., 1998b; Bortolato et al., 2011; Han et al., 2011). However, microdialysis techniques measuring extracellular dopamine levels are influenced by both release and uptake, and the increased release found in SI animals may partially offset the increased uptake rates. This balance between release and uptake may help explain the seemingly paradoxical lack of effect of isolation on basal dopamine levels reported previously.
Dopamine activity in the NAc is thought to play an important role in cue-related learning for positive and negative reinforcers (for review, see Wheeler & Carelli, 2009). Considering the aforementioned increases in dopamine activity in isolates, it is notable that SI rearing also produces increases in acquisition of Pavlovian conditioning. For example, SI animals show enhanced cue-conditioned responding for food and discriminative learning for a sucrose reward (Jones et al., 1990, 1992; Harmer & Phillips, 1998; Lapiz et al., 2003). Footshock-conditioned contextual cues also induce greater increases in dopamine levels in the NAc of SI rats compared with GH cohorts (Fulford & Marsden, 1998; Lapiz et al., 2003). The increased dopamine release and dopamine transporter activity observed in this study may be partly responsible for the increased sensitivity to Pavlovian conditioning, and greater dopamine responses to cues (Fulford & Marsden, 1998; Lapiz et al., 2003).
Relationship between anxiety and dopamine
Although the amygdala, hippocampus and prefrontal cortex are the focus of many anxiety studies in humans and rodents, the mesolimbic dopamine system also appears to play a critical role in anxiety-like behavior. The relationship between anxiety and dopamine activity is somewhat inconsistent, however, and may vary by brain region. For example, while intra-amygdala infusion of either D1-like or D2-like dopamine receptor agonists results in increased anxiety-like behaviors in rats (Bananej et al., 2012), systemic administration of dopamine agonists decreases anxiety-like behavior, an effect that can be blocked by D2-like receptor antagonists (Bartoszyk, 1998; Garcia et al., 2005). Also, dopamine is thought to have opposing roles in the central vs. the basolateral amygdala (Perez de la Mora et al., 2012). Although dopamine interactions with anxiety-like behavior are still being elucidated, it has been established that acute stressors increase dopamine in the nucleus accumbens (Abercrombie et al., 1989; Pei et al., 1990; Imperato et al., 1992), possibly through corticotrophin-releasing factor and/or glucocorticoid receptors (Angulo & McEwen, 1994; Piazza et al., 1996; Piazza & Le Moal, 1997; Lemos et al., 2012), and injections of dopamine receptor antagonists or temporary inactivation of VTA dopamine cell bodies prior to footshock stress prevent the later expression of anxiety-related behaviors (Corral-Frias et al., 2013). In addition, administration of l-DOPA, the dopamine precursor, in an animal model of Parkinson's disease increases anxiety-like behavior (Eskow Jaunarajs et al., 2012). Because of the growing body of literature suggesting that circuits involved in anxiety-like and reinforcing behaviors are highly interconnected (Pezze & Feldon, 2004; Price & Drevets, 2010), we examined the relationship between our accumbal dopamine function and anxiety measures. Our finding of significant negative correlations between EPM open-arm time and both dopamine release and uptake, regardless of housing condition, reinforces the notion that elevated accumbal dopamine signaling is associated with increased anxiety-like behavior.
SI rearing and dopamine D2-type autoreceptors
Dopamine terminal release and reuptake in the NAc are highly regulated processes, and both are modulated by feedback mechanisms such as dopamine D2-type autoreceptor activity (Joseph et al., 2002). D2 autoreceptors regulate dopamine activity by decreasing vesicular release through inhibition of dopamine synthesis and inhibition of exocytosis by hyperpolarization of dopamine cell bodies and terminals (Cubeddu & Hoffmann, 1982; Wolf & Roth, 1990; Mercuri et al., 1997). Additionally, presynaptic D2 receptor activation can affect uptake by increasing dopamine transporter surface expression as well as increasing the rate of uptake for each dopamine transporter by hyperpolarization of the presynaptic membrane (Meiergerd et al., 1993; Sonders et al., 1997; Dickinson et al., 1999; Bolan et al., 2007). Although SI has consistently produced increases in general dopamine activity, studies examining D2 levels have shown mixed results in the NAc core. For example, SI rearing has been reported to increase D2 receptor numbers (King et al., 2009) and the proportion of D2 receptors in a high-affinity state (Han et al., 2012), suggesting overall increases in striatal D2 activity. In contrast, several other studies have shown decreases (Hall et al., 1998b) or no changes in striatal D2 levels after SI rearing (Jones et al., 1992; Del Arco et al., 2004; Djouma et al., 2006). To further complicate the interpretation, these previous studies do not distinguish between pre- and post-synaptic D2 receptor expression, nor are the assays utilized capable of measuring changes in dopamine terminal function. To overcome these problems, in the current study, we examined presynaptic D2 receptor activity after SI rearing using voltammetry, which can be used to measure autoreceptor activity as agonist-induced (quinpirole) inhibition of evoked dopamine release (Joseph et al., 2002; Mateo et al., 2005; Maina & Mathews, 2010). Our results suggest that there are no SI rearing-induced differences in presynaptic D2 dopamine autoreceptor activity.
Conclusions
In the present study, we demonstrated that SI rearing results in long-lasting increases in anxiety-like behavior, and that these behavioral alterations are accompanied by changes in NAc dopamine kinetics, including increased evoked dopamine overflow and increased reuptake rates. These functional increases may produce greater dopamine responses in SI-reared rats, compared with GH animals, in the presence of a stimulus or challenge, such as during cue-conditioned learning or stimulant drug administration. Because SI rearing is a model of schizophrenia (Scheller-Gilkey et al., 2004; Nugent et al., 2011), the increased dopamine responses observed in SI rats may be related to the impaired latent inhibition and reduced PPI observed in humans with schizophrenia. Our finding that increased dopamine function in isolates is associated with increased anxiety-like behavior suggests that individuals who have experienced early life stress, and suffer from anxiety disorders, may also have disrupted dopamine system function. These differences in dopamine terminal activity are enduring, lasting well into adulthood. Additionally, there appears to be a protective effect of juvenile GH rearing conditions, such that adult isolation in GH animals does not engender an SI-like behavioral and neurochemical phenotype, further supporting the concept that in humans, stressors may be more deleterious in early life.
Acknowledgments
The authors would like to thank Dr Mark J. Ferris for his help in running statistical analyses, and Ann Chappell and Eugenia Carter for assistance in animal rearing and behavioral procedures. These studies were supported by F31 AA020439 (J.T.Y.), P01 AA017056 (J.L.W., S.R.J.), R01 AA017531 (J.L.W.) and U01 AA014091 (S.R.J.).
Abbreviations
- [DAp]
stimulated dopamine release
- EPM
elevated plus-maze
- GH
group housed
- Km
apparent affinity
- NAc
nucleus accumbens
- PD
postnatal day
- PPI
pre-pulse inhibition
- SI
socially isolated
- Vmax
maximal rate of uptake
References
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Angulo JA, McEwen BS. Molecular aspects of neuropeptide regulation and function in the corpus striatum and nucleus-accumbens. Brain Res Rev. 1994;19:1–28. doi: 10.1016/0165-0173(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Bananej M, Karimi-Sori A, Zarrindast MR, Ahmadi S. D1 and D2 dopaminergic systems in the rat basolateral amygdala are involved in anxiogenic-like effects induced by histamine. J Psychopharmacol. 2012;26:564–574. doi: 10.1177/0269881111405556. [DOI] [PubMed] [Google Scholar]
- Barr AM, Young CE, Sawada K, Trimble WS, Phillips AG, Honer WG. Abnormalities of presynaptic protein CDCrel-1 in striatum of rats reared in social isolation: relevance to neural connectivity in schizophrenia. Eur J Neurosci. 2004;20:303–307. doi: 10.1111/j.0953-816X.2004.03457.x. [DOI] [PubMed] [Google Scholar]
- Bartoszyk GD. Anxiolytic effects of dopamine receptor ligands: I. Involvement of dopamine autoreceptors. Life Sci. 1998;62:649–663. doi: 10.1016/s0024-3205(97)01160-0. [DOI] [PubMed] [Google Scholar]
- Bassi GS, Nobre MJ, Carvalho MC, Brandão ML. Substance P injected into the dorsal periaqueductal gray causes anxiogenic effects similar to the long-term isolation as assessed by ultrasound vocalizations measurements. Behav Brain Res. 2007;182:301–307. doi: 10.1016/j.bbr.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Beites CL, Xie H, Bowser R, Trimble WS. The septin CDCrel-1 binds syntaxin and inhibits exocytosis. Nat Neurosci. 1999;2:434–439. doi: 10.1038/8100. [DOI] [PubMed] [Google Scholar]
- Benoit-Marand M, Borrelli E, Gonon F. Inhibition of dopamine release via presynaptic D2 receptors: time course and functional characteristics in vivo. J Neurosci. 2001;21:9134–9141. doi: 10.1523/JNEUROSCI.21-23-09134.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M, Fone KFC, Azmi N, Heidbreder CA, Hagan JJ, Marsden CA. Isolation rearing induces recognition memory deficits accompanied by cytoskeletal alterations in rat hippocampus. Eur J Neurosci. 2006;24:2894–2902. doi: 10.1111/j.1460-9568.2006.05170.x. [DOI] [PubMed] [Google Scholar]
- Bolan EA, Kivell B, Jaligam V, Oz M, Jayanthi LD, Han Y, Sen N, Urizar E, Gomes I, Devi LA, Ramamoorthy S, Javitch JA, Zapata A, Shippenberg TS. D2 receptors regulate dopamine transporter function via an extracellular signal-regulated kinases 1 and 2-dependent and phosphoinositide 3 kinase-independent mechanism. Mol Pharmacol. 2007;71:1222–1232. doi: 10.1124/mol.106.027763. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Devoto P, Roncada P, Frau R, Flore G, Saba P, Pistritto G, Soggiu A, Pisanu S, Zappala A, Ristaldi MS, Tattoli M, Cuomo V, Marrosu F, Barbaccia ML. Isolation rearinginduced reduction of brain 5α-reductase expression: relevance to dopaminergic impairments. Neuropharmacology. 2011;60:1301–1308. doi: 10.1016/j.neuropharm.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Murray A, Wise RA. Influence of housing conditions on the acquisition of intravenous heroin and cocaine self-administration in rats. Pharmacol Biochem Behav. 1989;33:903–907. doi: 10.1016/0091-3057(89)90490-5. [DOI] [PubMed] [Google Scholar]
- Chappell AM, Carter E, McCool BA, Weiner JL. Adolescent rearing conditions influence the relationship between initial anxiety-like behavior and ethanol drinking in male Long Evans rats. Alcohol Clin Exp Res. 2012 doi: 10.1111/j.1530-0277.2012.01926.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Reith ME. Substrates and inhibitors display different sensitivity to expression level of the dopamine transporter in heterologously expressing cells. J Neurochem. 2007;101:377–388. doi: 10.1111/j.1471-4159.2006.04384.x. [DOI] [PubMed] [Google Scholar]
- Cilia J, Reavill C, Hagan JJ, Jones DN. Long-term evaluation of isolation-rearing induced prepulse inhibition deficits in rats. Psychopharmacology. 2001;156:327–337. doi: 10.1007/s002130100786. [DOI] [PubMed] [Google Scholar]
- Corral-Frias NS, Lahood RP, Edelman-Vogelsang KE, French ED, Fellous JM. Involvement of the ventral tegmental area in a rodent model of post-traumatic stress disorder. Neuropsychopharmacol. 2013;38:350–363. doi: 10.1038/npp.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubeddu LX, Hoffmann IS. Operational characteristics of the inhibitory feedback mechanism for regulation of dopamine release via presynaptic receptors. J Pharmacol Exp Ther. 1982;223:497–501. [PubMed] [Google Scholar]
- Da Silva NL, Ferreira VM, Carobrez ADP, Morato GS. Individual housing from rearing modifies the performance of young rats on the elevated plus-maze apparatus. Physiol Behav. 1996;60:1391–1396. doi: 10.1016/s0031-9384(96)00254-5. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Zhu S, Terasmaa A, Mohammed AH, Fuxe K. Hyperactivity to novelty induced by social isolation is not correlated with changes in D2 receptor function and binding in striatum. Psychopharmacology. 2004;171:148–155. doi: 10.1007/s00213-003-1578-8. [DOI] [PubMed] [Google Scholar]
- Delaney HD, Maxwell SE. On using analysis of covariance in repeated measures designs. Multivar Behav Res. 1981;16:105–123. doi: 10.1207/s15327906mbr1601_6. [DOI] [PubMed] [Google Scholar]
- Dickinson SD, Sabeti J, Larson GA, Giardina K, Rubinstein M, Kelly MA, Grandy DK, Low MJ, Gerhardt GA, Zahniser NR. Dopamine D2 receptor-deficient mice exhibit decreased dopamine transporter function but no changes in dopamine release in dorsal striatum. J Neurochem. 1999;72:148–156. doi: 10.1046/j.1471-4159.1999.0720148.x. [DOI] [PubMed] [Google Scholar]
- Djouma E, Card K, Lodge DJ, Lawrence AJ. The CRF1 receptor antagonist, antalarmin, reverses isolation-induced up-regulation of dopamine D2 receptors in the amygdala and nucleus accumbens of fawnhooded rats. Eur J Neurosci. 2006;23:3319–3327. doi: 10.1111/j.1460-9568.2006.04864.x. [DOI] [PubMed] [Google Scholar]
- Domeney A, Feldon J. The disruption of prepulse inhibition by social isolation in the Wistar rat: how robust is the effect? Pharmacol Biochem Behav. 1998;59:883–890. doi: 10.1016/s0091-3057(97)00534-0. [DOI] [PubMed] [Google Scholar]
- Duchesne A, Dufresne MM, Sullivan RM. Sex differences in corticolimbic dopamine and serotonin systems in the rat and the effect of postnatal handling. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:251–261. doi: 10.1016/j.pnpbp.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Einon DF, Morgan MJ. A critical period for social isolation in the rat. Dev Psychobiol. 1977;10:123–132. doi: 10.1002/dev.420100205. [DOI] [PubMed] [Google Scholar]
- Enoch MA. The influence of gene-environment interactions on the development of alcoholism and drug dependence. Curr Psychiatry Rep. 2012;14:150–158. doi: 10.1007/s11920-011-0252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskow Jaunarajs KL, George JA, Bishop C. L-DOPA-induced dysregulation of extrastriatal dopamine and serotonin and affective symptoms in a bilateral rat model of Parkinson's disease. Neuroscience. 2012;218:243–256. doi: 10.1016/j.neuroscience.2012.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricius K, Helboe L, Fink-Jensen A, Wörtwein G, Steiniger-Brach B, Sotty F. Increased dopaminergic activity in socially isolated rats: an electrophysiological study. Neurosci Lett. 2010;482:117–122. doi: 10.1016/j.neulet.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Fone KCF, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev. 2008;32:1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Fulford AJ, Marsden CA. Effect of isolation-rearing on conditioned dopamine release in vivo in the nucleus accumbens of the rat. J Neurochem. 1998;70:384–390. doi: 10.1046/j.1471-4159.1998.70010384.x. [DOI] [PubMed] [Google Scholar]
- Garcia AM, Martinez R, Brandão ML, Morato S. Effects of apomorphine on rat behavior in the elevated plus-maze. Physiol Behav. 2005;85:440–447. doi: 10.1016/j.physbeh.2005.04.027. [DOI] [PubMed] [Google Scholar]
- Gentsch C, Lichtsteiner M, Frischknecht HR, Feer H, Siegfried B. Isolation-induced locomotor hyperactivity and hypoalgesia in rats are prevented by handling and reversed by resocialization. Physiol Behav. 1988;43:13–16. doi: 10.1016/0031-9384(88)90091-1. [DOI] [PubMed] [Google Scholar]
- Hall FS, Humby T, Wilkinson LS, Robbins TW. The effects of isolation-rearing of rats on behavioural responses to food and environmental novelty. Physiol Behav. 1997;62:281–290. doi: 10.1016/s0031-9384(97)00115-7. [DOI] [PubMed] [Google Scholar]
- Hall FS, Huang S, Fong GW, Pert A, Linnoila M. Effects of isolation-rearing on locomotion, anxiety and responses to ethanol in Fawn Hooded and Wistar rats. Psychopharmacology. 1998a;139:203–209. doi: 10.1007/s002130050705. [DOI] [PubMed] [Google Scholar]
- Hall FS, Wilkinson LS, Humby T, Inglis W, Kendall DA, Marsden CA, Robbins TW. Isolation rearing in rats: pre- and postsynaptic changes in striatal dopaminergic systems. Pharmacol Biochem Behav. 1998b;59:859–872. doi: 10.1016/s0091-3057(97)00510-8. [DOI] [PubMed] [Google Scholar]
- Han X, Wang W, Shao F, Li N. Isolation rearing alters social behaviors and monoamine neurotransmission in the medial prefrontal cortex and nucleus accumbens of adult rats. Brain Res. 2011;1385:175–181. doi: 10.1016/j.brainres.2011.02.035. [DOI] [PubMed] [Google Scholar]
- Han X, Li N, Xue X, Shao F, Wang W. Early social isolation disrupts latent inhibition and increases dopamine D2 receptor expression in the medial prefrontal cortex and nucleus accumbens of adult rats. Brain Res. 2012;1447:38–43. doi: 10.1016/j.brainres.2012.01.058. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Phillips GD. Isolation rearing enhances the rate of acquisition of a discriminative approach task but does not affect the efficacy of a conditioned reward. Physiol Behav. 1998;63:177–184. doi: 10.1016/s0031-9384(97)00417-4. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G, Feldon J, Moran MC, Nelson P. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience. 2000;100:749–768. doi: 10.1016/s0306-4522(00)00336-5. [DOI] [PubMed] [Google Scholar]
- Hellemans KGC, Benge LC, Olmstead MC. Adolescent enrichment partially reverses the social isolation syndrome. Brain Res Dev Brain Res. 2004;150:103–115. doi: 10.1016/j.devbrainres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Holmes A, Rodgers RJ. Responses of Swiss-Webster mice to repeated plus-maze experience: further evidence for a qualitative shift in emotional state? Pharmacol Biochem Behav. 1998;60:473–488. doi: 10.1016/s0091-3057(98)00008-2. [DOI] [PubMed] [Google Scholar]
- Howes SR, Dalley JW, Morrison CH, Robbins TW, Everitt BJ. Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: relationship to extracellular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala-striatal FOS expression. Psychopharmacology. 2000;151:55–63. doi: 10.1007/s002130000451. [DOI] [PubMed] [Google Scholar]
- Imperato A, Angelucci L, Casolini P, Zocchi A, Puglisi-Allegra S. Repeated stressful experiences differently affect limbic dopamine release during and following stress. Brain Res. 1992;577:194–199. doi: 10.1016/0006-8993(92)90274-d. [DOI] [PubMed] [Google Scholar]
- John CE, Jones SR. Voltammetric characterization of the effect of monoamine uptake inhibitors and releasers on dopamine and serotonin uptake in mouse caudate-putamen and substantia nigra slices. Neuropharmacology. 2007;52:1596–1605. doi: 10.1016/j.neuropharm.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GH, Marsden CA, Robbins TW. Increased sensitivity to amphetamine and reward-related stimuli following social isolation in rats: possible disruption of dopamine-dependent mechanisms of the nucleus accumbens. Psychopharmacology. 1990;102:364–372. doi: 10.1007/BF02244105. [DOI] [PubMed] [Google Scholar]
- Jones GH, Hernandez TD, Kendall DA, Marsden CA, Robbins TW. Dopaminergic and serotonergic function following isolation rearing in rats: study of behavioural responses and postmortem and in vivo neurochemistry. Pharmacol Biochem Behav. 1992;43:17–35. doi: 10.1016/0091-3057(92)90635-s. [DOI] [PubMed] [Google Scholar]
- Joseph JD, Wang YM, Miles PR, Budygin EA, Picetti R, Gainetdinov RR, Caron MG, Wightman RM. Dopamine autoreceptor regulation of release and uptake in mouse brain slices in the absence of D (3) receptors. Neuroscience. 2002;112:39–49. doi: 10.1016/s0306-4522(02)00067-2. [DOI] [PubMed] [Google Scholar]
- King MV, Seeman P, Marsden CA, Fone KCF. Increased dopamine D2High receptors in rats reared in social isolation. Synapse. 2009;63:476–483. doi: 10.1002/syn.20624. [DOI] [PubMed] [Google Scholar]
- Kokare DM, Dandekar MP, Singru PS, Gupta GL, Subhedar NK. Involvement of [alpha]-MSH in the social isolation induced anxiety- and depression-like behaviors in rat. Neuropharmacology. 2010;58:1009–1018. doi: 10.1016/j.neuropharm.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Kramer PF, Christensen CH, Hazelwood LA, Dobi A, Bock R, Sibley DR, Mateo Y, Alvarez VA. Dopamine D2 receptor overexpression alters behavior and physiology in Drd2-EGFP mice. J Neurosci. 2011;31:126–132. doi: 10.1523/JNEUROSCI.4287-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapiz MD, Fulford A, Muchimapura S, Mason R, Parker T, Marsden CA. Influence of postweaning social isolation in the rat on brain development, conditioned behavior, and neurotransmission. Neurosci Behav Physiol. 2003;33:13–29. doi: 10.1023/a:1021171129766. [DOI] [PubMed] [Google Scholar]
- Lemos JC, Wanat MJ, Smith JS, Reyes BA, Hollon NG, Van Bockstaele EJ, Chavkin C, Phillips PE. Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature. 2012;490:402–406. doi: 10.1038/nature11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YP, Kao YC, Tung CS. Critical period exists in the effects of isolation rearing on sensorimotor gating function but not locomotor activity in rat. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1068–1073. doi: 10.1016/j.pnpbp.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Watt MJ, Lowry CA, Forster GL. Consequences of post-weaning social isolation on anxiety behavior and related neural circuits in rodents. Front Behav Neurosci. 2009;3:18. doi: 10.3389/neuro.08.018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina FK, Mathews TA. A functional fast scan cyclic voltammetry assay to characterize dopamine D2 and D3 autoreceptors in the mouse striatum. ACS Chem Neurosci. 2010;1:450–462. doi: 10.1021/cn100003u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo Y, Lack CM, Morgan D, Roberts DC, Jones SR. Reduced dopamine terminal function and insensitivity to cocaine following cocaine binge self-administration and deprivation. Neuropsychopharmacol. 2005;30:1455–1463. doi: 10.1038/sj.npp.1300687. [DOI] [PubMed] [Google Scholar]
- McCool BA, Chappell AM. Early social isolation in male Long-Evans rats alters both appetitive and consummatory behaviors expressed during operant ethanol self-administration. Alcohol Clin Exp Res. 2009;33:273–282. doi: 10.1111/j.1530-0277.2008.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiergerd SM, Patterson TA, Schenk JO. D2 receptors may modulate the function of the striatal transporter for dopamine: kinetic evidence from studies in vitro and in vivo. J Neurochem. 1993;61:764–767. doi: 10.1111/j.1471-4159.1993.tb02185.x. [DOI] [PubMed] [Google Scholar]
- Mercuri NB, Saiardi A, Bonci A, Picetti R, Calabresi P, Bernardi G, Borrelli E. Loss of autoreceptor function in dopaminergic neurons from dopamine D2 receptor deficient mice. Neuroscience. 1997;79:323–327. doi: 10.1016/s0306-4522(97)00135-8. [DOI] [PubMed] [Google Scholar]
- Miura H, Qiao H, Ohta T. Attenuating effects of the isolated rearing condition on increased brain serotonin and dopamine turnover elicited by novelty stress. Brain Res. 2002;926:10–17. doi: 10.1016/s0006-8993(01)03201-2. [DOI] [PubMed] [Google Scholar]
- Nugent NR, Tyrka AR, Carpenter LL, Price LH. Gene-environment interactions: early life stress and risk for depressive and anxiety disorders. Psychopharmacology. 2011;214:175–196. doi: 10.1007/s00213-010-2151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owesson-White CA, Roitman MF, Sombers LA, Belle AM, Keithley RB, Peele JL, Carelli RM, Wightman RM. Sources contributing to the average extracellular concentration of dopamine in the nucleus accumbens. J Neurochem. 2012;121:252–262. doi: 10.1111/j.1471-4159.2012.07677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Q, Zetterström T, Fillenz M. Tail pinch-induced changes in the turnover and release of dopamine and 5-hydroxytryptamine in different brain regions of the rat. Neuroscience. 1990;35:133–138. doi: 10.1016/0306-4522(90)90127-p. [DOI] [PubMed] [Google Scholar]
- Perez de la Mora M, Gallegos-Cari A, Crespo-Ramirez M, Marcellino D, Hannson AC, Fuxe K. Distribution of dopamine D(2)-like receptors in the rat amygdala and their role in the modulation of unconditioned fear and anxiety. Neuroscience. 2012;201:252–266. doi: 10.1016/j.neuroscience.2011.10.045. [DOI] [PubMed] [Google Scholar]
- Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol. 2004;74:301–320. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Phillips GD, Howes SR, Whitelaw RB, Wilkinson LS, Robbins TW, Everitt BJ. Isolation rearing enhances the locomotor response to cocaine and a novel environment, but impairs the intravenous self-administration of cocaine. Psychopharmacology. 1994;115:407–418. doi: 10.1007/BF02245084. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. Glucocorticoids as a biological substrate of reward: physiological pathophysiological implications. Brain Res Rev. 1997;25:359–372. doi: 10.1016/s0165-0173(97)00025-8. [DOI] [PubMed] [Google Scholar]
- Piazza PV, RougePont F, Deroche V, Maccari S, Simon H, Le Moal M. Glucocorticoids have state-dependent stimulant effects on the mesencephalic dopaminergic transmission. Proc Natl Acad Sci USA. 1996;93:8716–8720. doi: 10.1073/pnas.93.16.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Powell SB, Geyer MA, Preece MA, Pitcher LK, Reynolds GP, Swerdlow NR. Dopamine depletion of the nucleus accumbens reverses isolation-induced deficits in prepulse inhibition in rats. Neuroscience. 2003;119:233–240. doi: 10.1016/s0306-4522(03)00122-2. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacol. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahpour A, Ramsey AJ, Medvedev IO, Kile B, Sotnikova TD, Holmstrand E, Ghisi V, Nicholls PJ, Wong L, Murphy K, Sesack SR, Wightman RM, Gainetdinov RR, Caron MG. Increased amphetamine-induced hyperactivity and reward in mice overexpressing the dopamine transporter. Proc Natl Acad Sci USA. 2008;105:4405–4410. doi: 10.1073/pnas.0707646105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller-Gilkey G, Moynes K, Cooper I, Kant C, Miller AH. Early life stress and PTSD symptoms in patients with comorbid schizophrenia and substance abuse. Schizophr Res. 2004;69:167–174. doi: 10.1016/s0920-9964(03)00188-9. [DOI] [PubMed] [Google Scholar]
- Schenk S, Lacelle G, Gorman K, Amit Z. Cocaine self-administration in rats influenced by environmental conditions: implications for the etiology of drug abuse. Neurosci Lett. 1987;81:227–231. doi: 10.1016/0304-3940(87)91003-2. [DOI] [PubMed] [Google Scholar]
- Shao F, Jin J, Meng Q, Liu M, Xie X, Lin W, Wang W. Pubertal isolation alters latent inhibition and DA in nucleus accumbens of adult rats. Physiol Behav. 2009;98:251–257. doi: 10.1016/j.physbeh.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Simpson J, Kelly JP. An investigation of whether there are sex differences in certain behavioural and neurochemical parameters in the rat. Behav Brain Res. 2012;229:289–300. doi: 10.1016/j.bbr.2011.12.036. [DOI] [PubMed] [Google Scholar]
- Sonders MS, Zhu SJ, Zahniser NR, Kavanaugh MP, Amara SG. Multiple ionic conductances of the human dopamine transporter: the actions of dopamine and psychostimulants. J Neurosci. 1997;17:960–974. doi: 10.1523/JNEUROSCI.17-03-00960.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Li B, Fumushima S, Fukui A, Arime Y, Kasahara Y, Tomita H, Ikeda K. Monoamine transporter as a target molecule for psychostimulants. Int Rev Neurobiol. 2009;85:29–33. doi: 10.1016/S0074-7742(09)85003-4. [DOI] [PubMed] [Google Scholar]
- Varty GB, Braff DL, Geyer MA. Is there a critical developmental ‘window’ for isolation rearing-induced changes in prepulse inhibition of the acoustic startle response? Behav Brain Res. 1999;100:177–183. doi: 10.1016/s0166-4328(98)00129-6. [DOI] [PubMed] [Google Scholar]
- Walker QD, Rooney MB, Wightman RM, Kuhn CM. Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience. 2000;95:1061–1070. doi: 10.1016/s0306-4522(99)00500-x. [DOI] [PubMed] [Google Scholar]
- Wallace DL, Han MH, Graham DL, Green TA, Vialou V, Iñiguez SD, Cao JL, Kirk A, Chakravarty S, Kumar A, Krishnan V, Neve RL, Cooper DC, Bolaños CA, Barrot M, McClung CA, Nestler EJ. CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat Neurosci. 2009;12:200–209. doi: 10.1038/nn.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner I, Arad M. Using the pharmacology of latent inhibition to model domains of pathology in schizophrenia and their treatment. Behav Brain Res. 2009;204:369–386. doi: 10.1016/j.bbr.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Wheeler RA, Carelli RM. Dissecting motivational circuitry to understand substance abuse. Neuropharmacology. 2009;56(Suppl. 1):149–159. doi: 10.1016/j.neuropharm.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman RM, Zimmerman JB. Control of dopamine extracellular concentration in rat striatum by impulse flow and uptake. Brain Res Brain Res Rev. 1990;15:135–144. doi: 10.1016/0165-0173(90)90015-g. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Amatore C, Engstrom RC, Hale PD, Kristensen EW, Kuhr WG, May LJ. Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience. 1988;25:513–523. doi: 10.1016/0306-4522(88)90255-2. [DOI] [PubMed] [Google Scholar]
- Wilkinson LS, Killcross SS, Humby T, Hall FS, Geyer MA, Robbins TW. Social isolation in the rat produces developmentally specific deficits in prepulse inhibition of the acoustic startle response without disrupting latent inhibition. Neuropsychopharmacol. 1994;10:61–72. doi: 10.1038/npp.1994.8. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Roth RH. Autoreceptor regulation of dopamine synthesis. Ann NY Acad Sci. 1990;604:323–343. doi: 10.1111/j.1749-6632.1990.tb32003.x. [DOI] [PubMed] [Google Scholar]
- Wongwitdecha N, Marsden CA. Social isolation increases aggressive behaviour and alters the effects of diazepam in the rat social interaction test. Behav Brain Res. 1996;75:27–32. doi: 10.1016/0166-4328(96)00181-7. [DOI] [PubMed] [Google Scholar]
- Wright IK, Upton N, Marsden CA. Resocialisation of isolationreared rats does not alter their anxiogenic profile on the elevated X-maze model of anxiety. Physiol Behav. 1991;50:1129–1132. doi: 10.1016/0031-9384(91)90572-6. [DOI] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Kuhar MJ, Carroll FI, Garris PA. Preferential increases in nucleus accumbens dopamine after systemic cocaine administration are caused by unique characteristics of dopamine neurotransmission. J Neurosci. 2001;21:6338–6347. doi: 10.1523/JNEUROSCI.21-16-06338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajie D, Lin K, Baoming L, Lan M. Enhanced cocaine self-administration in adult rats with adolescent isolation experience. Pharmacol Biochem Behav. 2005;82:673–677. doi: 10.1016/j.pbb.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Yorgason JT, España RA, Jones SR. Demon Voltammetry and Analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods. 2011;202:158–164. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]