Abstract
Elephant endotheliotropic herpesviruses (EEHVs) can cause fatal hemorrhagic disease in juvenile Asian elephants (Elephas maximus); however, sporadic shedding of virus in trunk washes collected from healthy elephants also has been detected. Data regarding the relationship of viral loads in blood compared with trunk washes are lacking, and questions about whether elephants can undergo multiple infections with EEHVs have not been addressed previously. Real-time quantitative polymerase chain reaction was used to determine the kinetics of EEHV1 loads, and genotypic analysis was performed on EEHV1 DNA detected in various fluid samples obtained from five Asian elephants that survived detectable EEHV1 DNAemia on at least two separate occasions. In three elephants displaying clinical signs of illness, preclinical EEHV1 DNAemia was detectable, and peak whole-blood viral loads occurred 3–8 days after the onset of clinical signs. In two elephants with EEHV1 DNAemia that persisted for 7–21 days, no clinical signs of illness were observed. Detection of EEHV1 DNA in trunk washes peaked approximately 21 days after DNAemia, and viral genotypes detected during DNAemia matched those detected in subsequent trunk washes from the same elephant. In each of the five elephants, two distinct EEHV1 genotypes were identified in whole blood and trunk washes at different time points. In each case, these genotypes represented both an EEHV1A and an EEHV1B subtype. These data suggest that knowledge of viral loads could be useful for the management of elephants before or during clinical illness. Furthermore, sequential infection with both EEHV1 subtypes occurs in Asian elephants, suggesting that they do not elicit cross-protective sterilizing immunity. These data will be useful to individuals involved in the husbandry and clinical care of Asian elephants.
Keywords: Elephant, EEHV, herpesvirus, Proboscivirus, real-time polymerase chain reaction, viral load
INTRODUCTION
Elephant endotheliotropic herpesviruses (EEHVs) are members of the genus Proboscivirus in the subfamily Betaherpesvirinae.1,13 Herpesvirus infection of African elephants (Loxodonta africana) and Asian elephants (Elephas maximus) was first associated with mild disease after being identified in benign lung and skin nodules.7,9 However, beginning with the first report of the death of an Asian elephant calf associated with a herpesvirus infection,10 this group of viruses has emerged as one of the most significant causes of morbidity and mortality, especially in juvenile Asian elephant populations in human care in North America,4,12 Europe,2,3 and Asia.11,17 In addition, without improved fecundity rates or importation of animals from range countries, captive Asian elephants will be demographically extinct in North America within 40–50 yr.16 Overall, the impact of EEHV infection on Asian elephants is of critical concern, because E. maximus is listed as an endangered species by the International Union for Conservation of Nature.6
Despite these emerging data, the pathogenesis of EEHV1 infection remains poorly described. For example, data are lacking regarding the kinetics of viral loads from various fluid samples during the course of infection with EEHV1, and characterization of the viral genotypes associated with these infections has not been carried out. Viral load data are routinely used in the clinical management of human herpesvirus–associated diseases, such as Epstein-Barr virus–associated malignancies and lymphoproliferative disorders in immunocompromised patients5 and thus also might be clinically relevant for elephant disease. In addition, if EEHV1 DNAemia is detectable before the onset of clinical signs, treatment could be initiated before the onset of irreversible disease progression. Finally, knowledge of the specific viral genotypes involved in individual episodes of infections not only provides information about the number and variety of strains involved at each facility but also has importance for epidemiologic questions about origins and transmission, as well as having the potential to provide insight about viral pathogenesis.
The purpose of this study was to determine the kinetics of EEHV1 viral loads and to perform genotypic analysis on EEHV1 DNA detected in various fluid samples obtained from five Asian elephants with detectable EEHV1 DNAemia. An EEHV1-specific quantitative polymerase chain reaction (qPCR) assay was used to monitor absolute viral loads in DNA purified from whole blood, serum, urine, and trunk washes from three elephants that were clinically ill with EEHV-associated disease and from two elephants whose infection remained subclinical during the course of DNAemia. Viral loads were monitored during the course of treatment with herpesvirus-specific antiviral medications in four of the five elephants. Finally, viral gene subtyping analysis was performed on samples positive for EEHV1 DNA. These data will be useful to the husbandry and clinical care of Asian elephants.
MATERIALS AND METHODS
Animals
Institutional Animal Care and Use Committees at Baylor College of Medicine, Houston, Texas, USA, and the Saint Louis Zoo, St. Louis, Missouri, USA, reviewed and approved the research described in this study. Research oversight committees at the Houston Zoo, Inc., Houston, Texas, USA, and Feld Entertainment, Vienna, Virginia, USA, also reviewed and approved the research described in this study. All elephants in the study were Asian elephants; Table 1 summarizes the age, sex, and pregnancy status of the cohort. During the study period (February 2009–March 2010), elephants 1 (Jade) and 2 (Maliha) were located at the Saint Louis Zoo, where eight Asian elephants in total where housed. During the study period (January 2010–February 2011), elephant 3 (Barack) was part of a traveling group of 11 elephants owned by Feld Entertainment. Upon diagnosis of EEHV1 DNAemia, elephant 3 was relocated to the Center for Elephant Conservation in Polk City, Florida, USA, for treatment. During the course of the study (November 2009–July 2010), elephants 4 (Shanti) and 5 (Tucker) were located at the Houston Zoo, Inc., where five Asian elephants in total were housed but increased to six with the birth of a calf in May 2010. The institutions provided famciclovir, ganciclovir, or both used to treat the elephants in their collection.
Table 1.
Five captive-born Asian elephants that survived endotheliotropic herpesvirus-1 (EEHV1) infection: clinical signs, viral loads, and antiviral chemotherapy.
| Case no. |
Case date | Age (yr) |
Sex | Weighta (kg) |
Presenting clinical signs |
Whole-blood viral load at presentation (VGEs/mlb) |
Peak whole-blood viral load (VGEs/ml) |
Peak serum viral load (VGEs/ml) |
Antiviral chemotherapy (route) |
|---|---|---|---|---|---|---|---|---|---|
| 1c | Nov 2009 | 2.6 | Female | 500 | Lethargic | NAd | 635,992 | 292,000 | Ganciclovir (i.v., p.o.) |
| 2c | Nov 2009 | 3.3 | Female | 700 | Slightly lethargic | 10,000 | 11,019 | Undetectable | Famciclovir (p.o.) Ganciclovir (i.v., p.o.) |
| 3e | Jan 2010 | 1 | Male | 500 | Mildly lethargic | 30,000 | 300,017 | 995 | Famciclovir (p.o.) |
| 4f | Mar 2010 | 19.5 | Gravid female | 4,064 | None | NA | 2,437 | Undetectable | Famciclovir (p.r.g) |
| 5f | Jun 2010 | 5 | Male | 1,636 | None | NA | 407 | Undetectable | None |
Weight at time of diagnosis of EEHV1 DNAemia.
Viral genome equivalents per milliliter of whole blood as determined by EEHV1 quantitative polymerase chain reaction assay.
Located at the Saint Louis Zoo during surveillance period.
Not applicable.
Located at the Center for Elephant Conservation during surveillance period.
Located at the Houston Zoo during surveillance period.
By rectum.
Sample collection and processing
All biologic materials were collected as part of routine health surveillance programs or as part of the management of EEHV-associated disease. Whole blood and trunk washes were collected and stored, and DNA extraction was performed as described previously.15 Urine samples were collected in sterile 50-ml conical tubes and stored at 4°C until processed for DNA within 48 hr of collection. Serum was prepared from blood collected in Corvac serum separator tubes (Tyco Healthcare, Mansfield, Massachusetts 02048, USA). Tissue was obtained from the placenta of elephant 4 immediately upon delivery from the uterus, rinsed with sterile 0.9% NaCl solution, and stored at −80°C until processed for DNA. DNA was isolated from urine using a QIAamp Viral RNA Minikit (QIAGEN, Valencia, California 91355, USA) according to the manufacturer’s recommended protocol, and DNA from serum was isolated as described for whole blood. Starting volumes for urine and serum were 3 ml and 200 μl, respectively. DNA concentrations were quantitated according to the optical density260 as determined using a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, Massachusetts 02454, USA). All sample DNAs were stored at −20°C until analysis by real-time qPCR.
qPCR
Real-time qPCR for EEHV1 was performed as described previously.15 The intra-assay coefficient of variation of the quantification cycle (Cq) values for the EEHV1 standards ranged from 0.17 to 1.28%. The interassay variation ranged from 0.4 to 0.96%. The EEHV1 assay recognizes a region that is 100% conserved between known EEHV1A and EEHV1B major DNA binding protein genes.15 As an internal amplification control for purified DNA integrity and PCR conditions, a qPCR assay was developed that detects the Asian elephant tumor necrosis factor (TNF) α gene (GenBank accession AJ55708) by using the following oligonucleotides: hydrolysis probe, VICCCAGCTAGAGAAGGGT-MGB/NFQ; forward primer 5′-CCCATCTACCTGGGAGGAGTCT-3′; and reverse primer, 5′-TCGAGATAGTCAGGCAGATTGATC-3′. The TNFα real-time qPCR was used instead of the interferon-γ qPCR control used in our previous study15 because the latter assay was prone to sporadic failure, and the newer assay is more reliable. All primers used in qPCR were obtained from Integrated DNA Technologies (Coralville, Iowa 52241, USA). All hydrolysis probes were obtained from Applied Biosystems (Life Technologies, Inc., Carlsbad, California 92008, USA).
For trunk wash, urine, and serum DNA preparations, 5 μl of purified DNA was included in the qPCR assay. To assess purified DNA sample integrity and to control for PCR conditions, every DNA sample tested for EEHV1 also was tested for TNFα in a separate reaction. Due to the consistency in DNA samples prepared from blood samples, only DNA preparations that produced a TNFα reaction quantification cycle (Cq) at the expected cycle (22–23) were considered valid assays and were included in the data set. Due to the variability in DNA isolated from trunk wash and serum samples, any TNFα Cq was sufficient for inclusion into the data set. Urine samples rarely produced positive TNFα reactions, but all tested samples were included in the data set.
DNA sequence analysis from whole-blood and trunk-wash preparations
Samples used for DNA sequencing were selected on the basis of the results of the quantitation provided by EEHV1 qPCR assays. When possible, samples with the highest viral DNA loads were selected for DNA sequencing as described previously15 and first subjected to whole-genome amplification by use of a commercial kit (GenomiPhi HY DNA Amplification Kit, GE Healthcare Life Sciences, Piscataway, New Jersey 08854, USA). Two microliters of trunk-wash DNA preparation was used in a 20-μl total amplification reaction volume. This initial step, presumed to have the principal effect of diluting PCR inhibitors while maintaining approximately the same viral DNA concentration, proved to be essential for obtaining efficient PCR assay results with the trunk-wash samples. Two microliters of amplified DNA was then used for each of four first-round PCR reactions, by using outside primer pairs specific for four selected EEHV1 gene loci representing subsegments of the U38 (DNA POL), U71-U72 (gM), U76-U77 (HEL), and U51 (vGPCR1) genes. The strategy for amplification of each loci was with a standard seminested approach. If first-round PCR succeeded in generating sufficient DNA for sequencing, no further amplification was done. If insufficient DNA was detected after first-round PCR, it was followed by seminested second-round amplification, and if necessary a third round of seminested PCR from appropriate second-round products. For multiple-round PCR reactions, 2 μl of a 20-μl PCR reaction was used in subsequent PCR reactions. All PCR amplification reactions involved the following conditions: one cycle consisting of 95°C for 2 min; then 45 cycles consisting of 95°C for 40 sec, 50°C for 45 sec, and 73°C for 1 min; then 1 cycle consisting of 73°C for 5 min. All PCR product bands were purified as isolated agarose gel bands by use of a Qiagen II gel extraction kit (QIAGEN). DNA sequencing was carried out either by direct cycle sequencing on both strands with a cycle sequencing kit (ABI PRISM BigDye Terminator cycle sequencing kit 3.1, Applied Biosystems/Life Technologies, Inc.) and analyzed on a DNA sequencer (ABI-310, Applied Biosystems/Life Technologies, Inc.) or at Macrogen, Inc. (Rockville, Maryland 20850, USA). The PCR primers used to amplify the EEHV1 POL locus were as follows: first round (525 base pairs [bp]), LGH7445 (GATTTTGCGAG[C/T]CTGTA[C/T]CC) and LGH7446 (CACGCTGTCAGTATCTCCGTA); second-round A (507 bp), LGH7446 and LGH7447 (CCCAGTATCATTCAAGCATAC); second-round B (479 bp), LGH7445 and LGH7448 (CTGTCTACAGGGCA[A/G]TCAAC); and third round (461 bp), LGH7447 and LGH7448. The PCR primers used to amplify the EEHV1 U71-U72/gM locus were as follows: first round (679 bp), LGH6749 (CTATGGGATCCGAACTTTC) and LGH6751 (GAAGTCCTGCTAGCCCC[C/T]TAC; second-round A (663 bp), LGH6749 and LGH6752 (CTACATGCCCATGCAGATAGG); second-round B (664 bp), LGH6751 and LGH6750 (CTTTCTAAGGGGGTTTGTTGC); and third round (648 bp), LGH6750 and LGH6752. The PCR primers used to amplify the EEHV1 U76-U77/HEL locus were as follows: first round (986 bp), LGH6649 (CCAGTCAACGTATAGCTCTAG) and LGH3198 (CACACAGCGTTGTAGAACC); second-round A (950 bp), LGH6649 and LGH7885 (CTGCGTGTAACATGTGTTC); second-round B (576 bp), LGH6743 (GCAAGGT[A/G]GAACGTATCGTCG) and LGH3198; and third-round (540 bp), LGH6743 and LGH7885. The PCR primers used to amplify the EEHV1 vGPCR1 locus were as follows: first round (910 bp), LGH7506 (GATTGTGAACGCTGTATGTC) and LGH4963 (GACTTTCTTCGTCGTAGCCCTCGTCTT); second-round A (726 bp), LGH7506 and LGH5200 (CGTGATACGCTTCCAAACATACA); second-round B (747 bp), LGH7470 (GGTGGTACTGTATGATGTGC) and LGH4963; and third-round A (689 bp) LGH7506 and LGH5201 (GCCAGGGTAGATAGAATCAAGGGAA); third-round B (550 bp) LGH7471 (CGGTTACACCGTACCGTGGCTTGC) and LGH4963; and third-round C (563 bp) LGH7470 and LGH5200.
Viral gene subtype and phylogenetic analysis
Viral gene subtypes were characterized using the following criteria. All eight known EEHV1 strains that fall into the EEHV1B category are readily distinguishable from the 28 known strains in the EEHV1A category because of the presence of 32, 15, and 19 common characteristic nucleotide polymorphisms, respectively, across the three most conserved regions analyzed, representing the 658-bp U71/gM, 492-bp POL, and 953-bp HEL loci for a combined total of 2,103 bp. There are also usually (but not always) a small number of additional single, but characteristic, nucleotide polymorphic variations (or inserted or deleted codons) between different strains of each subtype at all three loci, thereby allowing them to be distinguished as A, A*, A1,or A$, for example, as used in this study. In addition, there is much greater variability within the 855-bp vGPCR1 locus at both the nucleotide and amino acid levels, thereby allowing all 36 currently known strains of EEHV1 to be classified into five major clusters of subtypes here that differ from each other, on average, by 15 characteristic scattered nucleotide polymorphisms, together with a single hypervariable nine amino-acid block with up to five amino changes, including deletions and insertions. Together with additional minor variant clusters, all vGPCR1 subtypes are defined as either A1, A2, B1, B2, B3, C, D1, D2, E, or E/A 18 (and unpublished data). All of these polymorphisms are highly stable and do not change between multiple samples collected from the same animal in blood or trunk wash.
Sequences obtained from trunk washes and blood were compared with previous sequencing data for the same four gene loci from selected reference samples8 from North American elephants with acute EEHV-associated hemorrhagic disease, including EEHV1A (North American Proboscivirus [NAP] 11 and NAP18), EEHV1B (NAP14), EEHV2 (NAP12), and EEHV6 (NAP35 and NAP42). Graphical DNA sequence alignments were prepared in Geneious PRO version 5.4.6 (Biomatters, Auckland 1010, New Zealand).
GenBank accession numbers
For consistency between publications, unique genotypes representing individual viruses found in each elephant during a single infectious episode have been designated as NAP case numbers (Table 2). For example, successive and distinct viruses detected in samples from elephant 1 are designated as NAP33 for the WB first episode (2/09) and as NAP39 for both the WB and TW for the subsequent second episode (11/09 and 12/09). Files for all sequence data regarding the gene subtyping analysis were deposited in GenBank under the following accession numbers: U71-U72/gM, JN633871–JN633882; U38/POL, JN633883–JN633895; U76-U77/HEL, JN633896–JN633912; and U51/vGPCR1, JN633913–JN633926.
Table 2.
Viral gene subtype analysis of five Asian elephants demonstrating sequential recurrent and/or recurrent infection of individual elephants with both the EEHV1A and EEHV1B subtypes over time.
| Polymerase chain reaction locus genotypea |
EEHV subtype |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Case no. | Date | Sample source | Commentsb | POL | HEL | vGPCR1 | gM | NAP no. | |
| 1 | Feb 2009 | WBc | Clinically ill | B | B | B2 | B | 1B | 33 |
| Nov 2009 | WB | Clinically ill | A* | A | A1 | A* | 1A | 39 | |
| Dec 2009 | TWd | Convalescent | A* | A | A1 | A* | 1A | 39 | |
| 2 | Feb 2009 | WB | Subclinical | B | B | B2 | B | 1B | 34 |
| Nov 2009 | WB | Clinically ill | A* | A | A1 | A* | 1A | 40 | |
| Dec 2009 | TW | Convalescent | nde | A | nd | nd | 1A | 40 | |
| 3 | Jan 2010 | WB | Clinically ill | A# | A# | E/A | A* | 1A | 41 |
| Jan 2010 | TW | Convalescent | A# | A# | E/A | A* | 1A | 41 | |
| Feb 11 | WB | Clinically ill | B | B* | B1 | B* | 1B | 49 | |
| 4 | Sep 2009 | TW | Subclinical | A$ | A* | D2 | A* | 1A | 37f |
| Mar 2010 | WB | Subclinical | B | B | B3 | B | 1B | 45g | |
| May 2010 | TW | Subclinical | B | B | B3 | B | 1B | 45 | |
| 5 | Nov 2009 | TW | Subclinical | B | B | B3 | B | 1B | 44 |
| May 2010 | WB | Subclinical | A$ | A* | D2(S)h | nd | 1A | 48 | |
| Jul 2010 | TW | Subclinical | A$ | A* | D2(S) | nd | 1A | 48 | |
Letters denoting viral gene subtypes at each locus have been assigned to denote the characteristic nucleotide sequence cluster pattern of each DNA sample. For example, in the case of POL, A, A*, A#, and A$ represent distinct genotypes and indicate that the four elephant endotheliotropic herpesvirus (EEHV) 1 strains each have unique distinguishable polymorphisms in the POL gene sequence.
Comments reflect the clinical condition of the elephant at the time the viral DNA was detected in the sample.
Whole blood.
Trunk wash.
Not determined.
Identical EEHV1A sequence to North American Proboscivirus (NAP) 32 and NAP36.17
Identical EEHV1B sequence to NAP38.15
Short 554-base pair (bp) version of vGPCR1 D2 gene subtype compared with the standard intact 910-bp version of the vGPCR1 D2 gene subtype.
RESULTS
Kinetics of EEHV1 viral loads in fluid samples from three elephants clinically ill secondary to EEHV1 infection
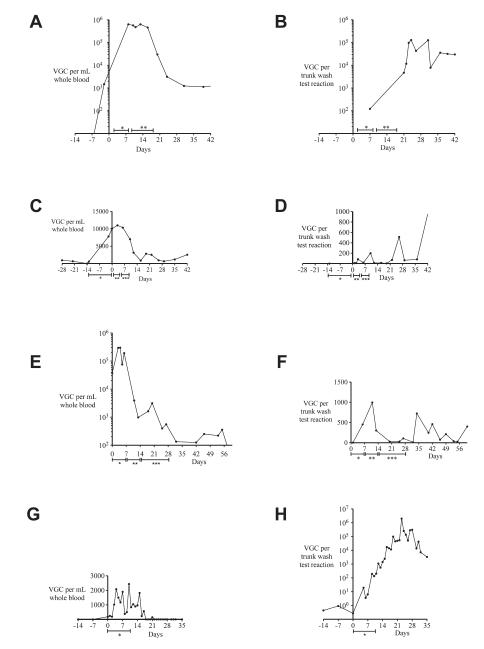
Using a real-time qPCR assay that detects EEHV1A and 1B,15 EEHV1 DNA loads were monitored in various fluid samples from three elephants considered clinically ill at the time of EEHV1 infection (Table 1). Elephant 1 experienced two distinct episodes of EEHV1-associated illness with detectable EEHV1 DNAemia, with the first episode beginning in February 2009 and the second episode beginning in November 2009 (Table 2). The viral load kinetic data described in this study were associated with the November 2009 episode of illness (Fig. 1A, B). Because of limited sample availability, viral load kinetic data are not available for the February 2009 event. At the onset of clinical signs, denoted as day 0, elephant 1 was reported to be slightly lethargic and had detectable EEHV1 DNAemia diagnosed at the National Elephant Herpesvirus Laboratory at the Smithsonian National Zoo, Washington, D.C., USA. As shown in Figure 1A, this elephant had a detectable whole-blood EEHV1 DNA load of 1,464 viral genome copies (VGCs)/ml 2 days before the onset of clinical signs and a peak viral load of 635,992 VGCs/ml at day 8. Samples between days−2 and 8 were unavailable for testing. This elephant had detectable EEHV1 DNAemia in whole blood until the conclusion of the study at 96 days. Viral DNA was detectable in two available serum samples on day 8 (292,002 VGCs/ml serum) and day 13 (174 VGCs/ml serum). EEHV1 DNA in trunk secretions was measured at 122 VGCs/test reaction on day 7 (the first available sample) and then increased to a peak of 128,563 VGCs/test reaction on day 24 and remained detectable until the completion of the study (Fig. 1B). Although this elephant was treated with intravenous ganciclovir from days 2 to 8 after the onset of clinical signs, it is difficult to determine whether EEHV1 viral loads changed substantially during this period, especially because there is a lack of critical time points during this period.
Figure 1.
Kinetics of EEHV1 viral loads detected in whole-blood and trunk-wash preparations from four elephants. DNA prepared from whole blood (A) and trunk washes (B) from elephant 1 in November 2009 was screened for the presence of EEHV1 nucleic acid by using a real-time quantitative polymerase chain reaction (qPCR) assay. Day 0 represents the first day that clinical signs of illness were observed. * Ganciclovir (3,500 mg) administered intravenously twice daily for days 2–8 after the onset of clinical signs. ** Ganciclovir (3,500 mg)
Elephant 2 also experienced two distinct episodes of EEHV1 DNAemia, and both episodes occurred within weeks of DNAemia in elephant 1, located at the same facility. The current study describes the kinetics of viral loads in elephant 2 during the November 2009 episode (Fig. 1C, D), during which the elephant displayed very slight signs of clinical illness. In response to the diagnosis of EEHV1 DNAemia in elephant 1, elephant 2 was treated with oral famciclovir prophylaxis. Elephant 2 had detectable EEHV1 DNA in whole-blood samples beginning 28 days before the onset of clinical signs and 14 days before famciclovir prophylaxis (Fig. 1C). Retrospectively, an increase in her whole-blood viral load to 10,000 VGCs/ml over 14 days of famciclovir prophylaxis was observed. On the 14th day of famciclovir prophylaxis, she was reported to be slightly lethargic, so oral famciclovir was discontinued, and she was treated with intravenous ganciclovir as described for elephant 1 (Fig. 1A, B). Her whole-blood viral load peaked at 11,019 VGCs/ml on day 3 and then remained positive at a low level for the remainder of the study. EEHV1 DNA was not detectable in serum samples from elephant 2 during the study period. EEHV1 DNA was not detectable in a trunk wash from elephant 2 collected 13 days before the onset of clinical signs. However, EEHV1 DNA was detectable in trunk washes collected from elephant 2 beginning 1 day after the onset of clinical signs and remained detectable at varying levels until the completion of the study (Fig. 1D).
Elephant 3 also experienced two distinct episodes of EEHV1 DNAemia and EEHV-associated illness. The viral load kinetics and treatment of the first event, January 2010, are described in this study. Initially, elephant 3 was observed to be mildly lethargic, and treatment with oral famciclovir was initiated as shown in Figure 1E, F. The whole-blood EEHV1 DNA load at the onset of clinical signs was 37,477 VGCs/ml and peaked at 300,017 VGCs/ml on day 4. The whole-blood viral load then decreased from 191,875 VGCs/ml on day 6 to 3,919 VGCs/ml on day 11 and remained positive at a low level of detection until the conclusion of the study on day 60. Three serum samples, from days 4, 11, and 20, were tested for the presence of EEHV1 DNA. Only the serum sample from day 4 was positive at a load of 995 VGCs/ml serum. EEHV1 DNA was detectable at relatively low levels in urine sediments prepared on days 6 (23 VGCs/test reaction) and 18 (<1 VGC/test reaction) but was negative in urine sediment preparations from days 11 and 13. A second significant EEHV1 DNAemia was detected in elephant 3 in February 2011, 13 mo after the EEHV1 DNAemia described in this report. Although the viral load kinetic data from the February 2011 event are not shown in this article, EEHV1 DNA from the second bout of DNAemia also was used for gene subtyping analysis (Table 2).
Kinetics of EEHV1 viral loads in fluid samples from two elephants subclinical for EEHV1 infection
In March 2010, during a routine EEHV1 herd-monitoring program, EEHV1 DNA was detectable at low levels in whole-blood samples from elephant 4, an apparently healthy, near-term gravid female elephant. As shown in Figure 1G, H, EEHV1 DNA was detectable in whole-blood preparations from elephant 4 for a period of 3 wk over a range of 0–2,432 VGCs/ml whole blood. At the time of diagnosis, EEHV1 DNAemia was 182 VGCs/ml whole blood, and treatment with famciclovir by rectum was initiated as shown in Figure 1G, H. During the 10-day course of treatment with famciclovir, no consistent change was observed in the whole-blood viral loads, and a substantial increase in the level of EEHV1 detection in trunk washes, from 0.27 to 194.6 VGCs/test reaction, was observed. Detection of EEHV1 DNA in trunk secretions peaked at day 21, having increased by 6 orders of magnitude to a peak of 1,984,028 VGCs/test reaction since diagnosis of EEHV1 DNAemia. Parturition occurred 36 days after EEHV1 DNAemia was detected, and the calf was born apparently healthy. DNA from 5 (19%) of 26 urine sediments prepared from elephant 4 during the course of this study tested positive for EEHV1 DNA; however, the apparent magnitude of detection of EEHV1 (range, 0.91–3.21 VGCs/test reaction) was substantially lower than that observed in trunk washes.
In May 2010, during routine twice-weekly whole blood monitoring, subclinical EEHV1 DNAemia also was detected in elephant 5. EEHV1 DNAemia was detectable in elephant 5 for 7 days at a relatively low level, ranging from 112 to 407 VGCs/ml whole blood (data not shown). EEHV1 DNA detection in trunk washes from elephant 5 remained at a very low level of detection during and after the period of EEHV1 DNAemia. Of significance is that during routine herd monitoring in November 2009, EEHV1 DNA was previously detected in trunk washes at a level of 33,390 VGCs/test reaction from elephant 5. At no time before May 2010 had EEHV1 DNAemia been detected in elephant 5. Prior positive trunk washes from elephant 5 were interpreted as demonstrating prior exposure to, and possibly infection with, EEHV1. Therefore, in response to detection of EEHV1 DNAemia in May 2010, elephant 5 was placed on close observation and monitoring of EEHV1 whole-blood viral load daily until the EEHV1 DNAemia ceased. No antiviral medications were given to elephant 5. The elephant remained apparently healthy during the course of EEHV1 DNAemia, and EEHV1 DNAemia resolved spontaneously.
Genotypic analysis of EEHV1 infection among this cohort of elephants
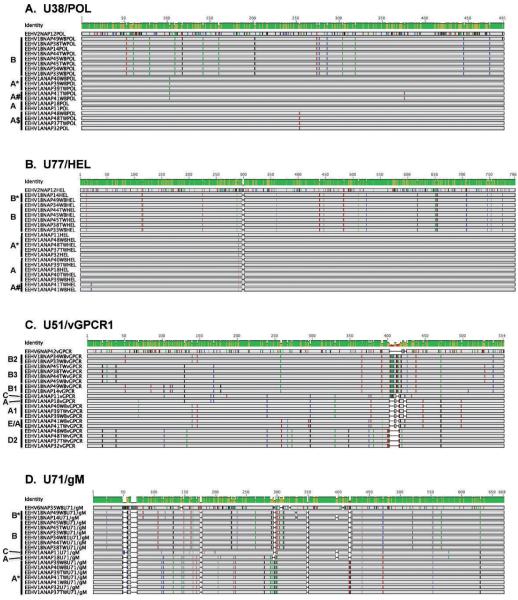
Using DNA samples from at least three trunk wash or whole-blood preparations representing three distinct time points from each of the five elephants included in this study, a genotypic analysis was conducted by direct DNA sequencing of PCR products from each of four different genetic loci. Although this analysis was not possible from several of the samples containing very low amounts of viral DNA (Table 2), informative results were obtained from a total of 15 independent DNA samples and 55 PCR products. The results are presented in the form of graphical presentations showing the nucleotide polymorphisms, genetic relatedness, and subtype patterns of all of the various NAP cases studied here at four EEHV genomic PCR loci in Figure 2 and are summarized in Table 2. These results illustrate which samples are identical and which are slightly different as well as which are EEH-V1A versus EEHV1B and compare them with prototype strains from both subtypes and from EEHV2 or EEHV6.
Figure 2.
Graphical presentations showing the nucleotide polymorphisms, genetic relatedness, and subtype patterns of all of the various North American Proboscivirus (NAP) cases studied here at four EEHV genomic PCR loci. Comparisons are made with representative standard prototype strains of EEHV1A (NAP11, 18), EEHV1B (NAP14), EEHV2 (NAP12), or EEHV6 (NAP35, 42) where appropriate (GenBank accession numbers are listed below). (A) The four genetic loci involved are highly conserved DNA polymerase locus (U38/POL). EEHV1A (NAP11) = HM568510, EEHV1B (NAP18) = HM568523, EEHV1B (NAP14) = HM568536, EEHV2 (NAP12) = HM568563. (B) Highly conserved helicase locus (U77/HEL). EEHV1A (NAP11) = HM568515, EEHV1A(NAP18) = HM568529, EEHV1B(NAP14) = HM568542, EEHV2(NAP12) = HM568564. (C) Variable viral G-protein–coupled receptor 1 locus (U51/vGPCR1). EEHV1A(NAP11) = GU350757, EEHV1A(NAP18) = GU350758, EEHV1B(NAP14) = GU350759, EEHV6(NAP42) = JN633926. (D) Variable intergenic myristylated tegument plus glycoprotein-M locus (U71/gM). EEHV1A(NAP11) = GU350764, EEHV1A(NAP18) = GU350750, EEHV1B(NAP14) = GU350767, EEHV6(NAP35) = JN913124. Substitutions from the consensus sequences are color coded (A = green, C = blue, G = black, T = red), and deletions are shown as gaps. Brackets to the left of the alignments indicate the specific gene subtype clusters assigned to the grouped cases based on the previously determined nomenclature system for 35 EEHV1 strains from cases of elephant hemorrhagic disease (Zong, unpubl. data). Cases NAP37, 38, 44, and 45 occurred at the Houston Zoo, Inc.; cases 33, 34, 39, and 40 occurred at the Saint Louis Zoo; and cases 41 and 49 occurred at Feld Entertainment. TW = trunk wash samples; WB = whole blood samples; others including NAP32 (accessions JF692760, GU350766, GU350760, JN633911) at the Houston Zoo in 2007 = necropsy tissue samples.
Over the course of 1 yr, elephants 1 and 2 each experienced two distinct episodes of EEHV1 DNAemia associated with clinical signs of illness. The first episode occurred in February 2009 and was associated with EEHV1B strains, with identical sequences across all four loci in both elephants (and with vGPCR1 = B2), with elephant 1 (NAP33) being diagnosed before elephant 2 (NAP34). In fact, these two sequences were also identical when the analysis was extended out over 10,000 bp, including the entire glycoprotein-B and DNA polymerase genes (data not shown). The second episode of EEHV1 DNAemia, detected in November 2009, was instead associated with EEHV1A strains having identical sequences (vGPCR1 = A1) in both calves and was first identified in elephant 1 (NAP39) and then in elephant 2 (NAP40). In December 2009, 1 mo after the second episode of EEHV1 DNAemia, an EEHV1A strain was identified in trunk washes from the same two elephants. The sample from elephant 1 had identical DNA sequences at all four loci examined to the EEHV1A DNA present in whole-blood samples (elephant 2 is presumed to be the same, although because there was a lower amount of virus only the HEL locus was able to be amplified in this sample).
Similarly, two separate episodes of EEHV1 DNAemia were identified in elephant 3, with each episode associated with distinct EEHV1 subtypes (Table 2). The first episode of EEHV1 DNAemia in January 2010 was associated with a previously unidentified EEHV1A strain (NAP41, vGPCR1 = E/A) and was followed by detection of the identical viral strain in trunk washes. This episode was followed 13 mo later, in February 2011, by another episode of EEHV1 DNAemia that was shown this time to be associated with a previously unidentified EEHV1B strain (NAP49, vGPCR1 = B1).
In September 2009 during a previous study,15 EEHV1 DNA was detected in 3 of 15 trunk washes from elephant 4, and DNA sequence analysis determined that this virus (NAP37) was identical in sequence at the POL, U71, and vGPCR1 loci to the EEHV1A strain (vGPCR1 = D2) previously associated with the death of a calf (NAP32) at the same facility 2 yr earlier. However, when the EEHV1 gene subtypes identified were compared from elephant 4 in the previous study with the virus identified in the current study, the EEHV1 DNAemia that occurred in March 2010 proved to be associated instead with a strain of the EEHV1B subtype (NAP45, vGPCR1 = B3). Interestingly, an EEHV1B strain with an identical sequence in POL, U71, and vGPCR1 also had been identified at very low levels previously within another herd mate (NAP38) from this same collection of elephants, however not at that time within this particular elephant.15 When gene-subtyping analysis was performed on the EEHV1 DNA present in trunk washes collected in May 2010, 2 mo after EEHV1 DNAemia, it was the identical EEHV1B (vGPCR1 = B3) virus strain as identified earlier in the peripheral blood samples from this elephant (Table 2).
Finally, gene-subtyping analysis was performed on the EEHV1 DNA identified in three different samples from elephant 5 and again identified two distinct EEHV1 strains referred to as NAP44 and NAP48 (Table 2). EEHV1 DNA had been detected at high levels in trunk secretions (up to 33,390 VGCs/test reaction) from elephant 5 in November 2009, approximately 8 mo before the May 2010 EEHV1 DNAemia. The viral DNA sequence here was once again determined to be identical to the EEHV1B (vGPCR1 = B3) strain detected previously in trunk washes from another elephant (NAP38) in the same herd in the original study.15 However, during the May 2010 episode of EEHV1 DNAemia, the same identical EEHV1A strain was instead identified that had previously been found in the calf (NAP32, vGPCR1 = D2) that died 2 yr earlier of EEHV-associated disease at this facility, and also later in trunk washes from two other adult herd mates (NAP36 and NAP37).15 In the case of elephant 5, the infection remained subclinical and in July 2010, 2 mo after EEHV1A DNAemia, the same unchanged EEH-V1A (vGPCR1 = D2) strain in trunk washes collected from this elephant was identified again. Note that for both the whole-blood and trunk-wash samples of NAP48, the U71/gM locus was not able to be amplified, and only a short 554-bp version of the vGPCR1 locus rather than the standard 870-bp version was analyzed (Table 2).
Therefore, over the total 2 yr of the study, each of the five monitored elephants suffered sequential infections with two distinct EEHV1 viruses, one from each of the two subtypes. These successive episodes occurred at intervals of 9, 9, 13, 6, and 7 mo apart in elephants 1, 2, 3, 4, and 5, respectively. Furthermore, the viruses found at different times in both whole blood and later trunk washes from at least one episode in each animal proved to be identical when compared at all loci and across the approximately 3,000 bp examined. However, each of the six EEHV1A or EEHV1B viruses detected here was both distinguishable from all other strains that have been examined in detail worldwide,18 as well as unique to the particular facility where it was found. In contrast, four of the total of six distinctive EEHV1A or EEHV1B strains observed here were found to have infected two or more herd mates at the same facility and to also have retained identical nucleotide sequences in each of them.
DISCUSSION
In this study, the kinetics of EEHV1 viral loads was monitored in various fluid samples collected from elephants with detectable EEHV1 DNAemia. It was found that DNAemia is detectable in elephants before the onset of clinical illness, that DNAemia generally precedes the detection of EEHV1 DNA in trunk washes, and that the identical virus that produces DNAemia is detectable in later trunk washes from the same elephant. In addition, each of the five elephants in the study had evidence of sequential infection with two distinct EEHV1 genotypes, one genotype from each of the EEHV1A and EEHV1B subtypes, providing evidence for the ability of multiple EEHVs to infect a single elephant.
EEHV infection of Asian elephants often presents with vague clinical signs and can progress very rapidly, making clinical management of elephants infected with EEHV very challenging. As described in this study, EEHV1 DNAemia is detectable in elephants up to 28 days before the onset of clinical signs. Previous studies have shown that elephants that die as a result of EEHV-associated disease often have whole-blood viral loads in excess of 106 VGCs/ml whole blood.8,15 Clinical signs of EEHV-associated disease were observed starting at 104 VGCs/ml whole blood, a value well above the limit of detection of the assay (103 VGCs/ml whole blood).15 The finding that preclinical DNAemia progresses to clinical illness over several days provides justification to the idea that monitoring susceptible elephants may lead to the ability to treat EEHV-associated disease before the onset of irreversible disease.
In the case of elephants 1, 2, and 3, whole-blood viral load information was not used to direct clinical decisions because these elephants were clinically ill at the time of diagnosis, and preclinical DNAemia was detectable in elephants 1 and 2 only on retrospective analysis. However, knowledge of viral load and previous EEHV testing was used in making clinical decisions regarding the management of elephants 4 and 5. Both of the elephants are enrolled in an EEHV1 herd-monitoring program and have routine screening of whole-blood and trunk-wash samples, screening of which led to the detection of subclinical EEHV1 DNAemia. In addition, previous data had demonstrated EEHV1 in trunk washes from elephants 4 and 5 before the DNAemia but that proved to have been from a previous infectious episode with a different EEHV1 subtype.
Previously published data have suggested that EEHV1 produces latent infection of Asian elephants, with occasional reactivation and shedding;14,15 however, the data presented in this study provide the strongest evidence to date for this ability of EEHV1. Using viral gene subtyping analysis, we demonstrated for the first time that the EEHV genotype that produces DNAemia is subsequently shed in trunk secretions. These data suggest that EEHV1, like other known betaherpesviruses, may infect a subset of leukocytes and become detectable in peripheral whole-blood samples during lytic replication. Subsequently active viral replication evidently then persists for at least several weeks in some cell type involved in producing nasal secretions before presumably establishing classical long-term quiescent latency. In some elephants, EEHV1 may be detectable at low levels in trunk secretions as the virus infection persists at a low level of lytic replication. However, the possibility that the low level of detection of EEHV1 in trunk-wash preparations from some elephants may represent environmental cross-contamination cannot be excluded. The actual pathogenesis of infection and nature of EEHV1 persistence or latency remains to be determined.
The genotyping data also provide evidence that an individual elephant can acquire multiple EEHV infections, a process termed “superinfection,” suggesting that prior infection with one EEHV subtype does not confer sterilizing immunity against subsequent infection with another EEHV subtype. All elephants in the study showed evidence of infection with at least two distinct EEHV genotypes over less than 1 yr. Most strikingly, all five of these elephants were infected sequentially with both one strain of EEHV1A followed by one strain of EEHV1B (or vice versa), but no animals have yet been observed to be simultaneously infected with or shedding two different strains of either the EEHV1A or EEHV1B subtypes. When the EEHV1 strain has been determined, EEHV1A strains have been found to cause systemic hemorrhagic disease significantly more often than EEHV1B strains (20 strains of EEHV1A vs. 3 strains of EEHV1B). Although it cannot be ruled out, there was no evidence obtained for simultaneous infections with both viruses in any of these animals; rather, it seemed that the first subtype was cleared to undetectable levels in blood or nasal secretions before detection of a new infection episode with the second subtype. Elephant 5 is also particularly interesting because he was previously shown by Latimer et al. (2010) to have had a low transient episode of presumed DNAemia with an elephant gammaherpesvirus (EGHV3A), viruses that are classified in the Gammaherpesvirinae, a group of elephant herpesviruses not associated with pathology. Whether preexisting infection with another elephant herpesvirus confers enough immunity to prevent catastrophic disease is a critical question that remains to be determined.
The data and methodology presented in this article represent a significant advance in the ability to detect subclinical EEHV infection and to characterize the viral species infecting healthy Asian elephants by screening individual elephants and elephant herds for the presence of previously undetectable EEHV infections. This advance has significant implications regarding the management of captive Asian elephant populations. Elephant managers and veterinarians can use information about the specific EEHV genotypes present among a population of elephants to make informed decisions about the clinical care, transfer, and breeding of captive elephants.
CONCLUSIONS
Using a real-time qPCR assay, EEHV1 DNAemia can be detected in Asian elephants before the onset of clinical signs and in elephants that do not display clinical signs of infection. DNAemia also precedes substantial shedding of EEHV1 in trunk secretions by approximately 2–3 wk. Finally, apparently healthy Asian elephants can be infected with more than one EEHV1 genotype.
Acknowledgments
The authors acknowledge the hard work and dedication of the elephant care staff at the Houston Zoo, Inc.; the Saint Louis Zoo; and the Ringling Bros. Center for Elephant Conservation. We thank Daryl Hoffman for advice regarding the management of elephants infected with EEHV; Virginia Mohlere for review of the manuscript, and Sarah Heaggans for assistance in managing the sequence data information and GenBank submissions. Work at Baylor College of Medicine was supported by Houston Zoo, Inc.; Elephant Managers Association; the Dan Duncan Family Foundation; and the National Institutes of Health training grant T-32-AI-07471. A student scholarship from the Morris Animal Foundation supported the work of C.E. The International Elephant Foundation supported work at the National Elephant Herpesvirus Laboratory. Work at Johns Hopkins School of Medicine was supported by the National Institutes of Health research grant NIAID AI24576, the International Elephant Foundation, and the Morris Animal Foundation.
LITERATURE CITED
- 1.Davison AJ, Eberle R, Ehlers B, Hayward GS, McGeoch DJ, Minson AC, Pellett PE, Roizman B, Studdert MJ, Thiry E. The order Herpesvirales. Arch. Virol. 2009;154:171–177. doi: 10.1007/s00705-008-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ehlers B, Burkhardt S, Goltz M, Bergmann V, Ochs A, Weiler H, Hentschke J. Genetic and ultrastructural characterization of a European isolate of the fatal endotheliotropic elephant herpesvirus. J. Gen. Virol. 2001;82:475–482. doi: 10.1099/0022-1317-82-3-475. [DOI] [PubMed] [Google Scholar]
- 3.Fickel J, Richman LK, Montali R, Schaftenaar W, Goritz F, Hildebrandt TB, Pitra C. A variant of the endotheliotropic herpesvirus in Asian elephants (Elephas maximus) in European zoos. Vet. Microbiol. 2001;82:103–109. doi: 10.1016/s0378-1135(01)00363-7. [DOI] [PubMed] [Google Scholar]
- 4.Garner MM, Helmick K, Ochsenreiter J, Richman LK, Latimer E, Wise AG, Maes RK, Kiupel M, Nordhausen RW, Zong JC, Hayward GS. Clinico-pathologic features of fatal disease attributed to new variants of endotheliotropic herpesviruses in two Asian elephants (Elephas maximus) Vet. Pathol. 2009;46:97–104. doi: 10.1354/vp.46-1-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gartner B, Preiksaitis JK. EBV viral load detection in clinical virology. J. Clin. Virol. 2010;48:82–90. doi: 10.1016/j.jcv.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 6.IUCN (International Union for Conservation of Nature) The IUCN Red List of Threatened Species. International Union for Conservation of Nature; Gland, Switzerland: 1986. Elephas maximus. [Google Scholar]
- 7.Jacobson ER, Sundberg JP, Gaskin JM, Kollias GV, O’Banion MK. Cutaneous papillomas associated with a herpesvirus-like infection in a herd of captive African elephants. J. Am. Vet. Med. Assoc. 1986;189:1075–1078. [PubMed] [Google Scholar]
- 8.Latimer E, Zong JC, Heaggans SY, Richman LK, Hayward GS. Detection and evaluation of novel herpesviruses in routine and pathologic samples from Asian and African elephants: identification of two new probosciviruses (EEHV5 and EEHV6) and two new gammaherpesviruses (EGHV3B and EGHV5) Vet. Microbiol. 2011;147:28–41. doi: 10.1016/j.vetmic.2010.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCully RM, Basson PA, Pienaar JG, Erasmus BJ, Young E. Herpes nodules in the lung of the African elephant (Loxodonta africana (Blumebach, 1792)) Onderstepoort J. Vet. Res. 1971;38:225–235. [PubMed] [Google Scholar]
- 10.Ossent P, Guscetti F, Metzler AE, Lang EM, Rubel A, Hauser B. Acute and fatal herpesvirus infection in a young Asian elephant (Elephas maximus) Vet. Pathol. 1990;27:131–133. doi: 10.1177/030098589002700212. [DOI] [PubMed] [Google Scholar]
- 11.Reid CE, Hildebrandt TB, Marx N, Hunt M, Thy N, Reynes JM, Schaftenaar W, Fickel J. Endotheliotropic elephant herpes virus (EEHV) infection. The first PCR-confirmed fatal case in Asia. Vet. Q. 2006;28:61–64. doi: 10.1080/01652176.2006.9695209. [DOI] [PubMed] [Google Scholar]
- 12.Richman LK, Montali RJ, Garber RL, Kennedy MA, Lehnhardt J, Hildebrandt T, Schmitt D, Hardy D, Alcendor DJ, Hayward GS. Novel endotheliotropic herpesviruses fatal for Asian and African elephants. Science. 1999;283:1171–1176. doi: 10.1126/science.283.5405.1171. [DOI] [PubMed] [Google Scholar]
- 13.Richman LK, Hayward GS. Elephant herpesviruses. In: Fowler M, editor. Fowler’s Zoo and Wild Animal Medicine. W. B. Saunders Co.; Philadelphia, Pennnsylvania: 2011. pp. 496–502. [Google Scholar]
- 14.Schaftenaar W, Reid C, Martina B, Fickel J, Osterhaus AD. Nonfatal clinical presentation of elephant endotheliotropic herpes virus discovered in a group of captive Asian elephants (Elephas maximus) J. Zoo Wildl. Med. 2010;41:626–632. doi: 10.1638/2009-0217.1. [DOI] [PubMed] [Google Scholar]
- 15.Stanton JJ, Zong JC, Latimer E, Tan J, Herron A, Hayward GS, Ling PD. Detection of pathogenic elephant endotheliotropic herpesvirus in routine trunk washes from healthy adult Asian elephants (Elephas maximus) by use of a real-time quantitative polymerase chain reaction assay. Am. J. Vet. Res. 2010;71:925–933. doi: 10.2460/ajvr.71.8.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiese RJ. Asian elephants are not self-sustaining in North America. Zoo Biol. 2000;19:299–309. [Google Scholar]
- 17.Zachariah A, Richman LK, Latimer E, Hayward GS, Kalaivannan N, Zachariah A, Balan S, Gafoor A, Easwaran EK. Fatal endotheliotropic elephant herpes virus mortality in free ranging and captive Asian elephants in south India. 2008 International Elephant Conservation and Research Symposium; Bangkok, Thailand. 2008. p. 60. [Google Scholar]
- 18.Zong JC, Latimer E, Heaggans S, Richman LK, Hayward GS. Pathogenesis and molecular epidemiology of fatal elephant endotheliotropic disease associated with the expanding Proboscivirus genus of the Betaherpesvirinae. International Elephant Conservation and Research Symposium; Orlando, Florida. 2007. pp. 23–35. [Google Scholar]