Summary
Islet transplantation has been shown to be a viable treatment option for patients afflicted with Type 1 diabetes. However, the severe shortage of human pancreas and the need to use risky immunosuppressive drugs to prevent transplant rejection remain two major obstacles to routine use of islet transplantation in diabetic patients. Successful development of a bioartificial pancreas using the approach of microencapsulation with perm-selective coating of islets in hydrogels for graft immunoisolation holds tremendous promise for diabetic patients because it has great potential to overcome these two barriers. In this review article, we will discuss the need for bioartificial pancreas, provide a detailed description of the microencapsulation process, and review the status of the technology in clinical development. We will also critically review the various factors that need to be taken into consideration in order to achieve the ultimate goal of routine clinical application.
Keywords: islets, alginate, microencapsulation, immunoisolation, diabetes, transplantation
1. Introduction
Polyuria, polydipsia, polyphagia, weight loss, and fatigue are early symptoms of diabetes mellitus, a common disorder of glucose homeostasis marked by a deficiency or lack of a small polypeptide known as insulin. Before the discovery of insulin, patients suffering from diabetes literally starved to death, their tissues awash in glucose, but the cells lacking the ability to admit this simple sugar. In the late 1880s, von Mering and Minkowski observed that pancreatectomized dogs developed glycosuria, implicating the pancreas in the disorder causing diabetes. Subsequently, Opie and others identified pathologic changes in the islets of Langerhans by histologic study of pancreatic tissue taken from patients with diabetes (1). Nearly 90 years ago, Banting, Best, Colip, and Macleod won the race to discover the pancreatic “principle” largely responsible for glucose homeostasis, and Banting and Macleod shared the Nobel Prize for this discovery with their colleagues (1).
Prior to the discovery of insulin by Banting and Best, effective treatment of diabetes mellitus was limited to dietary manipulation. The discovery of insulin converted an often rapidly fatal disease (particularly for patients with the clinical equivalent of Type 1 diabetes) to a chronic condition requiring life-long treatment. At the time, many thought that the ability to administer insulin exogenously would prove to cure diabetes, but the long-term imperfections in glycemic control present even with state of the art insulin management results in the so-called secondary complications of diabetes (diabetic nephropathy, retinopathy, neuropathy, and vascular disease). The secondary complications of diabetes significantly diminish life expectancy and quality of life in many patients (2). Current treatment for diabetes, both Type 1 and Type 2, includes exogenous insulin administration and endocrine replacement by solid organ or islet allotransplantation. Both insulin administration and pancreas transplantation have considerable inherent drawbacks, driving the clinical need for new approaches such as the bioartificial pancreas (2).
2. Therapeutic options for Type 1 diabetes
2.1 Exogenous Insulin Therapy
Exogenous insulin administration to control blood glucose has been the standard therapy since the discovery of insulin. In this therapy, the amount of carbohydrates consumed is estimated by measuring food, and this is used to determine the amount of insulin necessary to cover the meal. The calculation is based on a simple open-loop model based on past success. Calculated insulin is then adjusted based on pre-meal blood glucose measurement, such that, insulin bolus is increased for high blood glucose or decreased for low-blood glucose. Insulin is injected or infused subcutaneously and enters the blood stream in approximately 15 min. Then blood glucose can be tested again and adjusted by additional insulin bolus or eating more carbohydrates, until balance is achieved. Needless to say, this procedure leads to rapid blood glucose fluctuations and highly inefficient both in terms of patient convenience and health. While it has been useful, insulin treatment also has a negative impact on personal and social functioning as well. The poor control of blood glucose fluctuations with this therapy leads to many severe secondary complications such as retinopathy, neuropathy, nephropathy, and cardiovascular diseases (2,3). According to the Diabetes Control and Complications Trial (DCCT), strict control of blood glucose may only delay the onset of new diabetes-related complications and the progression of existing ones, but would not ultimately prevent the development of secondary diseases associated with diabetes (4).
2.2 Pancreas Transplantation
Kelly and Lillehei performed the first clinical pancreas transplant at the University of Minnesota in 1966 (5). Currently, pancreas transplantation is the only option therapeutically available that reproducibly achieves normoglycemia. Pancreas transplantation re-establishes endogenous insulin secretion that is responsive to normal feedback regulation. Since 1966, more than 30,000 pancreas transplants have been performed worldwide. According to the Scientific Registry of Transplant Recipients (SRTR), the 1-year rate of graft survival is 86% when a pancreas and a kidney are transplanted together (SPK), 82% when pancreas is transplanted after kidney (PAK) and 75% when pancreas is transplanted alone. Most pancreatic grafts are from cadaveric donors, though transplantation of a segment of the pancreas donated by a living donor has also been reported (6). Transplantation, however, requires major surgery and dependence on life-long immunosuppression to prevent graft rejection. Most pancreas transplants are performed with immunosuppression induction therapy (usually monoclonal or polyclonal T-cell depleting antibody) and maintenance immunosuppression with a calcineurin inhibitor (cyclosporine or tacrolimus), an antimetabolite (mycophenolic acid) plus or minus corticosteroids (7, 8). Owing to the limited availability of human pancreases and the need for immunosuppression, relatively few pancreas transplants are done compared to the entire diabetic population. Improvements in surgical technique or immunotherapy are unlikely to make whole organ pancreas transplantation available to the majority of patients with diabetes.
2.2.1 Islet Transplantation
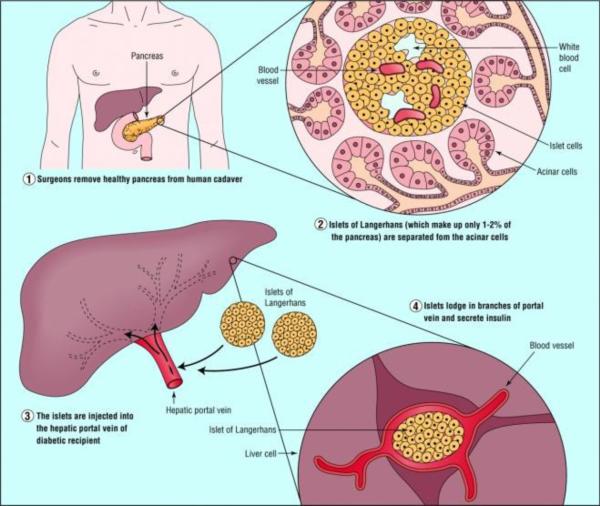
Islet transplantation promises to be a cure at least as effective as pancreas transplantation, while being much less invasive. The efficiency of islet recovery from the whole organ pancreas and the susceptibility of allogeneic islet to immune attack (both alloimmunity and autoimmunity) are the two major barriers to successful islet transplantation. There are approximately 1 million islets in an adult human pancreas. However, only half or fewer of these are successfully isolated on a consistent basis. Thus, islet transplantation usually requires islets isolated from two or more donor pancreases. Since islet isolation requires manipulation of human tissue, the process must be carried out in a good manufacturing process (GMP) facility, which adds to the expense of the procedure. Islets are transplanted by transfusion into the portal vein and embolization into the liver. The transplanted islets engraft in the distal portal triad (Figure 1). Allogeneic human islets have been successfully transplanted using the Edmonton immunosuppression (steroid-free) protocol (9). In investigations with this protocol, glycemic control has been restored for extended periods of greater than 5 years in a few patients, but at the expense of immunosuppression of the transplant recipient. The necessary life-long adherence to an immunosuppression drug regimen is inconvenient and associated with side effects and complications of over-immunosuppression.
Figure 1.
Illustration of islet transplantation (Serup et al., 2001)(with permission).
2.3 Artificial Pancreas
The artificial pancreas is a technological development to enable Type 1 diabetic patients to automatically control their blood glucose, acting in essence like a healthy pancreas. The goals of the artificial pancreas are: i) to improve presently popular but inefficient insulin therapy to attain a better glycemic control, thus avoiding the complications due to blood glucose fluctuations, and ii) to mimic normal stimulation of the liver by the pancreas and to normalize carbohydrate and lipid metabolism. There are various approaches to the artificial pancreas:
a) Medical equipment approach
This is basically an insulin pump under closed loop control utilizing real-time data from a continuous blood glucose sensor.
b) Gene therapy approach
This involves therapeutic infection of a diabetic person by a genetically engineered virus causing a DNA transformation of few intestinal cells to become insulin-producing cells. It has even been suggested as a strategy to tackle the cause of beta cell destruction itself hence curing the patients before full and irreversible β cells destruction (10). While novel and potentially able to treat diabetes, this approach is still in infancy with a lot of unanswered questions.
c) Bioengineering approach
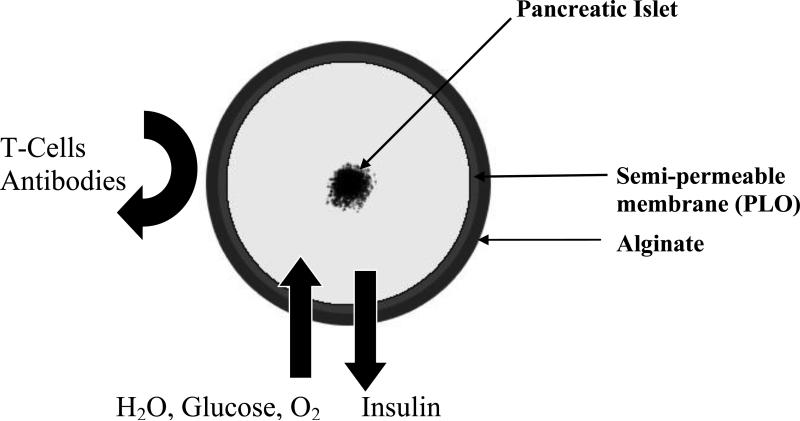
The bioengineering approach to designing a bioartificial pancreas has generally involved the development of either microcapsules, or macrocapsules, or other devices such as biocompatible sheet of encapsulated islets. When implanted, these constructs would substitute for the defective native endocrine pancreas (11). However, this review will focus on the microencapsulated islet construct, as it has advanced into the stage of clinical trials (12-15) and has significant promise to be a good alternative to pancreas transplantation. Using alginate as the encapsulation polymer, the concept of islet immunoisolation is illustrated in Figure 2, which essentially incorporates a semipermeable membrane into the process because alginate does not have any appreciable perm-selectivity towards immune cells and other immunological factors such as antibodies that can potentially destroy the encapsulated cells.
Figure 2.
Illustration of the principle of immunoisolation by microencapsulation
3. A Bioartificial Pancreas
3.1 Materials
i) Alginate
The chemical structure of alginate consists of unbranched binary copolymers of 1-4-linked β-d-mannuronic acid (M) and α-l-guluronic acid (G) whose structures are illustrated in Figure 3. Both the composition and block structure vary in different types of alginate, but the length of the G blocks is the main structural feature that contributes to gelation because the diaxially linked G residues form cavities that function as binding sites for ions (16). As already mentioned, alginate gels easily in the presence of divalent cations, such as Ca2+, Sr2+ and Ba2+, which interact with Na+ ions from the G –monomers in the polymer chains to form ionic bridges between adjacent polymers (16,17). Among the possible cross-linking cations for alginate microbeads, Ba2+ and Ca2+ have been the most researched in cell microencapsulation studies. For most laboratories, Ca2+ is the preferred divalent cation for alginate cross-linking during cell microencapsulation because in contrast to Ca2+, Ba2+ binds to both the G and M molecules in alginate leading to a high degree of cross-linking and greater in vivo stability, albeit, leaving no room to equip the alginate microbeads with perm-selectively (18). There is also some concern about possible Ba2+ toxicity to cells since this divalent cation is a strong inhibitor of K+ channels (19), which are critically involved in the stimulus-secretion coupling of insulin secretion (20). It is also known that organic solutes used routinely in the generation of Ba2+-alginate beads are cytotoxic when released from microcapsules after transplantation and thus have to be replaced by biomolecules such as histidine (19). One major advantage of using Ca2+ as the crosslinking cation is that an inner alginate core encapsulating islets can be liquefied in order to enhance the diffusion of permissible molecules to and from the microcapsules (21). The process of liquefaction is pretty delicate and has to be performed with utmost caution in order to avoid capsule breakage caused by high internal colloid-osmotic pressure after the “degelling” (19).
Figure 3.
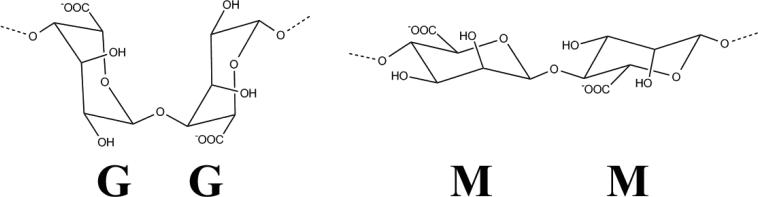
Structure of M and G chains in alginate
It has been shown that the chemical composition of alginate based on the ratio of G to M blocks, the gelling cation, and the purity of alginate have considerable effects on microbead size and morphology (22-24) as well as the host tissue response (25,26). For transplantation experiments, the purity of alginate is critical, as it is very well established that host tissue response is significantly reduced or even eliminated when highly purified alginate is used for encapsulation (18, 27). Ultrapure Keltone LV sodium alginate commercially-available as low viscosity high-mannuronic acid (LVM) and low viscosity high guluronic acid (LVG) preparations from Novamatrix, Sandvika, Norway, is routinely used for cell microencapsulation. In our experience these alginate preparations can be stored frozen at -80oC for many years without any change in their properties.
ii) Semi-permeable membrane fabrication
Effective immunoisolation of cells in alginate microcapsules is primarily achieved by the incorporation of a perm-selective membrane into the encapsulated cell device, and a number of biopolymers including, Poly-L-Lysine (PLL), Poly-L- Ornithine (PLO) and Chitosan-Polyvinylpyrrolidone have been used for this purpose (18,25,26). Of these materials, PLL has been routinely used, but evidence is emerging that the use of PLO may result in better mechanical strength and smaller pore-size exclusion (28) as well as enhanced stability of microcapsules (29,30). The preferred molecular weight range for both PLL (Sigma-Aldrich catalogue # P4957) and PLO (Sigma-Aldrich catalogue # P5061) for the purpose of perm-selective coating of alginate microbeads is 15 – 30KDa. Both PLL and PLO are polycationic polymers that require covering of their surface with a coat of the more biocompatible polyanionic alginate in order to prevent electrostatic interactions with cells and proteins after in vivo implantation. This external alginate coating of microcapsules has traditionally been performed by simple incubation of the PLL- and PLO-coated microcapsules in a low concentration of alginate solution for a short period of time, usually less than 10 minutes. However, this thin coating with alginate that is not cross-linked is prone to the risk of degradation in long-term experiments. Recent studies have described a new procedure for cross-linking the external alginate coating with Ca2+ (2, 31), which should enhance the long-term stability of the external alginate coat.
3.2 Microencapsulation of islets
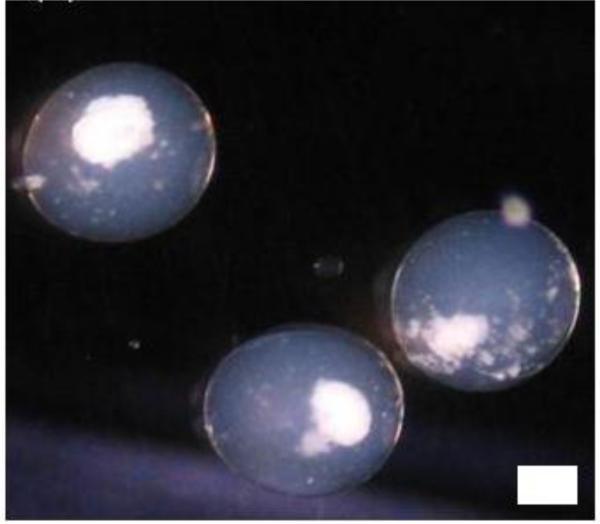
Based on the issues raised above, our laboratory adopts a 4-step process in islet microencapsulation. In the first step, islets are suspended in a solution of sodium alginate (usually from 1.2 – 1.8% w/v) and microspheres of alginate containing one or two islets/microsphere (depending upon the alginate-islet ratio in the suspension), are generated and allowed to gel into microbeads in a bath of 100 mM CaCl2 solution. Following a couple of washings with normal saline, the microbeads are then perm-selectively coated with variable concentrations of PLL or PLO for variable duration of time depending on the desired pore-size exclusion limit in the second step. The third step is the liquefaction of the alginate core of the microcapsules achieved by a brief incubation in 55 mM sodium citrate solution. After washings with normal saline again, the final step in the microencapsulation process is the external coating with a lower concentration (routinely about 10% of the concentration used in generating the initial microspheres), but our new procedure utilizes only a slightly lower concentration than the initial alginate concentration used to generate the microspheres (2, 31). To cross-link the external alginate coat, a solution of normal saline supplemented with 22 mM calcium chloride has been recently described for the final washings of microcapsules (31), otherwise normal saline is routinely used. Figure 4 shows some islets encapsulated in alginate-PLO-alginate (APA) microcapsules in our laboratory using the 4-step process.
Figure 4.
Encapsulated islets in an alginate microcapsule. Scale=100 μm.
3.3 Microencapsulation devices
The currently available devices for the microencapsulation of islets have been designed based on one of these techniques: i) Interfacial precipitation, ii) Phase Inversion, and iii) Polyelectrolyte coacervation. Phase inversion has been mostly used for making macrocapsules while interfacial precipitation has been applied in the microcapsule generation. Polyelectrolyte coacervation is a modification of alginate-calcium interfacial precipitation system, in which complexation of oppositely charged polymers leads to formation of a hydrogel membrane encapsulating the islets (17). The two most widely used devices for microencapsulation are the air-syringe pump droplet generator and the electrostatic bead generator (2). Each of these devices is fitted with a single needle through which droplets of cells suspended in alginate solution are produced and cross-linked into spherical microbeads. A major drawback in the design of these instruments is that they are incapable of producing sufficient numbers of microcapsules in a short-time period to permit mass production of encapsulated and viable cells for transplantation in large animals and humans. It is noteworthy that a prolonged process of encapsulation of cells adversely affects their viability.
A multi-needle approach to producing more than one encapsulated cell at a time as a scale up of the process has also been described with four needles (32). While this scale up is a step forward in accelerating the production of encapsulated cells, production rates at several orders of magnitude higher are required to meaningfully produce sufficient quantities of encapsulated and viable cells to serve millions of patients requiring cell transplantation. For instance, for transplantation in human subjects, it has been estimated that for the 1 million islets needed for transplantation in a diabetic human subject, about 100 hours would be required to complete the encapsulation of this number of islets, assuming one islet/microcapsule. In practice, it has actually been estimated that the duration of the process would be closer to 200 hours (33) because of the additional steps involved in the encapsulation procedure, following the generation of the initial cell-containing alginate microspheres. This situation raises an urgent need for a radically different approach to rapidly producing viable encapsulated cells in sufficient quantities for routine application in human cell therapy. To address this need, we have recently designed and tested the efficiency of a new scalable prototype device for cell encapsulation using a microfluidic approach (34, 35).
3.4 Other critical factors affecting the function of encapsulated islets
In addition to the need for effective immunoisolation by incorporating a well-characterized perm-selective membrane in the alginate microcapsule, as discussed above, there are other critical factors to be considered when using microcapsules to encapsulate islets for transplantation, and some of these include:
i) Islet requirements for oxygen and the need for revascularization of encapsulated islets
Although islets constitute approximately 1 % of the pancreas, they receive about 6 -10% of its blood flow (36, 37), indicating a disproportionate level of perfusion in which islets receive and consume lots of oxygen. The usual high oxygen requirement of islets is interrupted during the process of islet isolation and processing when islets are used for transplantation, and studies haveshown that hypoxia has significant deleterious effects on the survival and function of islets (38). In the immediate post-transplant period, isolated islets are forced to depend upon diffusion of oxygen and nutrients through peripheral perfusion from the surrounding tissue within the site of transplantation (39), until revascularization by angiogenesis, a process that requires 7-10 days (40). Routinely, microencapsulated islets are transplanted in the peritoneal cavity, where norevascularization takes place, thus subjecting the islet grafts to extended periods of hypoxia and eventual death. Therefore, the death of most of the encapsulated islet grafts owing to severe hypoxia results in the need for large quantities of microencapsulated islets to achieve normoglycemia in studies performed in large animals and humans (2).
In consideration of the oxygen need of encapsulated islets, the size of the microcapsule is crucial for the function of the encapsulated islets. The major drawback of macrocapsules is their relative low surface to volume ratio, which interferes with optimal diffusion of nutrients and oxygen. A small size of microcapsule would benefit the islet and also exponentially decrease the total transplant volume. A significant amount of work has been done with various new technologies to make beads as small as 185 μm (diameter), which is about four times smaller than conventional beads (800 μm). The smaller the diameter of the capsules the better the diffusion of nutrients to the islets, and Omer et al. have demonstrated that capsules with a diameter of 600±100 μm showed improved stability in vivo over larger capsules with diameters of 1000±100 μm (41). In most tissues, it has been shown that the maximum diffusion distance for effective oxygen and nutrient diffusion from blood capillary to cells is 100 μm, which is exceeded when islets with an average diameter <200 μm are enclosed in conventional microcapsules with average diameters ~800 μm. The absence of this convection inside a capsule induces a nutrient-gradient from the capsule surface to center of islet. However the caveat is that with reduction in capsule size the number of capsules containing partially protruding islets also proportionally increases, and this in turn increases the number of capsules affected by an inflammatory response. Decreasing the islet density in alginate can solve this problem, as it has been shown that each capsule size has an optimal islet density. Usually this is associated with a slight increase in empty capsules but minimizing protruding islets is of utmost priority. In many cases, the inner alginate bead will be either completely or partially liquefied by the removal of calcium ions with calcium quenching reagents such as sodium citrate, which allows for improved diffusion in the microbeads (21).
Investigators are currently examining different approaches to address the problem of inadequate supply of oxygen to encapsulated islet transplants. In a recent study, the effect of a combination of growth hormone releasing (GHRH) agonist and controlled oxygen supply on the function of a bioartificial macrochamber was examined. In this study islets were encapsulated and maintained within alginate slab configuration adjacent to an oxygen-permeable membrane to create an immune barrier and allow for oxygenation of the islet graft. The minimally invasive implantable chamber was shown to normalize blood glucose in Streptozotocin-induced diabetic rodents for up to 3 months after subcutaneous transplantation (42). In another study, investigators showed that encapsulation of solid calcium peroxide within hydrophobic polydimethylsiloxane resulted in sustained oxygen generation that lasted for more than 6 weeks and was enough to prevent hypoxia-induced cell dysfunction and death in insulin-producing cells (43). Another important factor that affects the function of encapsulated islets is the morphology of the microcapsules used for the encapsulation. Spherical microcapsules are necessary for long term functionality; irregularities or imperfections in the microcapsules can cause an immune response and inefficiencies in the delivery of nutrients resulting in loss of islet functionality (44).
ii) Transplantation Site
Based on the issue of adequate nutrient supply as discussed above, it is necessary to find a site where encapsulated islets are in close contact with the blood stream. Unfortunately, it is difficult to find such a site since it should combine the capacity to bear a large graft volume with immediate vicinity to blood vessels. Transplantation of encapsulated islets is most commonly done intraperitoneally, as it offers the advantages of laparoscopic implantation or through injection, and allows ample room to implant numerous microcapsules (13, 45). However, there are several disadvantages to this site. In addition to the problem of avascular supply discussed earlier, another major disadvantage is that microcapsules that are implanted intraperitoneally are vulnerable to an immune response from intra-peritoneal T -cells and macrophages (27,46-48), and have less access to the vasculature. This results in an increased likelihood of fibrotic growth over encapsulated islets, a loss of graft functionality, and a delay in insulin uptake into the blood circulation (49).
Consequently, alternative transplantation sites have been investigated, including transplanting into liver (50), kidney capsule (51), subcutaneously (52), and into an omentum pouch (2, 34, 53, 54). In the study conducted by Toso et al., microcapsules were injected into the portal veins of rats; however, the results of the study showed that immunosuppressants were necessary to prevent fibrotic overgrowth, and the risk of hepatic thrombosis makes this approach impractical. The studies by Dufrane et al. that investigated implant sites such as subcutaneous and the kidney capsule showed that encapsulated islets implanted in these two sites had less cellular overgrowth compared to encapsulated islets implanted intraperitoneally. The studies by Dufrane and colleagues demonstrated the functionality of encapsulated islets implanted within the kidney capsule of primates (51); however, clinical application would be difficult given the limited space within this site (55). The attraction for the omentum pouch is that like the kidney capsule, it offers a well-vascularized site for transplantation, but has more space for microcapsules and is easier to access (56). In addition, microencapsulated islets transplanted in the omentum pouch are easily retrievable for post-transplant evaluation (2).
4. Summary & Conclusion
It is clear that the microencapsulated islet technology has enormous potential to achieve the ultimate goal in transplantation, which is to routinely perform islet transplantation in patients without the need for risky immunosuppressive drugs to prevent transplant rejection. The modern era of the islet microencapsulation technology began with the report by Lim and Sun that a single implantation of microencapsulated islets into rats with Streptozotocin-induced diabetes corrected the diabetic state for 2 to 3 weeks. In addition, the paper showed that the microencapsulated islets remained morphologically and functionally intact throughout long-term culture studies lasting over 15 weeks (57). Since that publication, interest in the technology has waxed and waned as various groups obtained variable results with their microencapsulated islet constructs in pre-clinical studies sometimes performed with insufficient consideration of the critical factors required for optimal function of the encapsulated islets. Thus, we have seen studies performed with islets microencapsulated in microcapsules without perm-selectivity, and studies performed with microcapsules fabricated without due consideration for enhanced diffusion of nutrients and oxygen. The high oxygen requirements of islets need to be taken into consideration during all processes involved in the development of the technology. These processes include the islet isolation process as well as the microencapsulation technique itself as encapsulated dead cells have no functional value. We have recently discussed the various factors that are necessary for optimal function of encapsulated islets (2, 11). One of these limiting factors is the absence of high throughput devices for timely mass production of viable islets for studies in large animals and humans. Recent developments in the cell microencapsulation described by our group have now provided the much needed procedure to enhance clinical application of the microencapsulated islet technology (34, 35).
Although, as we have pointed out in this article, the microencapsulated islet technology as we presently know it has not been optimized, studies have since been initiated in humans beginning with the first experiment performed by Soon-Shiong and colleagues that reported insulin independence in a type 1 diabetic patient after encapsulated islet transplantation (12). In the experiment, encapsulated human islets were injected intraperitoneally in the diabetic patient with a functioning kidney graft, and insulin independence with tight glycemic control was demonstrated 9 months after the procedure. Since the report by Soon-Shiong et al., other groups have performed studies with microencapsulated islets in human subjects and have obtained variable results (13, 14, 58). When evaluating these studies, it is very important to consider if the critical factors such as method of encapsulation including adequate perm-selectivity and mechanical strength of the microcapsules, the site of transplantation, and delivery of oxygen to the encapsulated islets were adequately addressed in the design of each study. An on-going clinical trial with encapsulated neonatal pig islets in diabetic patients has shown some promise and is currently in phase II in New Zealand and Argentina (15). One can only hope that with adequate optimization, the microencapsulated islet technology will some day offer a cure for Type 1 diabetes.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge generous financial support from the National Institutes of Health (grant # R01DK080897) and the Vila Rosenfeld Estate, Greenville, NC for the studies on the bioartificial pancreas in Dr. Opara's laboratory.
REFERENCES
- 1.Bliss M. The discovery of insulin. The University of Chicago Press; 1982. [Google Scholar]
- 2.Opara EC, Mirmalek-Sani S-H, Khanna O, Moya ML, Brey EM. Design of a bioartificial pancreas. J Investig Med. 2010;58(7):831–837. doi: 10.231/JIM.0b013e3181ed3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kort HD, Koning EJD, Rabelink TJ, Bruijn JA, Bajema IM. Islet transplantation in type 1 diabetes. BMJ. 2011;342:d217. doi: 10.1136/bmj.d217. [DOI] [PubMed] [Google Scholar]
- 4.THE DIABETES CONTROL AND COMPLICATIONS TRIAL RESEARCH GROUP The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 5.Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery. 1967;61(6):827–37. [PubMed] [Google Scholar]
- 6.Sutherland DE, Radosevich D, Gruessner R, Gruessner A, Kandaswamy R. Pushing the envelope: living donor pancreas transplantation. Curr Opin Organ Transplant. 2012;17(1):106–15. doi: 10.1097/MOT.0b013e32834ee6e5. [DOI] [PubMed] [Google Scholar]
- 7.Robertson RP. Successful islet transplantation for patients with diabetes - fact or fantasy? N Engl J Med. 2000;343(4):289–290. doi: 10.1056/NEJM200007273430409. [DOI] [PubMed] [Google Scholar]
- 8.Sutherland DER, Gruessner RWG, Gruessner AC. Pancreas transplantation for treatment of diabetes mellitus. World Journal of Surgery. 2001;25(4):487–496. doi: 10.1007/s002680020342. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro AMJ, Lakey JRT, Ryan EA, et al. Islet Transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 10.Rothman S, Tseng H, Goldfine I. Oral gene therapy: A novel method for the manufacture and delivery of protein drugs. Diabetes Technology & Therapeutics. 2005;7(3):549–557. doi: 10.1089/dia.2005.7.549. [DOI] [PubMed] [Google Scholar]
- 11.Pareta RA, McQuilling JP, Farney A, Opara EC. Organ Donation. INTECH Publishers; 2012. Bioartificial pancreas: evaluation of crucial barriers to clinical application. pp. 241–266. ISBN 979-953-307-081-9; Chapter 14. [Google Scholar]
- 12.Soon-Shiong P, Feldman E, Nelson R, et al. Successful reversal of spontaneous diabetes in dogs by intraperitoneal microencapsulated islets. Transplantation. 1992;54:769–774. doi: 10.1097/00007890-199211000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Calafiore R, Calabrese G, Basta G, et al. Microencapsulated pancreatic islet allograft into non-immunosuppressed patients with Type 1 diabetes. Diabetes Care. 2006:137–138. doi: 10.2337/diacare.29.1.137. [DOI] [PubMed] [Google Scholar]
- 14.Elliott RB, Escobar L, Tan PLJ, et al. Live encapsulated porcine islets from type 1 diabetic patient 9.5 yr after xenotransplantation. Xenotransplantation. 2007;14:157–161. doi: 10.1111/j.1399-3089.2007.00384.x. [DOI] [PubMed] [Google Scholar]
- 15. http://www.lctglobal.com/
- 16.Smidsrod O, Skjak-Braek G. Alginate as immobilization matrix for cells. Trends in Biotechnology. 1990;8:71–78. doi: 10.1016/0167-7799(90)90139-o. [DOI] [PubMed] [Google Scholar]
- 17.Holtan S, Zhang Q, Strand WI, Skjåk-Braek G. Characterization of the hydrolysis mechanism of polyalternating alginate in weak acid and assignment of the resulting MG-oligosaccharides by NMR spectroscopy and ESI-mass spectrometry. Biomacromolecules. 2006;7(7):2108–21. doi: 10.1021/bm050984q. [DOI] [PubMed] [Google Scholar]
- 18.Uludag H, de Vos P, Tresco PA. Technology of mammalian cell encapsulation. Adv Drug Deliv Rev. 2000;42:29–64. doi: 10.1016/s0169-409x(00)00053-3. [DOI] [PubMed] [Google Scholar]
- 19.Zimmermann U, Mimietz S, Zimmermann H, et al. Hydrogel-based non-autologous cell and tissue therapy. Biotechniques. 2000;29:564–581. doi: 10.2144/00293rv01. [DOI] [PubMed] [Google Scholar]
- 20.Cook DL, Satin LS, Ashford ML, Hales CN. ATP-sensitive K+ channels in pancreatic beta-cells. Spare-channel hypothesis. Diabetes. 1988;37(5):495–8. doi: 10.2337/diab.37.5.495. [DOI] [PubMed] [Google Scholar]
- 21.Garfinkel MR, Harland RC, Opara EC. Optimization of the microencapsulated islet for transplantation. J Surg Res. 1998;76:7–10. doi: 10.1006/jsre.1997.5258. [DOI] [PubMed] [Google Scholar]
- 22.Wandrey C, Espinosa D, Rehor A, Hunkeler D. Influence of alginate characteristics on the properties of multi-component microcapsules. J Microencapsul. 2003;20(5):597–611. doi: 10.1080/0265204031000148022. [DOI] [PubMed] [Google Scholar]
- 23.Kendall WF, Jr., Darrabie MD, El-Shewy H, Opara EC. Effect of composition and purity of alginate on microspheres. J Microencapsul. 2004;21:821–828. doi: 10.1080/02652040400015452. [DOI] [PubMed] [Google Scholar]
- 24.Darrabie MD, Kendall WF, Opara EC. Effect of alginate composition and gelling cation on microbead swelling. J Microencapsul. 2006;23(#1):29–37. doi: 10.1080/02652040600687621. [DOI] [PubMed] [Google Scholar]
- 25.de Vos P, Hoogmoed CG, Busscher HJ. Chemistry and biocompatibility of alginate-PLL capsules for immunoprotection of mammalian cells. J Biomed Mater Res. 2002;60:252–259. doi: 10.1002/jbm.10060. [DOI] [PubMed] [Google Scholar]
- 26.King A, Sandler S, Andersson A. The effect of host factors and capsule composition on the cellular overgrowth on implanted alginate capsules. J Biomed Mater Res. 2001;57:374–383. doi: 10.1002/1097-4636(20011205)57:3<374::aid-jbm1180>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 27.De Vos P, Van Straaten JFM, Nieuwenhuizen AG, et al. Why do microencapsulated islet grafts fail in the absence of fibrotic overgrowth? Diabetes. 1999;48:1381–1388. doi: 10.2337/diabetes.48.7.1381. [DOI] [PubMed] [Google Scholar]
- 28.Darrabie MD, Kendall WF, Opara EC. Characteristics of poly-L-ornithine-coated alginate microcapsules. Biomaterials. 2005;26/34:6846–6852. doi: 10.1016/j.biomaterials.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Thanos CG, Bintz BE, Bell WJ, Qian H, Schneider PA, MacArthur DH, Emerich DF. Intraperitoneal stability of alginate-polyornithine microcapsules in rats: an FTIR and SEM analysis. Biomaterials. 2006;27(19):3570–9. doi: 10.1016/j.biomaterials.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 30.Thanos CG, Calafiore R, Basta G, Bintz BE, et al. Formulating the alginate–polyornithine biocapsule for prolonged stability: Evaluation of composition and manufacturing technique. J Biomed Mater Res A. 2007;83(1):216–24. doi: 10.1002/jbm.a.31472. [DOI] [PubMed] [Google Scholar]
- 31.Khanna O, Moya ML, Opara EC, Brey EM. Synthesis of multi-layered alginate microcapsules for the sustained release of fibroblast growth factor-1. J Biomed Mater Res A. 2010;95(2):632–40. doi: 10.1002/jbm.a.32883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu BR-S, Chen H-C, Fu S-H, et al. The use of field effects to generate calcium alginate microspheres and its application in cell transplantation. J Formos Med Assoc. 1994;93:240–245. [PubMed] [Google Scholar]
- 33.De Vos P, De Haan BJ, Schilfgaarde R. Upscaling the production of microencapsulated pancreatic islets. Biomaterials. 1997;18:1085–1090. doi: 10.1016/s0142-9612(97)00040-9. [DOI] [PubMed] [Google Scholar]
- 34.McQuilling JP, Arenas-Herrera J, Childers C, Pareta RA, Khanna O, Jiang B, Brey EM, Farney AC, Opara EC. New alginate microcapsule system for angiogenic protein delivery and immunoisolation for transplantation in the rat omentum pouch. Transplant Proc. 2011;43:3262–3264. doi: 10.1016/j.transproceed.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tendulkar S, Mirmalek-Sani S-H, Childers C, Saul J, Opara EC, Ramasubramanian MK. A three- dimensional microfluidic approach to scaling up microencapsulation of cells. Biomed Microdevices. 2012 Jan 14; doi: 10.1007/s10544-011-9623-6. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lifson N, Lassa CV, Dixit PK. Relation between blood flow and morphology in islet organ of rat pancreas. Am J Physiol. 1985;249:E43–E48. doi: 10.1152/ajpendo.1985.249.1.E43. [DOI] [PubMed] [Google Scholar]
- 37.Jansson L, Hellerstrom C. Stimulation by glucose of the blood flow to the pancreatic islets of the rat. Diabetologia. 1983;25:45–50. doi: 10.1007/BF00251896. [DOI] [PubMed] [Google Scholar]
- 38.Dionne KE, Colton CK, Yarmush ML. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes. 1993;42:12–21. doi: 10.2337/diab.42.1.12. [DOI] [PubMed] [Google Scholar]
- 39.Davalli AM, Scaglia L, Zangen DH, Hollister J, Bonner-Weir S, Weir GC. Vulnerability of transplanted islets in the immediate posttransplantation period; dynamic changes in structure and function. Diabetes. 1996;45:1161–1167. doi: 10.2337/diab.45.9.1161. [DOI] [PubMed] [Google Scholar]
- 40.Menger MD, Jaeger S, Walter P, et al. Angiogenesis and hemodynamics of microvasculature of transplanted islets of Langerhans. Diabetes. 1989;36(Suppl. 1):199–201. doi: 10.2337/diab.38.1.s199. [DOI] [PubMed] [Google Scholar]
- 41.Omer A, Duvivier-Kali V, Fernandes J, et al. Long-term normoglycemia in rats receiving transplants with encapsulated islets. Transplantation. 2005;79(1):52–58. doi: 10.1097/01.tp.0000149340.37865.46. [DOI] [PubMed] [Google Scholar]
- 42.Ludwig B, Rotem A, Schmid J, Weir GC, Colton CK, et al. Improvement of islet function in a bioartificial pancreas by enhanced oxygen supply and growth hormone agonist. Proc Natl Acad Sci (USA) 2012;109(#13):5022–5027. doi: 10.1073/pnas.1201868109. Epub 2012 March 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pedraza E, Coronel MM, Fraker CA, Ricordi C, Stabler CL. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated oxygen generating biomaterials. Proc Natl Acad Sci (USA) 2012;109(#11):4245–4250. doi: 10.1073/pnas.1113560109. Epub 2012 Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hobbs HA, Kendall WF, Darrabie M, Opara EC. Prevention of morphological changes in alginate microcapsules for xenotransplantation. J Invest Med. 2001;49:572–575. doi: 10.2310/6650.2001.33722. [DOI] [PubMed] [Google Scholar]
- 45.Elliott RB, Escobar L, Garkavenko O, Croxson MC, Schroeder BA, McGregor M, et al. No evidence of infection with porcine endogenous retrovirus in recipients of encapsulated porcine islet xenografts. Cell Transplant. 2000;9(6):895–901. doi: 10.1177/096368970000900616. [DOI] [PubMed] [Google Scholar]
- 46.de Groot M, Schuurs TA, van Schilfgaarde R. Causes of limited survival of microencapsulated pancreatic islet grafts. J Surg Res. 2004;121:141–150. doi: 10.1016/j.jss.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 47.de Vos P, Smedema I, van Goor H, Moes H, et al. Association between macrophage activation and function of micro-encapsulated rat islets. Diabetologia. 2003;46(5):666–673. doi: 10.1007/s00125-003-1087-7. [DOI] [PubMed] [Google Scholar]
- 48.Safley SA, Kapp LM, Tucker-Burden C, Hering B, Kapp JA, Weber CJ. Inhibition of cellular immune responses to encapsulated porcine islet xenografts by simultaneous blockade of two different costimulatory pathways. Transplantation. 2005;79(4):409–418. doi: 10.1097/01.tp.0000150021.06027.dc. [DOI] [PubMed] [Google Scholar]
- 49.de Vos P, Vegter D, de Haan BJ, Strubbe JH, Bruggink JE, VanSchilfgaarde R. Kinetics of intraperitoneally infused insulin in rats - Functional implications for the bioartificial pancreas. Diabetes. 1996;45(8):1102–1107. doi: 10.2337/diab.45.8.1102. [DOI] [PubMed] [Google Scholar]
- 50.Toso C, Mathe Z, Morel P, Oberholzer J, et al. Effect of microcapsule composition and short-term immunosuppression on intraportal biocompatibility. Cell Transplantation. 2005;14(2-3):159–167. doi: 10.3727/000000005783983223. [DOI] [PubMed] [Google Scholar]
- 51.Dufrane D, Goebbels RM, Saliez A, Guiot Y, Gianello P. Six-month survival of microencapsulated pig islets and alginate biocompatibility in primates: Proof of concept. Transplantation. 2006;81(9):1345–1353. doi: 10.1097/01.tp.0000208610.75997.20. [DOI] [PubMed] [Google Scholar]
- 52.Dufrane D, Steenberghe M, Goebbels RM, Saliez A, Guiot Y, Gianello P. The influence of implantation site on the biocompatibility and survival of alginate encapsulated pig islets in rats. Biomaterials. 2006;27(17):3201–3208. doi: 10.1016/j.biomaterials.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 53.Kobayashi T, Aomatsu Y, Iwata H, Kin T, Kanehiro H, Hisanga M, et al. Survival of microencapsulated islets at 400 days posttransplantation in the omental pouch of NOD mice. Cell Transplant. 2006;15(4):359–365. doi: 10.3727/000000006783981954. [DOI] [PubMed] [Google Scholar]
- 54.Moya ML, Garfinkel MR, Liu X, Lucas S, Opara EC, Greisler HG, Brey EM. Fibroblast growth factor-1 (FGF-1) loaded microbeads enhance local capillary neovascularization. J Surg Res. 2010;160:208–212. doi: 10.1016/j.jss.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Vos P, Hamel AF, Tatarkiewicz K. Considerations for successful transplantation of encapsulated pancreatic islets. Diabetologia. 2002;45(2):159–173. doi: 10.1007/s00125-001-0729-x. [DOI] [PubMed] [Google Scholar]
- 56.Kin T, Korbutt GS, Rajotte RV. Survival and metabolic function of syngeneic rat islet grafts transplanted in the omental pouch. Am J Transplant. 2003;3(3):281–285. doi: 10.1034/j.1600-6143.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- 57.Lim F, Sun A. Microencapsulated islets as bioartificial pancreas. Science. 1980;210:908–910. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 58.Tuch BE, Keogh GW, Williams LJ, Wu W, et al. Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diabetes Care. 2009;32(10):1887–1889. doi: 10.2337/dc09-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]