Abstract
Hsp70 ATP binding induces substrate release, but the transiency of this state has inhibited its characterization. In this issue, Kityk et al. determine the Hsp70*ATP structure utilizing engineered disulfide bonds, providing insights into the workings of this essential molecular machine.
The study of transient protein conformations can be as important as the characterization of long-lived states, as the functional significance of a particular conformation is not determined by its persistence. This is certainly true for Hsp70s, a family of protein chaperones whose nucleotide regulated cycles of protein substrate binding mediate many of the cellular proteostatic and protein processing reactions from bacteria to humans. Hsp70s are comprised of a nucleotide binding domain (NBD) and a protein substrate binding domain (SBD). When ADP is bound to the NBD, the SBD holds tightly to protein substrates, which bind in a β sandwich pocket (SBDβ) capped by an α-helical lid (SBDα; Figure 1A). Upon release of ADP and binding of ATP– a reaction catalyzed by nucleotide exchange factors–the SBD releases its bound substrate. Binding of a new substrate, presented to the Hsp70 by a J cochaperone, stimulates ATP hydrolysis, resulting in a long-lived Hsp70*ADP*substrate complex. Structures of Hsp70s in apo- or ADP-bound states (Bertelsen et al., 2009) reveal the SBD in same tight binding (closed) conformation seen with an isolated SBD (Zhu et al., 1996), indicating that it is the ATP-bound NBD that induces conformational changes in the SBD (Swain et al., 2006). But a crystal structure of the Hsp70 ATP state had proven impossible to obtain, until now. In this issue of Molecular Cell, Mayer and colleagues captured the HSP*ATP state for crystallization by engineering disulfide bonds to lock in this normally transient conformation (Kityk et al., 2012). This is often a perilous path, as the engineering itself may artificially constrain the resulting conformation. However, these investigators also carry out solution experiments that validate their structure and provide further insight into the mechanism of this molecular machine.
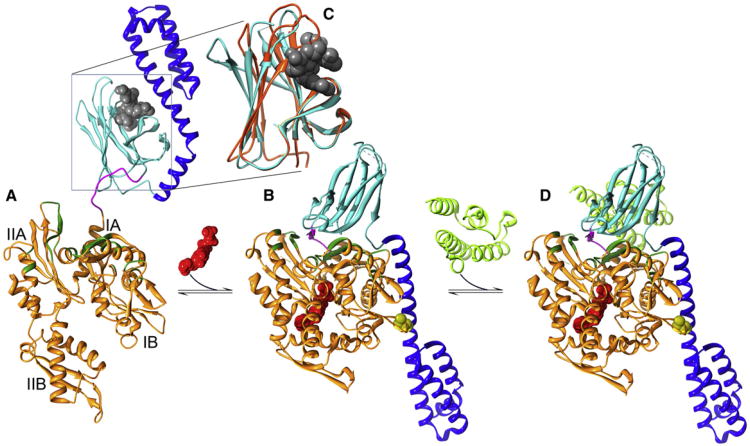
Figure 1. Conformational Transitions in the Hsp70 Functional Cycle.
(A) In the ADP/apo state, substrates (gray) are bound within the SBD β sandwich (cyan) and capped by the SBD helical lid (blue; Protein Data Bak [PDB] 2KHO [Bertelsen et al., 2009] with the substrate imported from PDB 1DKX [Zhu et al., 1996]).
(B) Upon binding ATP (red), the NBD (orange) closes, leading to widening of the crevice between subdomains 1A and IIA, allowing the NBD-SBD linker (magenta) to bind within it. Together with other changes in the disposition of NBD regions (in green) involved in interactions with SBDβ, this creates a surface on which SBDβ docks, leading to displacement of SBDα, which docks onto subdomain 1B (PDB 4B9Q [Kityk et al., 2012]; cysteines introduced to create a disulfide to lock in this conformation for crystallization are in yellow).
(C) Closed SBDβ (orange) wraps around bound substrates but is induced to open (cyan) and release substrate by interactions with NBD*ATP. Substrate rebinding induces SBDβ closure and transmits an ATPase stimulating signal to the NBD through the NBD:SBDβ interface.
(D) The ATPase stimulating signal is amplified by the J cochaperone (in light green and imported from PDB 2QWN [Jiang et al., 2007]), which binds so as to hold SBDβ and the linker against the NBD.
So how does HSP70 work? Befitting such an ancient protein, the Hsp70 NBD exhibits the familiar hexokinase/actin fold, and the ATP-induced conformational change in the NBD recapitulates the historically important induced fit change–predicted by Koshland, seen by Steitz–driven by glucose binding to hexokinase. In the case of both HSP70 and hexokinase, binding of the ligand in the center of the horseshoe-shaped protein domain induces it to close around that ligand. With Hsp70, this involves clockwise rotation (as defined by the view in Figure 1) of NBD lobe II (subdomains IIA and IIB), resulting in closure of the space between subdomains IB and IIB and widening of a crevice between subdomains IA and IIA. Widening of this crevice allows the linker that connects NBD and SBD to bind within it and, combined with changes in the relative orientation of subdomains IA and IIA, creates a surface on the NBD to which SBDβ binds. Binding of SBDβ to this surface displaces interactions between SBDβ and SBDα and sterically pushes SBDα away from SBDβ. The displaced SBDα settles onto a site on subdomain IB of the NBD (Figure 1B).
It was the interaction of SBDα and the subdomain IB of the NBD that Kityk et al. (2012) took advantage of to create their locked in ATP conformation. To guide their engineering, they modeled their bacterial Hsp70 (DnaK) on yeast Hsp110 (aka Sse1). Hsp110 is a distant Hsp70 homolog that acts as nucleotide exchange factor for Hsp70 and that also binds misfolded proteins. However, a key functional difference is that Hsp110 does not form long-lived complexes with substrates like Hsp70 does. The structure of ATP bound yeast Hsp110 had been previously determined and proposed to model the Hsp70 ATP state (Zhu et al., 1996). Kityk at al. introduced a cysteine at NBD residue 47 and paired it with cys substitutions at seven different positions on SBDα. Efficient disulfide formation was seen only for the two cys pairs (E47C/D526C and E47C/F529C) predicted by the Hsp110-based model to be closest. As expected, disulfide bond formation was enhanced by ATP binding. Additional evidence that these disulfidelinked species capture a relevant conformation is that, in their oxidized forms, they exhibit endogenous tryptophan fluorescence similar to that seen when WT Hsp70 binds ATP. In addition, ATP hydrolysis by E47C/F529C, in both its reduced and oxidized forms, is synergistically stimulated by substrate and the J cochaperone in a manner similar to that of the WT enzyme. It was oxidized E47C/F529C, in the presence of ATP and containing a mutation (T199A) to slow ATP hydrolysis, that yielded diffraction quality crystals.
Kityk et al. (2012) also characterize the kinetics of these ATP-driven transitions and find that the first step is binding of the linker in the crevice between subdomains IA and IIA, followed by docking of SBDβ, release of bound substrate, and, lastly, docking of SBDα onto the NBD. The release of substrates from Hsp70 is therefore understood to be due to the opening of the SBD that occurs when SBDα is driven off of SBDβ by the latter binding to the ATP stabilized NBD conformation. It is surprising, then, that Kityk et al., find that in the absence of ATP, SBDα becomes disulfide linked to the NBD at a rate only 2-fold slower than seen in the presence of ATP. Though it is difficult to interpret these rates in terms of differences in the populations of different conformations, this result is consistent with studies indicating that in the ADP/apo state the Hsp70 conformational ensemble includes substantial populations of molecules in which the SBD and NBD interact and that ATP merely increases this interacting population (Marcinowski et al., 2011; Schlecht et al., 2011). However, it is hard to understand how a few-fold increase in the population of Hsp70 molecules in which the NBD and SBD interact and the SBD is open can explain the 1,000-fold increase in substrate dissociation rates seen when ATP binds Hsp70 (Schmid et al., 1994). It is therefore possible that, even when SBD interacts with ADP/Apo NBD and SBDα and SBDβ separate, the conformational changes required for rapid substrate release are not fully induced in the SBD. Indeed, comparison of closed and open SBD conformations reveals that the open conformation differs from the closed not merely in that SBDα has moved, but also in the conformation of SBDβ, which displays a widened pocket that allows rapid substrate dissociation (Figure 1C). SBDβ interactions with ADP/apo NBD may not elicit these same conformational changes and, therefore, may not lead to substrate release.
Conformational changes in SBDβ, induced by substrate binding and transmitted to the NBD across the SBDβ:NBD interface, may also explain how substrate binding stimulates ATP hydrolysis and how the J cochaperone amplifies this stimulation by binding to Hsp70 in a manner that holds SBDβ and the linker against the NBD (Figure 1D). SBDα also plays a role in modulating ATP hydrolysis: Kityk et al., find that hydrolysis by oxidized E47C/F529C is stimulated by substrate and J cochaperone to a lesser degree than WT or reduced E47C/F529C Hsp70, from which they conclude that fast ATP hydrolysis requires that SBDα undock from the NBD. Because the rate of SBDα docking to the NBD is slower than substrate dissociation, they propose further that this provides Hsp70 with time to release the bound substrate and bind a new substrate before ATP is hydrolyzed. These mechanistic hypotheses may perhaps be believed: after all, they reveal an Hsp70 that can dance (Nietzsche, 1907).
References
- Bertelsen EB, Chang L, Gestwicki JE, Zuiderweg ER. Proc Natl Acad Sci USA. 2009;106:8471–8476. doi: 10.1073/pnas.0903503106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Maes EG, Taylor AB, Wang L, Hinck AP, Lafer EM, Sousa R. Mol Cell. 2007;28:422–433. doi: 10.1016/j.molcel.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kityk R, Kopp J, Sinning I, Mayer MP. Mol Cell. 2012;48:863–874. doi: 10.1016/j.molcel.2012.09.023. this issue. [DOI] [PubMed] [Google Scholar]
- Marcinowski M, Höller M, Feige MJ, Baerend D, Lamb DC, Buchner J. Nat Struct Mol Biol. 2011;18:150–158. doi: 10.1038/nsmb.1970. [DOI] [PubMed] [Google Scholar]
- Nietzsche FW. Tak govoril Zaratustra 1907 [Google Scholar]
- Schlecht R, Erbse AH, Bukau B, Mayer MP. Nat Struct Mol Biol. 2011;18:345–351. doi: 10.1038/nsmb.2006. [DOI] [PubMed] [Google Scholar]
- Schmid D, Baici A, Gehring H, Christen P. Science. 1994;263:971–973. doi: 10.1126/science.8310296. [DOI] [PubMed] [Google Scholar]
- Swain JF, Schulz EG, Gierasch LM. J Biol Chem. 2006;281:1605–1611. doi: 10.1074/jbc.M509356200. [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhao X, Burkholder WF, Gragerov A, Ogata CM, Gottesman ME, Hendrickson WA. Science. 1996;272:1606–1614. doi: 10.1126/science.272.5268.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]