Abstract
The authors sought to determine whether nebivolol treatment results in changes in blood pressure (BP), nitric oxide bioavailability, and vascular function in obese African Americans with recently diagnosed stage 1 hypertension. Forty‐three obese, hypertensive African Americans (mean BP: systolic, 148.8±14.3 mm Hg; diastolic, 90.4±8.2 mm Hg) were treated with nebivolol (5–10 mg/d) for 8 weeks. Primary outcomes were change in systolic and diastolic BP and efficacy in reaching normotensive BP. Mean systolic BP decreased by 9.2±14 mm Hg (P<.005) and diastolic BP decreased 6.8±9 mm Hg (P<.005) with 8 weeks of therapy. Significant improvements were seen in arterial compliance with nebivolol treatment as measured by aortic augmentation index (P<.005) and time to wave reflection (P=.013). Nebivolol treatment improved endothelial function as measured by flow‐mediated dilation (P<.005). Levels of erythrocyte cellular superoxide dismutase increased with nebivolol, indirectly suggesting increased bioavailability of nitric oxide (P<.005). Monotherapy with nebivolol in obese, hypertensive African Americans results in significant systolic and diastolic BP reduction by mechanisms that include improved vascular function and compliance.
Hypertension is a leading risk factor for morbidity and mortality globally, and approximately 13% of US deaths in 2000 were due to uncontrolled hypertension. 1 , 2 The current World Health Organization (WHO) definition of hypertension is the persistent elevation of blood pressure (BP) above 140/90 mm Hg at rest, on 3 separate measurements. 3 Hypertension can be a silent disease, rarely resulting in acute symptoms. However, if hypertension is not treated it can result in complications including coronary heart disease, heart failure, stroke, kidney disease, and mortality linked to cardiovascular disease. It has been estimated that approximately 50% of all hypertensive cases in the United States are not controlled. 3
Hypertension prevalence is highly variable among populations worldwide. In the United States there is a disproportionate burden of this disease and its complications in African Americans. 4 According to the 2003 to 2004 National Health and Nutrition Examination Survey (NHANES), hypertension prevalence is 39.1% in African Americans and 28.5% in white Americans. 5 African Americans have the highest prevalence of hypertension in the world, significantly higher than people of African origin living outside the United States. 6 Thus, the increased prevalence has been attributed to both genetic and environmental factors. 7 In addition, hypertension is usually observed at a younger age in African Americans and results in more severe disease complications. This finding results in a significantly higher hypertension‐related mortality rate for African Americans, 49.9% and 40.6% for African American men and women, respectively, compared with 17.9% for the overall US population in 2004. 4
Although medical intervention has been effective in some segments of the hypertensive population, <30% of African Americans achieve BP control with medications. 5 In addition, African Americans are frequently underrepresented in hypertension clinical trials, thereby limiting the applicability of the results to these patients. 8 Due to the high prevalence of hypertension and low efficacy of antihypertensive medications in African Americans, as well as the lack of data in this population, this study was limited to African American patients.
This study evaluates the effects of nebivolol therapy in obese, hypertensive African Americans. Obesity was an inclusion criteria for this study as approximately 30% of African Americans are obese, 5 and obesity and hypertension are hallmarks of the metabolic syndrome. Nebivolol is a highly cardioselective vasodilatory β1‐receptor blocker that lowers BP by reducing peripheral vascular resistance 9 and significantly increases stroke volume 10 with preservation of cardiac output 11 when compared with atenolol, another β1‐receptor blocker. The effects of nebivolol on systolic and diastolic BP and on endothelial function, arterial compliance, and erythrocyte superoxide dismutase (EC SOD), a biomarker of oxidation, were studied.
Methods
Participants
Obese African American men and women between the ages of 18 and 75 years (mean age 50.0 years) with recently diagnosed stage 1 hypertension were enrolled in the study. Obesity was defined as body mass index >30 kg/m2. Stage 1 hypertension was defined as a systolic BP of 140 to 159 mm Hg and diastolic BP of 90 to 99 mm Hg. Patients were excluded if they had any of the following: diabetes mellitus (positive glucose tolerance test or fasting glucose >126 mg/dL, hemoglobin A1c >8.0, or taking antidiabetic medications); known coronary heart disease, cerebrovascular disease, peripheral vascular disease, or renovascular disease; known history of cirrhosis or a serum total bilirubin level ≥10.0 mg/dL); history of chronic renal failure requiring dialysis or significant renal dysfunction (defined as serum creatinine >3.5 mg/dL and not receiving continuous renal replacement therapy or requiring acute hemodialysis; current smoker; uncontrolled hypertension (BP >179/110 mm Hg); uncontrolled hyperlipidemia (low‐density lipoprotein cholesterol >190 mg/dL); initiation of over‐the‐counter vitamins such as Vitamin A, C, or E within 6 months of study initiation; inability to walk on a treadmill because of arthritic conditions; reactive airways disease (including asthma) that required any regular medical therapy (including daily use of inhalers, oral medications, steroid therapy) or supplemental oxygen therapy; pregnant or nursing women; history of human immunodeficiency virus or acquired immune deficiency syndrome; and mental condition rendering the patient unable to understand the scope and possible consequences of the study. Women of childbearing age were required to use an acceptable method of birth control. All patients were informed of the purpose of this study and written informed consent was obtained from all participants. Complete confidentiality for each patient was provided throughout the study process.
Study Design
Enrolled patients were placed on 5 mg/d of nebivolol therapy and the dose was increased to 10 mg/d in patients whose BP did not normalize (<130 mm Hg systolic; <85 mm Hg diastolic) with 2 weeks of therapy on the lower dose. Patients were taking nebivolol therapy for a total of 8 weeks. Patients were provided physical activity and nutrition information sheets at each visit. Fasting blood samples were drawn at baseline, week 4, and week 8 at the same time of day. Changes in systolic and diastolic BP were the primary end points. BP was measured at baseline and 2, 4, 6, and 8 weeks. Brachial artery reactivity, arterial stiffness, exercise treadmill testing, and collection of urine sample were performed at baseline and at week 8. Pill counts were obtained at each visit to determine compliance. The study protocol complies with the Declaration of Helsinki and was approved by the institutional review board before its implementation.
Measurement of BP
An Omron sphygmomanometer (Schaumburg, IL) was used to measure systolic and diastolic BP. Measurements were taken at 5‐minute intervals and a total of 3 readings were obtained at each visit. The average of the 3 measurements was calculated for each visit.
Laboratory Analysis
Fasting blood samples were obtained to determine complete blood cell count, lipid profile, and levels of EC SOD. Plasma samples were centrifuged and stored at −80°C.
EC SOD Analysis
Activity of EC SOD was determined using hemolysates and commercially available kits. Briefly, superoxide radicals produced by xanthine and xanthine oxidase react with 2‐(4‐iodophenyl)‐3‐(4‐nitrophenol)‐5‐phenyltertazolium chloride to form a red formazan dye. 12 The EC SOD activity is then measured by the degree of inhibition of this reaction. EC SOD activity is expressed as units per mL. 13
Endothelial Function
Evaluation of endothelial function was made noninvasively using brachial artery reactivity testing, which uses ultrasound to evaluate endothelium‐dependent flow‐mediated dilation (FMD). The same vascular technician conducted the testing in all patients through the course of the study. Briefly, patients were positioned in the supine position with the arm in a comfortable position for imaging the brachial artery. A BP cuff was placed on the forearm, after which a baseline rest image was acquired using an ultrasonography machine with a high‐resolution 11‐mHz probe. The brachial artery was imaged above the antecubital fossa in the longitudinal plane. A segment with clear anterior and posterior intimal interfaces between the lumen and vessel wall was selected for continuous 2‐dimensional gray‐scale imaging. Blood flow velocity was estimated by time averaging the pulsed Doppler velocity signal obtained from a mid‐artery sample volume. The cuff was then inflated to ≥50 mm Hg above systolic BP to occlude arterial flow for 5 minutes. After cuff deflation, the longitudinal image of the artery was continuously recorded from 30 seconds before to 2 minutes after cuff deflation. A mid‐artery pulsed Doppler signal was obtained on immediate cuff release and no later than 15 seconds after cuff deflation to access hyperemic velocity. After 15 minutes, nitroglycerin 0.4 mg was given sublingually, and repeat images were obtained to determine endothelium‐independent vasodilation.
The diameter of the brachial artery was measured from longitudinal images in which the lumen‐intima interface was visualized on both the near (anterior) and far (posterior) walls. Once the image for analysis was chosen, the boundaries for diameter measurement were manually identified with electronic calipers (Medical Imaging Application Vascular Tools). The average diameter was determined from at least 3 different diameter measurements determined along a segment of the vessel. Brachial artery diameter was measured at the same time in the cardiac cycle by use of electrocardiography gating during image acquisition. FMD was measured as the percent change in diameter in response to reactive hyperemia or to nitroglycerin.
Arterial Stiffness
Arterial stiffness was assessed by means of pulse wave velocity and radial pulse wave analysis using the SphygmoCor Pulse Wave Velocity system (AtCor Medical, West Ryde, N, Australia) as described in the Conduit Artery Function Evaluation (CAFE) study. 14 Augmentation index (AIx) was defined as the ratio of augmentation to central pulse pressure and is expressed as a percentage; AIX = (ΔP/PP)×100, where P is pressure and PP is pulse pressure.
Statistical Analysis
Statistical analysis was performed using 2‐sample proportion test. All analyses were tested as 2‐sided, and P values <.05 were considered statistically significant.
Results
Study Demographics
Forty‐three participants were enrolled, of which 33 (15 men and 18 women) completed the 8‐week study. The treatment was well tolerated, and unwillingness to continue was the main reason for dropping out of the study. The follow‐up of all patients was 100% complete. None of the patients dropped out of the study due to side effects from the treatment. The Table shows patient characteristics and baseline demographics for those who completed the study.
Table.
Patient Demographics and Baseline Characteristics
| Women, No. (%) | 18 (54.5) |
| Age, y | 50.03 (12.56) |
| SBP, mm Hg | 148.8 (14.3) |
| DBP, mm Hg | 90.4 (8.2) |
| BMI, kg/m2 | 36.48 (5.36) |
| Total cholesterol, mg/dL | 189.28 (36.75) |
| LDL cholesterol, mg/dL | 112.56 (35.56) |
| HDL cholesterol, mg/dL | 52.09 (14.26) |
| Triglycerides, mg/dL | 123.38 (63.90) |
| Glucose, mg/dL | 93.84 (14.53) |
| Creatinine, mg/dL | 0.94 (0.19) |
| Bilirubin, mg/dL | 0.57 (0.20) |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SBP, systolic blood pressure. Data are mean (standard deviation) or No. (%).
Analysis of BP With Nebivolol Treatment
Figure 1 displays the systolic and diastolic BP change in patients with nebivolol treatment. Mean systolic BP decreased by 9.2±14 mm Hg and diastolic BP decreased by 6.8±9 mm Hg with 8 weeks of treatment (P<.005).
Figure 1.
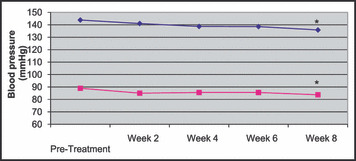
Systolic and diastolic blood pressure (mm Hg) before initiation of treatment and at 2, 4, 6, and 8 weeks of nebivolol treatment. * P<.005. ⋄, systolic; □, diastolic.
Analysis of Endothelial Function and Arterial Compliance With Nebivolol Treatment
With 8 weeks of nebivolol therapy, FMD of the brachial artery significantly increased as shown in Figure 2 (pretreatment: 3.4±0.4%, posttreatment: 11.0±1.3%; P<.005). Improvements were also seen in arterial compliance with nebivolol treatment. Figure 3a shows that aortic AIx significantly decreases with treatment (pretreatment: 16.6±2.2%, posttreatment: 11.1±1.7%; P<.005). There was a significant decrease in time to wave reflection with treatment as shown in Figure 3b (pretreatment: 164±22 ms, posttreatment: 137±16 ms; P=.013).
Figure 2.
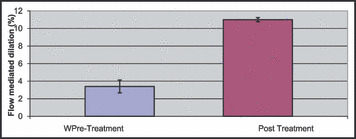
Change in flow‐mediated dilation (%) of the brachial artery before (pre) and after (post) nebivolol treatment. * P<.005.
Figure 3.
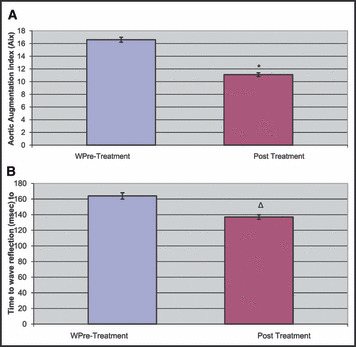
Change in aortic augmentation index (%) and time to wave reflection (milliseconds) before (pre) and after (post) nebivolol treatment. * P<.005. Δ P=.01.
Analysis of Antioxidant Defense With Nebivolol Treatment
Activity of EC SOD, an antioxidative marker, increased significantly with nebivolol treatment, as shown in Figure 4 (pretreatment: 465.2±50.6 U/mL, posttreatment: 537.4±54.4 U/mL; P<.005).
Figure 4.
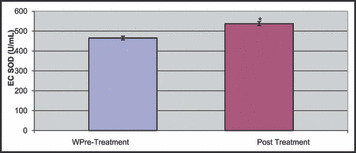
Erythrocyte superoxide dismutase (EC SOD) activity level changes before (pre) and after (post) nebivolol treatment. * P<.005.
Discussion
Although medical treatment has been effective in the treatment of hypertension, the incidence of this disorder continues to be high, particularly in African American patients. 15 , 16 This study shows a substantial benefit with nebivolol monotherapy in obese, hypertensive African American patients. Systolic and diastolic BP reductions were significant and patients were able to reach normotensive BP levels with therapy. Furthermore, effects including improved endothelial function and arterial compliance were observed. Nebivolol treatment also increased levels of EC SOD, thereby suggesting defense against oxidation. 17
Nebivolol is a highly cardioselective vasodilatory β1‐receptor blocker, and it has recently been approved in the United States for the treatment of hypertension. 18 , 19 It lowers BP by reducing peripheral vascular resistance and significantly increases stroke volume with preservation of cardiac output. 9 , 10 , 11 The net hemodynamic effect of nebivolol is the result of a balance between the depressant effects of β‐blockade and an action that maintains cardiac output. 9 , 10 , 11 It is thought that the antioxidative and nitric oxide–mediated mechanisms of action observed in nebivolol may be beneficial in reducing the progression of insulin resistance, diabetes mellitus, and cardiovascular diseases. 20 , 21
The present findings indicate that nebivolol therapy increased levels of erythrocyte superoxide dismutase in our study population. As an indirect marker of nitric oxide bioavailability, an increased level of EC SOD may be indicative of mechanism(s) of action of nebivolol. Furthermore, enhanced levels of EC SOD appear to induce an inhibitory effect against pro‐oxidative species, 17 suggesting a vasoprotective effect and overall enhanced vascular function. In addition, increased EC SOD activity with nebivolol may indicate increased nitric oxide bioavailability in the vasculature, making this a possible mechanism by which improvements in arterial compliance and endothelial function are achieved with nebivolol therapy.
Increased cardiovascular reactivity to stress, reported in young normotensive African Americans, may contribute to their greater susceptibility to hypertension. 22 More recently, research has focused on potential differences in arterial wall function between African Americans and Caucasians and their impact on vascular homeostasis. 23 Studies have demonstrated reduced nitric oxide–mediated vasodilation of forearm resistance vessels to mental stress and to endothelium‐dependent and ‐independent pharmacologic and physiologic stimuli. FMD was significantly lower in age‐matched African Americans compared with Caucasians (4.8% vs 8.9%, respectively; P<.0001). Nitroglycerin‐mediated dilation was also significantly lower in African Americans compared with whites (11% vs 15%, respectively; P<.0002). Thus, healthy African American men and women show reduced responsiveness of blood vessels to both endogenous and exogenous nitric oxide compared with whites. Body mass index correlated inversely with endothelium‐independent responses in several studies. 22 , 23 These findings expand our understanding of racial differences in vascular function and indicate a mechanistic explanation for the increased incidence and severity of cardiovascular disease observed in African Americans. This diminished response to vasodilators may result in a decreased pattern of hemodynamic reactivity that leads, in the long term, to increased vascular tone and hypertension. Importantly, the impact of the metabolic syndrome demonstrates progressive impairment of FMD with increasing exposure to metabolic syndrome risk factors. 24
Lifestyle changes, including an increased prevalence of obesity and the metabolic syndrome, contribute to the incidence of hypertension. 25 , 26 At the environmental level, barriers to healthy lifestyles include lack of access to exercise facilities at work or in the community, lack of bicycle and walking paths, and high traffic and crime in urban settings, which prevent access to safe walking areas. Seasonal variation, market availability, and affordability of fresh fruits and vegetables in small urban stores are issues, thus multilevel approaches incorporating both individual and policy level changes are advocated. While environmental change is beyond the scope of this proposal, problem solving to access existing resources will be reinforced. For individual African Americans, overall perceived and real barriers to engage in exercise and healthy diets may outweigh perceived positive outcomes of total lifestyle changes, underscoring the importance of individual effective problem solving to reduce barriers and information to influence outcome expectancies. 27 , 28 These important culturally relevant factors were incorporated into the nutrition and physical activity educational materials, which were provided to patients at each study visit.
Limitations
Our investigation is a single‐center, short‐term study (8 weeks) to determine the effects of nebivolol therapy on BP and vascular function changes. As an open‐label study, all study patients received treatment and there was no control group. Additionally, nebivolol is indicated only for hypertension even though our data suggests that this drug may also be efficacious in treating the components of the metabolic syndrome.
Conclusions
As many as 30% of adult African Americans are obese and may have characteristics of the metabolic syndrome (a constellation of factors that include obesity, hypertension, dyslipidemia, and diabetes). 22 , 29 , 30 These findings imply that the use of nebivolol by obese, hypertensive African Americans results in significant BP reduction as well as positive vascular changes, thereby protecting against the development of cardiovascular and renal disease. Therefore, such an agent would have therapeutic effects in treating BP and components of the metabolic syndrome.
Acknowledgment and disclosures: The authors thank Tahir Haque and Ibis Bridges of the Atlanta Vascular Research Foundation, Atlanta, GA, for their role in the preparation of this manuscript. This study was supported by the National Institutes of Health grant (DK‐ R03DK073190). Also, the investigators of the study received an unrestricted grant and the study drug (nebivolol) from Forest Pharmaceuticals. Drs Khan and Ferdinand are on the Scientific Advisory Board of Forest Pharmaceuticals.
References
- 1. Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–223. [DOI] [PubMed] [Google Scholar]
- 2. Lawes CM, Vander Hoorn S, Law MR, et al. Blood pressure and the global burden of disease 2000. Part II: estimates of attributable burden. J Hypertens. 2006;24(3):423–430. [DOI] [PubMed] [Google Scholar]
- 3. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206–1252. [DOI] [PubMed] [Google Scholar]
- 4. Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics – 2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115(5):e69–e171. [DOI] [PubMed] [Google Scholar]
- 5. Ong KL, Cheung BM, Man YB, et al. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension. 2007;49(1):69–75. [DOI] [PubMed] [Google Scholar]
- 6. Cooper R, Rotimi C. Hypertension in blacks. Am J Hypertens. 1997;10(7 Pt 1):804–812. [DOI] [PubMed] [Google Scholar]
- 7. Lawes CM, Vander Hoorn S, Law MR, et al. Blood pressure and the global burden of disease 2000. Part 1: estimates of blood pressure levels. J Hypertens. 2006;24(3):413–422. [DOI] [PubMed] [Google Scholar]
- 8. Ferdinand KC. Cardiovascular disease and African Americans: why determination of race is inadequate for research and practice. J Natl Med Assoc. 2007;99(6):686–689. [PMC free article] [PubMed] [Google Scholar]
- 9. Van de Water A, Janssens W, Van Neuten J, et al. Pharmacological and hemodynamic profile of nebivolol, a chemically novel, potent, and selective beta 1‐adrenergic antagonist. J Cardiovasc Pharmacol. 1988;11(5):552–563. [DOI] [PubMed] [Google Scholar]
- 10. Kamp O, Sieswerda GT, Visser CA. Comparison of effects on systolic and diastolic left ventricular function of nebivolol versus atenolol in patients with uncomplicated essential hypertension. Am J Cardiol. 2003;92(3):344–348. [DOI] [PubMed] [Google Scholar]
- 11. Ritter JM. Nebivolol: endothelium‐mediated vasodilating effect. J Cardiovasc Pharmacol. 2001;38(suppl 3):S13–S16. [DOI] [PubMed] [Google Scholar]
- 12. Erel O, Kocyigit A, Bulut V, Gurel MS Reactive nitrogen and oxygen intermediates in patients with cutaneous leishmaniasis. Mem Inst Oswaldo Cruz. 1999;94(2):179–183. [DOI] [PubMed] [Google Scholar]
- 13. McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969;244(22):6049–6055. [PubMed] [Google Scholar]
- 14. Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure‐lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113(9):1213–1225. [DOI] [PubMed] [Google Scholar]
- 15. Cutler JA, Sorlie PD, Wolz M, et al. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension. 2008;52(5):818–827. [DOI] [PubMed] [Google Scholar]
- 16. Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290(2):199–206. [DOI] [PubMed] [Google Scholar]
- 17. Landmesser U, Merten R, Spiekermann S, et al. Vascular extracellular superoxide dismutase activity in patients with coronary artery disease: relation to endothelium‐dependent vasodilation. Circulation. 2000;101(19):2264–2270. [DOI] [PubMed] [Google Scholar]
- 18. Cockcroft JR, Chowienczyk PJ, Brett SE, et al. Nebivolol vasodilates human forearm vasculature: evidence for an L‐arginine/NO‐dependent mechanism. J Pharmacol Exp Ther. 1995;274(3):1067–1071. [PubMed] [Google Scholar]
- 19. Van Bortel LM, Breed JG, Joosten J, et al. Nebivolol in hypertension: a double‐blind placebo‐controlled multicenter study assessing its antihypertensive efficacy and impact on quality of life. J Cardiovasc Pharmacol. 1993;21(6):856–862. [PubMed] [Google Scholar]
- 20. Taddei S, Virdis A, Ghiadoni L, Salvetti A The role of endothelium in human hypertension. Curr Opin Nephrol Hypertens. 1998;7(2):203–209. [DOI] [PubMed] [Google Scholar]
- 21. Tzemos N, Lim PO, MacDonald TM. Nebivolol reverses endothelial dysfunction in essential hypertension: a randomized, double‐blind, crossover study. Circulation. 2001;104(5):511–514. [DOI] [PubMed] [Google Scholar]
- 22. Hall WD, Clark LT, Wenger NK, et al. The Metabolic Syndrome in African Americans: a review. Ethn Dis. 2003;13(4):414–428. [PubMed] [Google Scholar]
- 23. Zion AS, Bond V, Adams RG, et al. Low arterial compliance in young African‐American males. Am J Physiol Heart Circ Physiol. 2003;285(2):H457–H462. [DOI] [PubMed] [Google Scholar]
- 24. Cardillo C, Kilcoyne CM, Cannon RO III, Panza JA Impairment of the nitric oxide‐mediated vasodilator response to mental stress in hypertensive but not in hypercholesterolemic patients. J Am Coll Cardiol. 1998;32(5):1207–1213. [DOI] [PubMed] [Google Scholar]
- 25. Greenlund KJ, Daviglus ML, Croft JB. Differences in healthy lifestyle characteristics between adults with prehypertension and normal blood pressure. J Hypertens. 2009;27(5):955–962. [DOI] [PubMed] [Google Scholar]
- 26. Nguyen NT, Magno CP, Lane KT, et al. Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: findings from the National Health and Nutrition Examination Survey, 1999 to 2004. J Am Coll Surg. 2008;207(6):928–934. [DOI] [PubMed] [Google Scholar]
- 27. Martin MY, Person SD, Kratt P, et al. Relationship of health behavior theories with self‐efficacy among insufficiently active hypertensive African‐American women. Patient Educ Couns. 2008;72(1):137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hildreth C, Saunders E. Hypertension in blacks: clinical overview. Cardiovasc Clin. 1991;21(3):85–96. [PubMed] [Google Scholar]
- 29. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) . Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 30. Hutchinson RG, Watson RL, Davis CE, et al. Racial differences in risk factors for atherosclerosis. The ARIC Study. Atherosclerosis risk in communities. Angiology. 1997;48(4):279–290. [DOI] [PubMed] [Google Scholar]


