Summary
During pancreas development, endocrine and exocrine cells arise from a common multipotent progenitor pool. How these cell fate decisions are coordinated with tissue morphogenesis is poorly understood. Here we have examined ductal morphology, endocrine progenitor cell fate and Notch signaling in Ngn3−/− mice, which do not produce islet cells. Ngn3 deficiency results in reduced branching and enlarged pancreatic duct-like structures, concomitant with Ngn3 promoter activation throughout the ductal epithelium and reduced Notch signaling. Conversely, forced generation of surplus endocrine progenitor cells causes reduced duct caliber and an excessive number of tip cells. Thus, endocrine progenitor cells normally provide a feedback signal to adjacent multipotent ductal progenitor cells that activates Notch signaling, inhibits further endocrine differentiation and promotes proper morphogenesis. These results uncover a novel layer of regulation coordinating pancreas morphogenesis and endocrine/exocrine differentiation, and suggest ways to enhance the yield of beta-cells from stem cells.
Keywords: Pancreas development, lateral inhibition, lineage tracing, branching morphogenesis, Notch, Jagged1, Neurogenin3
INTRODUCTION
The exocrine and endocrine compartments of the adult pancreas can be viewed as two independent organs. The distinct functions of the exocrine pancreas (release of digestive enzymes into the duodenum) and the endocrine pancreas (regulation of glucose homeostasis) are reflected by the different genetic programs responsible for the function of each tissue. Furthermore, the important pathologies associated with the pancreas can be divided into those that affect the endocrine (e.g. diabetes) or exocrine (e.g. pancreatic cancer, pancreatitis) compartments, and the clinical practice of islet transplantation shows that endocrine and exocrine functions are separable anatomically.
However, it is now clear that during development, a common group of multipotent progenitor cells in the embryonic gut, expressing specific markers such as Foxa1, Foxa2, Pdx1, Ptf1a and Sox9, give rise to both the endocrine and exocrine pancreas (Burlison et al., 2008; Gao et al., 2008; Gu et al., 2002; Kawaguchi et al., 2002; Martin et al., 2007; Seymour et al., 2007). Recently, Zhou and Melton proposed a “tiptrunk” model in an effort to explain tissue dynamics during pancreas development (Zhou et al., 2007). According to this model, tip cells expressing Carboxypeptidase A1 (Cpa1) constitute a multipotent progenitor pool; as they divide, these tip cells leave behind elongated branches (trunks) that contain the progenitors for both ducts and islets. Over time, Neurogenin3+ (Ngn3+) endocrine progenitor cells appear within the trunks, delaminate, and differentiate into hormone producing cells that later coalesce into the islets of Langerhans. In parallel, tip cells differentiate into acinar cells and subsequently secrete digestive enzymes into the ductal network. While this model provides a coherent description of pancreas development, it gives little insight into the regulatory signals that govern exocrine and endocrine differentiation or the mechanisms by which the size and form of the endocrine and exocrine compartments are coordinated.
One molecular pathway previously implicated in binary differentiation decisions of the developing pancreas is Notch signaling (Apelqvist et al., 1999; Gradwohl et al., 2000). Several studies have shown that Notch pathway activation in the epithelium prevents differentiation to either endocrine fates (Greenwood et al., 2007; Hald et al., 2003; Jensen et al., 2000a; Murtaugh et al., 2003), while down regulation of Notch causes a premature induction of Ngn3, leading to precocious endocrine differentiation and depletion of the progenitor pool (Ahnfelt-Ronne et al., 2007a; Apelqvist et al., 1999; Jensen et al., 2000b). By analogy to cell fate decisions in Drosophila, it has been proposed that the induction of Ngn3 expression occurs via the process of lateral inhibition, where Delta-Notch intercellular signaling between neighboring cells determines their fate (Apelqvist et al., 1999; Skipper and Lewis, 2000; Wang et al., 2010). However, this proposal still awaits experimental validation. Notch signaling has also been implicated in the development of the exocrine pancreas. Overexpression of Notch prevents acinar cell differentiation and causes the formation of enlarged, cystic and undifferentiated ducts (Esni et al., 2004; Hald et al., 2003; Murtaugh et al., 2003). In any case, Notch signaling is usually investigated in the context of one compartment only, namely exocrine or endocrine. Whether the morphogenesis of the endocrine and exocrine compartments of the pancreas is interdependent and coordinated, and whether this occurs via Notch signaling, remains unexplored.
Here we investigate how the embryonic endocrine pancreas affects the development of the exocrine pancreas, in particular the fundamental process of ductal branching morphogenesis. To this end we have employed genetic lineage tracing in mice deficient for Ngn3, which completely lack endocrine cells (Gradwohl et al., 2000). We report that endocrine progenitor cells, but not their differentiated progeny, are necessary for correct branching morphogenesis of the ductal tree. In addition, we provide evidence that endocrine progenitor cells inhibit further endocrine differentiation in adjacent epithelial cells through non-cell autonomous regulation of Notch signaling. These results demonstrate a novel mechanism whereby differentiation and cellular delamination are coordinated to balance tissue composition and morphogenesis during the formation of a mature organ. Moreover, these results suggest that Notch-mediated lateral inhibition may account for some of the difficulties encountered in attempts to derive beta cells from embryonic stem cells in vitro.
MATERIALS AND METHODS
Mice
Mouse strains used in this study were Ngn3-CreER (serving as Ngn3 nulls) (Wang et al., 2010), ROSA-LSL-YFP (Srinivas et al., 2001), BAC transgenic Ngn3-Cre (Schonhoff et al., 2004), Pax4−/− (Sosa-Pineda et al., 1997), Pax6−/− (St-Onge et al., 1997) and compound Pdx1-Cre; Rosa-LSL-Nicd (Murtaugh et al., 2003). The joint ethics committee (IACUC) of the Hebrew University and Hadassah Medical Center approved the study protocol for animal welfare. The Hebrew University is an AAALAC International accredited institute
Explant cultures
The dorsal pancreatic bud from embryonic day (e)12.5 mouse embryos was excised under a dissecting microscope in Hank’s balanced salts solution (HBSS). The explants were cultured in DMEM supplied with 10% fetal bovine serum, containing 100 Units/ml penicillin and 100 µg/ml streptomycin. Explants were cultured on microporous membranes and no medium was added on top of the filter, so that the tissue grew at the air/medium interface. Medium was changed every other day. Gamma Secretase Inhibitor (GSI) was added to explants for 15–42 hours. We used either Gamma secretase inhibitor XX, (Calbiochem, cat # 565789, used at a final concentration of 100nM), or LY-411575 (Stemgent, cat #04–0054, used at a final concentration of 100nM). To mark Ngn3+ cells from the Ngn3-CreER allele, explants were treated with 4-hydroxy tamoxifen (Sigma, used at a final concentration of 5µM).
Immunostaining
Staining was performed on 5 micron thick paraffin sections of dissected guts (e15.5) or isolated pancreata (e15.5-P3). Tissue was fixed in 4% buffered zinc-formalin for 2 hours at 4c, dehydrated in an ethanol series, cleared in histoclear and embedded in Paraplast (Kendall). When required, antigen retrieval was performed using a pressure cooker. Whole mount staining was performed essentially as described previously (Ahnfelt-Ronne et al., 2007b).
Primary antibodies used in this study included: guinea pig anti-insulin (1:500; DAKO), armenian hamster anti-muc1 (1:250; Labvision), rabbit anti-amylase (1:100; Sigma), biotinylated DBA (1:250; Vector), rabbit anti-carboxypepetidase1 (1:200; Rockland), guinea pig and rabbit anti-Pdx1 (1:2,500; a generous gift of Chris Wright), mouse anti–E-cadherin (1:50; BD), rabbit anti-ptf1a (1:2000), rabbit anti-Ngn3 (1:500) and mouse anti-Nkx6.1 (1:100) from Beta Cell Biology Consortium, rabbit anti Cleaved Notch Intracellular Domain (1:100; Cell Signaling), rabbit anti Hes1 (1:1500; (Zong et al., 2009)), rabbit anti Sox9 (1:250; Chemicon), guinea pig anti-Ngn3 (1:500; a generous gift of Maike Sander), goat anti-GFP antibody (1:500; Abcam), goat anti-Jagged1 (Santa Cruz, 1:100), Rabbit anti-Hnf-1beta (Santa cruz, 1:200), Rabbit anti-Hnf-6 (Santa cruz, 1:100), Goat anti-Amylase (Santa cruz, 1:100),rabbit anti-Cre (a generous gift of Christoph Kellendonk, 1:3000) (Kellendonk et al., 1999) and rabbit anti Cre (1:100, Novus). For DNA counterstain we used dapi (Sigma). Rabbit anti-Hes1 and rabbit anti-NICD require amplification using TSA kit (Perkin Elmer) (Ahnfelt-Ronne et al., 2007b). Secondary antibodies were from Jackson immunoresearch, used at 1:200. Immunofluorescence images were captured on a Nikon C1 confocal microscope or Olympus FV1000.
Morphometric analysis of duct volume in explants
Confocal z-stacks of pancreas explants were subjected to morphometric analysis using a custom MATLAB code: ducts were identified by three conditions (i) DAPI-negative regions, which are (ii) enclosed by, and include, a MUC1-bright boundary region, and are (iii) surrounded by DAPI-stained nuclei. Total duct volume per explant was then calculated as the total duct area per z-stack multiplied by the image separation, and the result was normalized by the total sample volume.
Morphometric analysis of network density in vivo
Confocal z-stacks for pancreas tissue aged e16.5-P1 were subjected to morphometric analysis using a custom MATLAB code. Ducts were first identified as described above. For each image, the fraction of pancreatic volume at a distance of x from the ducts was evaluated by calculating the size of a border region of thickness x pixels around the ducts (see schematic in Supplemental Fig. 3S). Results were averaged over all samples per genotype. High duct penetration predicts full coverage of pancreatic volume at smaller values of x than low duct penetration.
RESULTS
Ngn3 deficiency perturbs branching morphogenesis
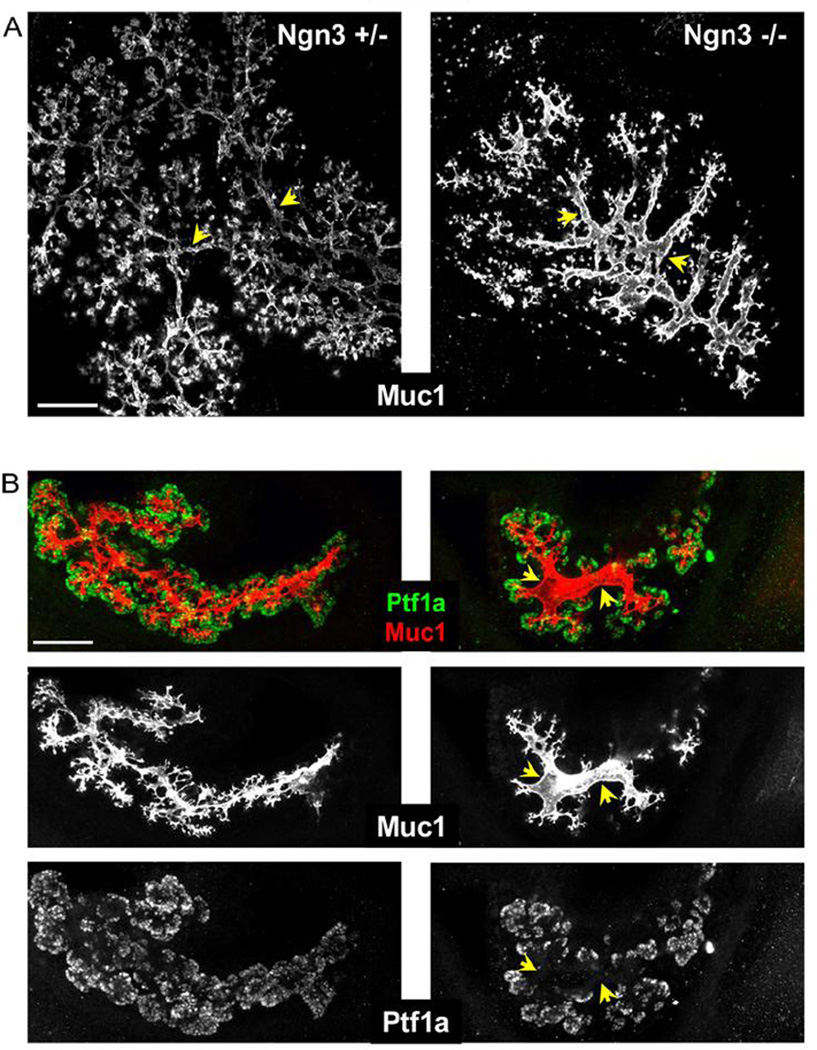
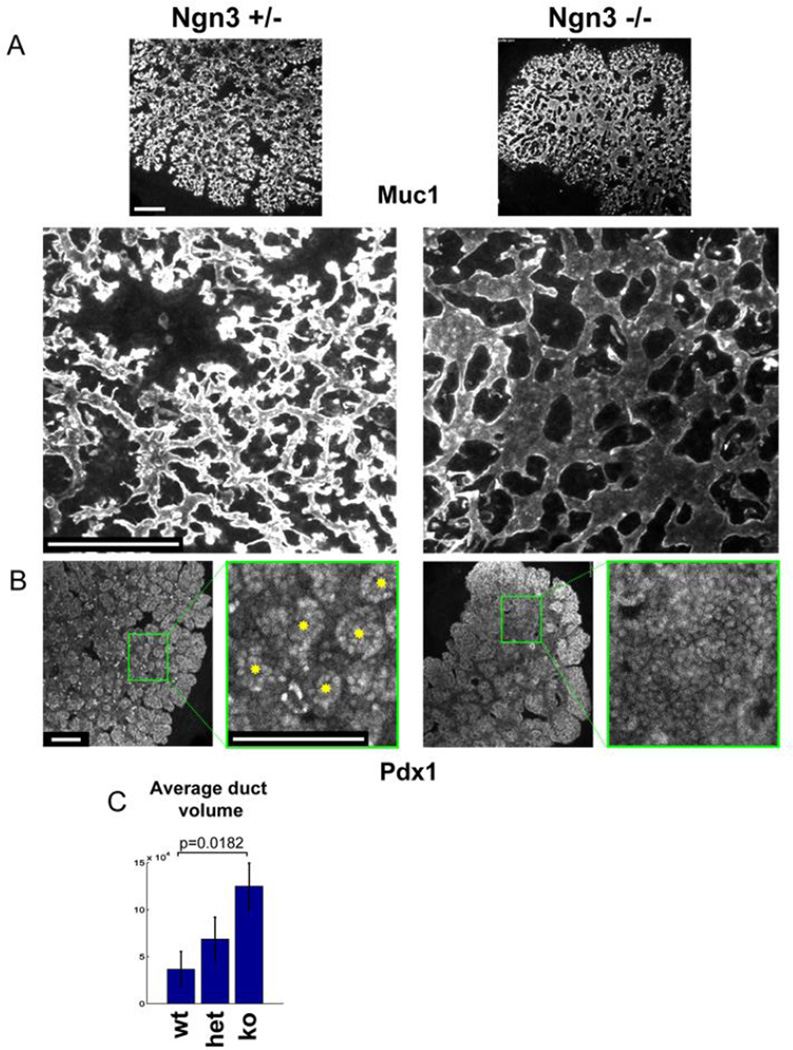
To determine if and how endocrine cell formation affects branching morphogenesis of the pancreas, we examined the structure of the ductal tree in Ngn3-deficient pancreata (Wang et al., 2010). As previously shown by Gradwohl and colleagues (Gradwohl et al., 2000), Ngn3-deficient embryos at all stages completely lack endocrine hormone-producing cells. Surprisingly, whole-mount staining for the ductal marker Muc1 revealed a dramatic alteration in the morphology of the developing ductal tree in the pancreas of e13.5 and e15.5 Ngn3-deficient embryos (Fig. 1A, Supplemental Fig. 1S and Supplemental movies 1+2). Mutant tubes were enlarged and dilated, and had fewer branches. The abnormal thickness of mutant ducts was most apparent in central areas of the pancreas, where Ngn3+ cells are normally located. Notably, mutant ducts were dilated, not elongated, and the overall size of the mutant pancreas was unchanged. The pattern of dilated ducts with larger lumens in Ngn3 mutants was apparent also when staining for other markers, including E-Cadherin, Hnf1beta and Hnf6 (Supplemental Fig. 2S). Mutant ducts appeared normal at e12.5, suggesting that the phenotype develops during the secondary transition of pancreas development, the time of massive Ngn3+ cell formation and delamination in wild type (Supplemental Fig. 2S). Staining for the tip markers Ptf1a and Cpa1 (Zhou et al., 2007) revealed that the mutant epithelium had fewer tip cells, particularly in central areas (Fig. 1B and Supplemental Fig. 2S). The duct phenotype in Ngn3−/− embryos was even more pronounced in explant cultures, probably due to their flatter structure that is more easily captured in microscopic analysis. As shown in Fig. 2A, the explanted mutant epithelium had thicker, less branched tubes. Furthermore, staining of wild type explants for Pdx1 revealed the emergence of discrete structural units, likely the precursors of acini; in contrast, the mutant epithelium stained homogenously for Pdx1 and lacked these units, consistent with reduced branching (Fig. 2B). Morphometric quantification of Muc1-stained tissue in explants confirmed that the duct volume was increased in Ngn3 mutants (Fig. 2C). Due to early postnatal lethality of Ngn3−/− mutants (Gradwohl et al., 2000), it was impossible to examine the functional impact of altered duct/exocrine morphology in adult mutant mice. 3D reconstruction of the ductal system in newborns suggested that Ngn3 mutants still have abnormal ducts (Supplemental movie 3). Visual inspection of confocal slices and Z-stacks from e16.5, e18.5 and p1 mutants revealed a sparser ductal network, indicative of reduced branching. However, quantification of network density did not assign statistical significance to these trends (supplemental Fig. 3S). It is possible that ductal morphology and branching pattern in Ngn3 mutants begin to normalize towards birth, likely related to the decline of Ngn3 expression at this stage. Regardless of the question of normalization, acinar differentiation beyond the tip formation stage appears to be normal in Ngn3 mutants, reflecting a specific role of Ngn3+ cells in the allocation of tip cells. Finally, to determine if duct morphology is affected by Ngn3+ endocrine progenitor cells or by their progeny, we examined the structure of ducts in Pax4−/− and Pax6−/− embryos, which have normal Ngn3+ cells but lack beta cells and alpha cells, respectively (Sosa-Pineda et al., 1997; St-Onge et al., 1997). The ductal tree in both mutants appeared normal, compared with wild type littermates (Supplemental Figure 4S). In sum, these results show that Ngn3-deficient pancreata have a defective ductal structure, and suggest that endocrine progenitor cells (but not their differentiated progeny) participate in shaping the morphogenesis of the exocrine pancreas.
Figure 1. abnormal branching of the pancreas in Ngn3 −/− embryos.
A, representative images of e15.5 Ngn3−/− (right) and control (left) littermates, stained for Muc1. Note thicker central tubes in mutants, marked by arrowheads. Whole mount staining, 5 optical confocal sections superimposed. A similar pattern was observed in a total of 10 Ngn3−/−embryos from 6 litters (see also Supplemental Fig. 1S). B, e13.5 Ngn3−/− and control littermates, stained for Muc1 and the tip marker Ptf1a. Central areas in the mutant epithelium have fewer Ptf1a+ cells (arrowheads), suggesting reduction in the number of tip cells. Amylase is not detected at this stage (data not shown), indicating that these are multipotent progenitors and not differentiated acinar cells. Wholemount staining, 7 optical confocal sections stacked. Scale bar 100µm.
Figure 2. abnormal branching in explanted Ngn3−/− pancreata.
A, e12.5 explants cultured on filters for 2 days and stained for Muc1. Note thicker, less branched epithelium in mutants. Whole mount staining, 5 optical confocal sections stacked. B, Pdx1 staining of explants. Note discrete acinar-like structures in heterozygotes (yellow astericks) and their absence in null mutants. Scale bars in A,B 100µm. C, quantification of duct volume in explants as a function of Ngn3 gene dosage, measured as the surface area stained for Muc1.
A gamma secretase inhibitor induces ductal thinning and excessive tip formation in wild type but not Ngn3 mutants
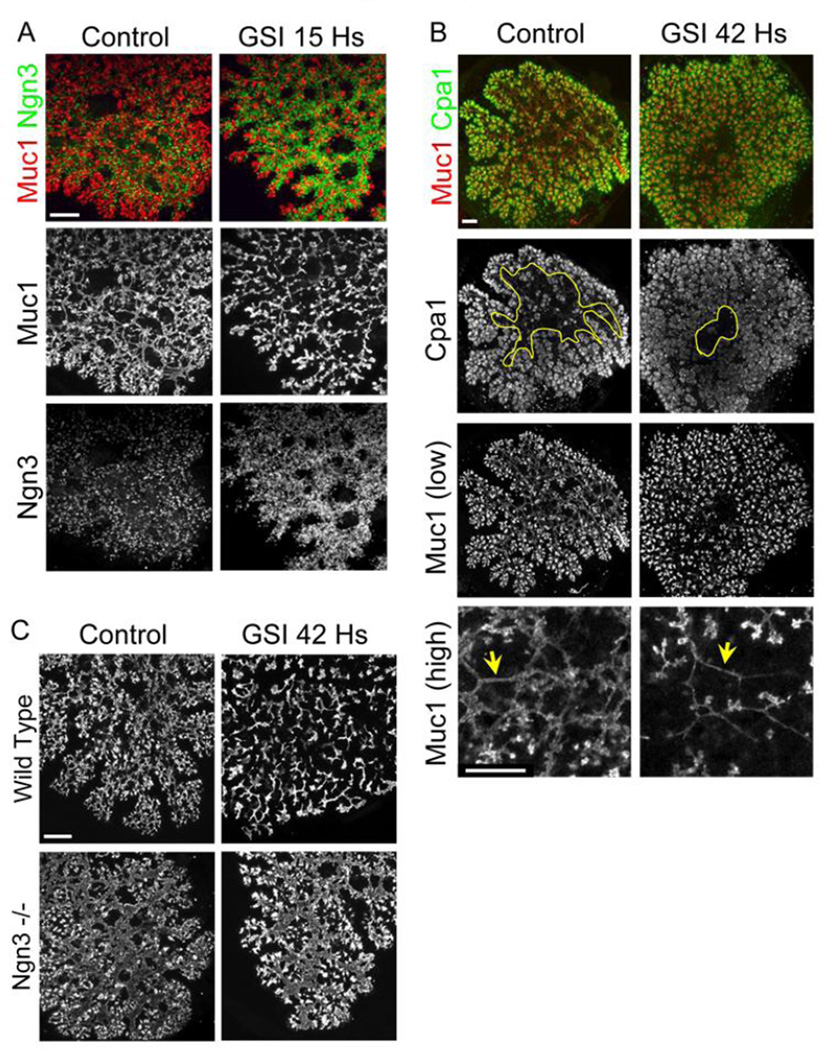
We reasoned that if endocrine progenitors are necessary for proper ductal morphogenesis, and their absence impedes branching, then forced generation of surplus Ngn3+ cells might cause opposite effects on ductal morphogenesis and branching. To test this prediction we treated wild type e12.5 explants with a gamma secretase inhibitor (GSI). As previous studies have shown (Duvillie et al., 2006), the application of GSI caused a rapid (observed within 15 hours) and significant increase in the number of Ngn3+ cells (Fig. 3A). This was accompanied by dramatic thinning of Muc1+ epithelial tubes (observed after ~2 days of treatment) (Fig. 3A,B), reflecting widespread Ngn3+ cell delamination. Strikingly, the expression domain of the tip marker Carboxypeptidase A1 (Cpa1), normally confined to the periphery of explants, expanded to central areas upon GSI treatment (Fig. 3B). Similar results were obtained with a chemically different GSI, supporting the specificity of the phenotype to inhibition of gamma secretase (Supplemental Figure 5S).
Figure 3. Gamma secretase inhibitor produces the reverse phenotype of Ngn3 deficiency: excessive Ngn3+ cells, ductal thinning and excessive Cpa1+ tip cells.
A, Muc1 and Ngn3 staining of e12.5 wild type explants cultured on filters for 2 days and incubated with GSI during the last 15 hours. Note excessive formation of Ngn3+ cells and thinning of duct-like structures in GSI-treated samples. B, Muc1 and Cpa1 staining of wild type e12.5 explants cultured on filters for 3 days and incubated with GSI during the last 42 hours. GSI-treated explants show more Cpa1+ cells in central areas (left panels, yellow circles) and thinner, sparser tubes (right panels, yellow arrowheads). Scale bars 100µm. C, the impact of gamma secretase inhibitor on the epithelium is Ngn3-dependent. Muc1 staining of e12.5 wild type and Ngn3−/− deficient explants, cultured on filters for 2 days and incubated with GSI during the last 42 hours. While GSI treatment causes dramatic ductal thinning in wild type explants, the thicker central tubes of Ngn3 mutants are resistant to the drug. Note that GSI does seem to have some effects in the periphery of Ngn3−/− explants, perhaps inducing the differentiation of committed acinar precursors. Scale bar 100µm.
Cpa1 marks both multipotent tip cells and differentiated acinar cells. To verify that GSI indeed enhances the formation of tip cells, we harvested explants after either 2 or 3 days treatment with GSI, and co-stained for Cpa1 and the acinar marker Amylase. After 2 days, treated explants had expanded areas of Cpa1+Amylase− cells; after 3 days, these cells were mostly Cpa1+ Amylase+ acinar cells (Supplemental Fig. 6S). These results are consistent with GSI triggering the initial formation of multipotent tip cells, followed by acinar differentiation.
To determine if GSI shifted Ngn3+ from an endocrine to a tip/acinar fate, as suggested by a recent study (Cras-Meneur et al., 2009), we treated explants for 2 days with GSI and co-stained for Ngn3, Insulin and Muc1. This treatment caused depletion of Ngn3+ cells, and the appearance of both excessive insulin+ cells and excessive tips (Supplemental Fig. 7S and Fig. 3B). Thus, our results suggest that GSI induces the formation of excessive Ngn3+ cells which go on to become endocrine, as previously shown; in parallel, GSI induces the formation of excessive tip cells.
Next, we considered two possibilities: GSI could impact the developing ducts and tips directly, or indirectly via an acceleration of Ngn3+ cell formation. To distinguish between these possibilities, we treated Ngn3−/− explants with GSI. As shown in Fig. 3C, Ngn3−/− explants were refractory to the duct-thinning effect of GSI, mostly in central areas where Ngn3+ cells form. This indicates that excessive formation of Ngn3+ cells is responsible for ductal thinning and increased tip formation in GSI-treated wild-type explants.
Taken together, these results demonstrate that Ngn3+ endocrine progenitor cells are important determinants of duct size and structure. Moreover, they support the notion that endocrine progenitors control tip formation and branching of the embryonic pancreas.
Fate of endocrine progenitor cells in the absence of Ngn3
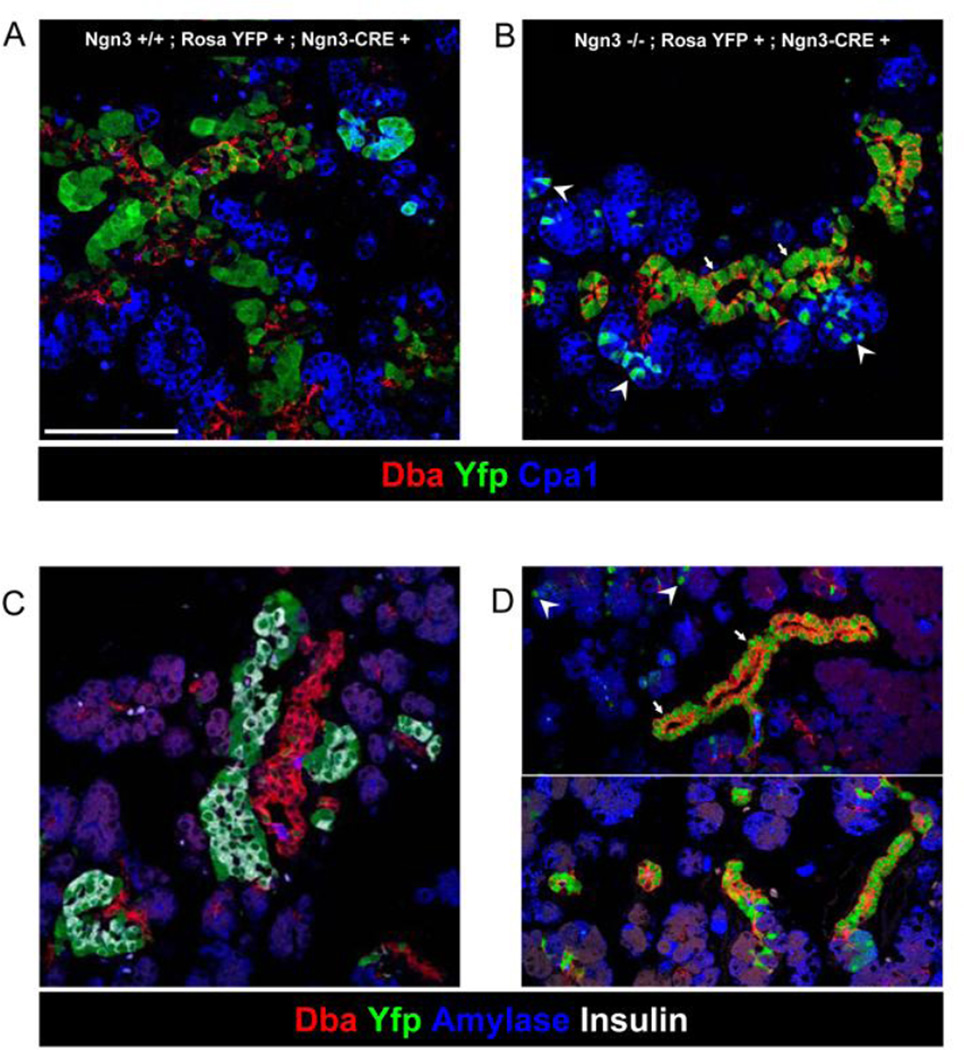
The most plausible explanation for the thicker ducts in Ngn3−/− pancreata is a mass effect: duct diameter could be a function of the number of cells delaminating from, or remaining within, the epithelium. This scenario requires that (1) Ngn3 expression is induced within the ductal epithelium prior to delamination; and (2) in Ngn3−/− mutants, cells activating the Ngn3 promoter survive, but fail to delaminate and are therefore retained within the epithelium, causing an increase in duct diameter. To test these predictions, we first immunostained e15.5 wild type embryonic pancreata for Ngn3, Muc1 and E-Cadherin (demarcating cell boundaries). Ngn3+ nuclei could clearly be identified in Muc1+ cells in contact with the lumen, indicating that Ngn3 expression precedes delamination (Supplemental Fig. 8S). This observation is consistent with a recent report that documented the initiation of delamination by Ngn3 (Gouzi et al., 2011). Therefore, delamination or retention of Ngn3+ cells contributes cell-autonomously to the thickness of ducts. To determine the fate of Ngn3-expressing cells in Ngn3−/− mice, we next employed genetic lineage tracing, allowing for the detection of these cells despite the absence of Ngn3 protein expression. Ngn3 deficiency was combined with a BAC transgene where the Ngn3 locus drives Cre recombinase (Ngn3-Cre) (Schonhoff et al., 2004) and a ROSA26-lox-stop-lox-YFP (Rosa-YFP) reporter for Cre activity (Srinivas et al., 2001). In e15.5 Ngn3−/−; Ngn3-Cre; Rosa-YFP embryos, cells that turn on the Ngn3 promoter are permanently labeled with YFP, such that their fate could be determined. As expected, YFP expression was strictly dependent on the presence of the Ngn3-Cre transgene (supplemental Fig. 9S). Recombination occurred in Ngn3+/+ e15.5 embryos specifically in the DBA-labeled epithelium and in the hormone-producing progeny of Ngn3+ cells (Fig. 4A). By postnatal day 3, YFP+ cells were confined to the nascent islets, with minimal labeling of ducts and acini (Fig. 4C). Ngn3-deficient e15.5 embryos (Fig. 4B) and newborn mice (Fig. 4D) had abundant YFP+ cells that were confined to ducts, with few scattered acinar cells. This result provides clear evidence that in the absence of Ngn3 protein, cells expressing the Ngn3 promoter survive, fail to delaminate and remain integrated within the ductal epithelium. To examine the fate of Ngn3+ cells lacking Ngn3 protein using another Cre driver, we took advantage of the CreER gene that was knocked into the Ngn3 locus for its disruption (Wang et al., 2010). Explants containing one or two copies of CreER (causing heterozygosity or deficiency of Ngn3, respectively) and a YFP reporter were treated with 4-hydroxy tamoxifen. The spectrum of labeled cells in these explants was more restricted, likely reflecting lower levels of nuclear Cre in these mice compared with the BAC transgenic (a combination of lower expression level and tamoxifen dependency). In heterozygous explants, recombination was observed in endocrine cells. In Ngn3-deficient explants (lacking endocrine cells), recombination was largely restricted to Muc1 ductal cells, with minimal labeling in acinar cells (Supplemental Fig. 9S). This observation is in agreement with a similar lineage tracing study published recently (Wang et al., 2010). Taken together, these results provide a plausible explanation for the impact of Ngn3+ endocrine progenitor cells on duct morphology: the delamination of these cells is a significant contributor to overall cell number in ducts, and hence to duct thickness. In addition, decreased tip formation in Ngn3−/− embryos may contribute to duct thickness. Therefore, the thickness of ducts in wild type mice, in Ngn3−/− mice and in GSI-treated explants is determined by the number of Ngn3+ cells that have delaminated from the epithelium.
Figure 4. Fate of endocrine progenitor cells lacking the Ngn3 gene.
A, B, e15 5 Ngn3-Cre; Rosa-YFP mice containing wild type or null Ngn3 alleles, stained for Dba (duct marker), Cpa1 (acinar marker at this age) and YFP (marks historical activation of Ngn3 promoter). In Ngn3−/− embryos, most progeny of Ngn3-expressing cells remain confined within the Dba+ epithelium, while a minority appear to be acinar. C, D, postnatal day 3, wild type and Ngn3−/− littermates, stained for Dba, YFP, Insulin and Amylase. Most ductal cells in mutants but not in controls are YFP+, indicating that most mutant duct cells have activated the Ngn3 promoter sometime in their history. Scale bar 100µm. In panels B+D, arrows point to labeled ducts and arrowheads point to scattered labeled acinar cells.
Ngn3 protein restricts the number of ductal cells turning on the Ngn3 gene
A closer examination of the lineage tracing data revealed that in embryonic and newborn Ngn3−/− mice, the majority of DBA-labeled duct cells were YFP+, indicating that they or their ancestors have activated the Ngn3 promoter at one time or another (fig. 4B,D, Supplemental Fig. 11S). This is in sharp contrast to the situation in wild type animals, where Ngn3+ cells are born in the ductal epithelium, but delaminate such that postnatal ducts contain mostly cells that have never activated the Ngn3 gene promoter. Specifically, in e15.5 embryos we found that 25% of DBA+ cells in wild type animals were YFP+, while 78% of DBA+ cells in Ngn3−/− littermates were YFP+ (p=0.0007). This observation suggests that in wild type pancreata, cells expressing the Ngn3 protein prevent the activation of the Ngn3 gene promoter in adjacent ductal epithelial cells. According to this view, Ngn3−/− mutants lack this non-cell autonomous feedback signal, and hence most ductal epithelial cells turn on the Ngn3 promoter. Since this does not result in Ngn3 protein accumulation in the mutant, there are no phenotypic consequences (other than Cre-mediated activation of the YFP reporter in Ngn3-Cre; Rosa-YFP mice), and Ngn3−/− cells that turn on the Ngn3 promoter remain in ducts. This result is consistent with the hypothesis that endocrine differentiation in pancreatic epithelium is controlled by a process of lateral inhibition, as proposed by many studies of pancreas development (Apelqvist et al., 1999; Jensen et al., 2000a; Wang et al., 2010). Moreover, it shows that most trunk epithelial cells and not just a small subset have the potential to become endocrine cells, though in practice very few do.
Evidence for Ngn3-mediated lateral inhibition of cell fate via Notch
In the central nervous system, lateral inhibition is mediated via Notch signaling. The core pathway involves up-regulation of Notch ligand expression in differentiating epithelial cells (becoming neuronal progenitors), which acts to maintain high Notch activity in adjacent cells and thus prevent their differentiation (Bray, 2006; Fortini, 2009; Kopan and Ilagan, 2009; Lewis, 1998). This process causes a mosaic distribution of delaminating neuronal progenitors, while maintaining a self-renewing population of neuronal stem cells within the epithelium. Ngn3 expression is known to be repressed by the Notch target Hes1, suggesting that a similar mechanism might be operating in pancreatic epithelium (Lee et al., 2001).
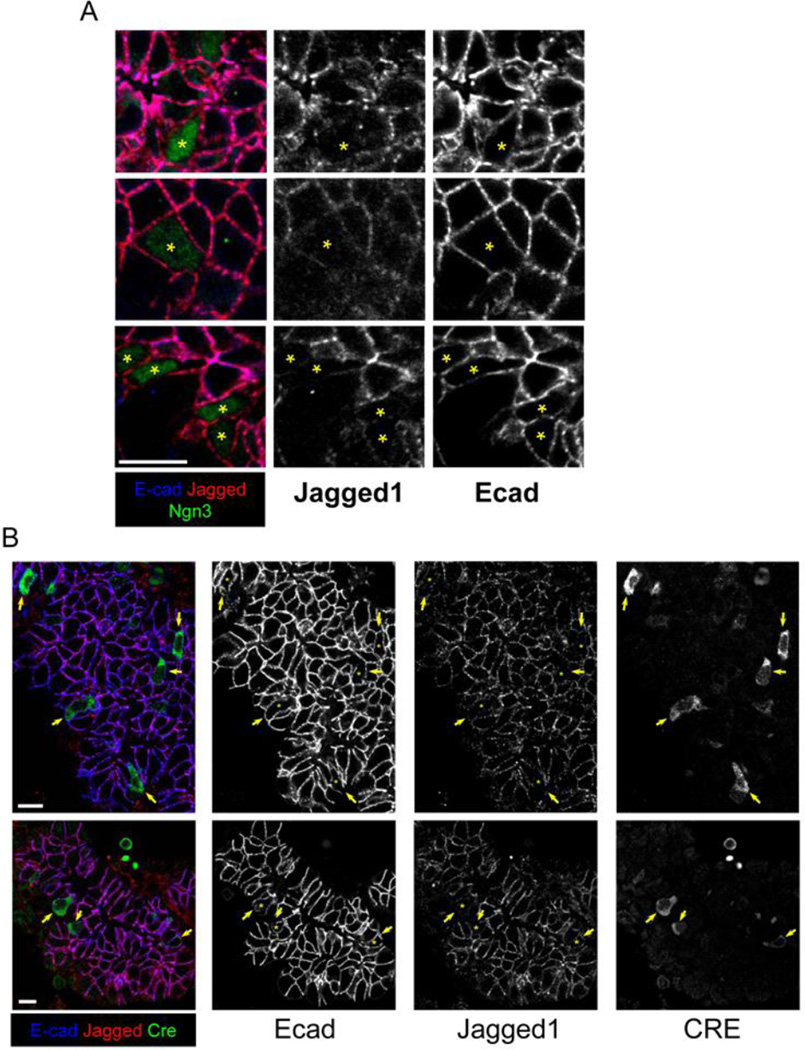
We sought to test the hypothesis that inhibition of endocrine differentiation is mediated by direct communication of Ngn3-expressing cells with adjacent ductal epithelial cells prior to delamination. Specifically, we speculated that Ngn3+ endocrine progenitors (which have low Notch activity) turn on a Notch ligand, which acts to enhance Notch activity in immediately adjacent ductal epithelial cells and thus prevent Ngn3 expression and endocrine differentiation. This idea is supported by a previous study suggesting that Delta-like 1 (Dll1), a key Notch ligand, restrains the number of Ngn3+ cells in the developing pancreas (Apelqvist et al., 1999). The predictions of such a model are that (1) Ngn3+ cells should express Notch ligand/s; (2) Notch signaling should be active in epithelial cells adjacent to Ngn3+ cells; and (3) Notch signaling should be reduced in Ngn3−/− mutants. Using commercially available antibodies directed against Dll1 we failed to obtain immunostaining of sufficient quality (not shown). In a previous study no reduction in the mRNA level of Dll1 was found in Ngn3+ cells expressing different levels of Ngn3 protein (Wang et al., 2010), although others have shown that Dll1 mRNA can be induced by Ngn3 protein (Gasa et al., 2004; Treff et al., 2006). An alternative means for inducing Notch ligand activity in Ngn3+ cells would be to downregulate an inhibitor. It was shown recently that Jagged1 acts as a competitive inhibitor of Notch signaling in the pancreas and in blood vessels, by competing with Delta-like ligands (Benedito et al., 2009; Golson et al., 2009). We therefore examined Jagged1 expression in the pancreatic epithelium. As shown in Fig. 5A, Ngn3+ cells have reduced levels of Jagged1 protein. Co-staining for Jagged1 and Cre in embryos expressing Cre under the Ngn3 promoter generated similar results, namely lower levels of Jagged1 in Cre+ epithelial cells (Fig. 5B). These results are consistent with two recent reports that showed higher levels of Jagged1 mRNA in Ngn3-negative cells versus Ngn3-positive cells (Soyer et al., 2010) and in Ngn3+ cells expressing lower than normal levels of Ngn3 protein (Wang et al., 2010). These data provide a potential molecular mechanism for increased Notch ligand activity (e.g. Dll1) in Ngn3+ cells.
Figure 5. Jagged1 is downregulated in Ngn3-expressing epithelial cells.
A Paraffin sections from e15.5 wild type pancreata stained for Ngn3, E-Cad and Jagged1. Asterisks point to Ngn+ cells. B. Paraffin sections from e14.5 pancreata of Ngn3-CreER+/− mice stained for Cre, E-cad and Jagged1. Arrows and asterisks point to Cre+ cells. Scale bars 10µm.
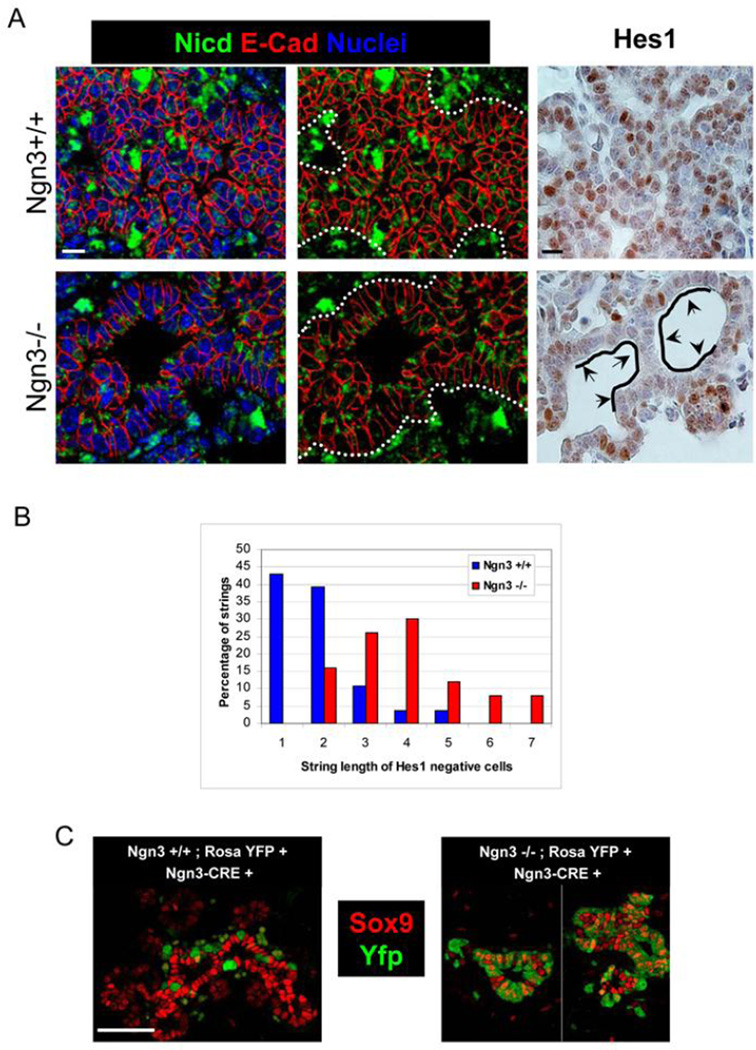
To examine Notch activity as a function of Ngn3 expression more directly, we determined the expression pattern of the Notch intracellular domain (Nicd), as a readout for cells in which Notch is active and hence endocrine differentiation is unlikely. In addition, we documented the expression pattern of Hes1, a repressor of Ngn3 expression and a direct target of Notch signaling. As shown in Fig. 6A,B, multiple cell nuclei in the wild type ductal epithelium were Hes1+ and Nicd+. Ngn3+ cells were never Hes1+ or Nicd+, consistent with the notion that these cells have low Notch pathway activation, and supporting the validity of our immunostaining (Supplemental Fig. 10S). Furthermore, Nicd+ and Hes1+ cells were never clustered but rather interspersed between Nicd/Hes1-negative cells that were often Ngn3+; this pattern is consistent with a lateral inhibition paradigm. As an additional control, we examined sections of transgenic embryos that overexperssed Nicd throughout the pancreas (Pdx1-Cre; Rosa-LSL-Nicd) (Murtaugh et al., 2003). As expected and further supporting the validity of immunostaining, transgenic sections had more homogenous staining for both Nicd and Hes1 compared with wild types (Supplemental Fig. 10S). Strikingly, the ductal epithelium in Ngn3−/− embryos had reduced levels of Hes1 and Nicd (Fig. 6A,B), suggesting that Ngn3+ cells enhance Notch activity in adjacent cells to prevent their endocrine differentiation. The fact that GSI, a known inhibitor of Notch signaling, induces Ngn3 expression in the majority of pancreatic epithelial cells (Fig. 3) provides further evidence that interference with Notch signaling disrupts the lateral inhibition mechanism that prevents the formation of Ngn3 cells within the ductal epithelium.
Figure 6. Ngn3 protein controls expression of Notch signaling components in the developing pancreatic epithelium.
A, paraffin sections from e15.5 pancreata stained for Notch intracellular domain (Nicd, green) and Hes1 (brown). Note a reduction in the level of Nicd and Hes1 in the Ngn3−/− epithelium. Black lines and arrows highlight abnormally long stretches of Hes1-negative ductal epithelial cells in Ngn3−/− mice. Scale bars 10Dm. B, quantification of Hes1-negative ductal epithelial cells in Ngn3 wild type vs. Ngn3 null embryos (n=3 embryos per genotype). We counted the number of lumen-contacting cells in a row that are Hes1-negative. Bars represent the length distribution of such strings. Note that Ngn3−/− ducts have much longer strings of Hes1-negative epithelial cells. C, epithelial cells in Ngn3−/− e15.5 embryos express normal levels of Sox9 despite past activation of the Ngn3 promoter, indicating that Ngn3 protein is required for downregulation of Sox9 expression in endocrine progenitor cells. Scale bars 50µm.
Ngn3 protein is required for downregulation of Sox9 in endocrine progenitor cells
Sox9, a transcription factor marking the early pancreatic epithelium, has emerged as an important regulator of Ngn3 expression, endocrine differentiation, and a target of Notch signaling (Seymour et al., 2008; Seymour et al., 2007; Zong et al., 2009). Normally, Ngn3 and Sox9 expression in the epithelium are mutually exclusive ((Seymour et al., 2008; Seymour et al., 2007) and Fig. 6C). This suggests that Sox9 expression is shut-off after the onset of the endocrine differentiation program, but the mechanism underlying this downregulation is not known. Strikingly, we found that epithelial cells which have turned on the Ngn3 promoter, but failed to accumulate Ngn3 protein, remained Sox9+ (Fig. 6C). This result is consistent with the recent finding that Sox9 mRNA is upregulated in Ngn3-deficient cells (Wang et al., 2010), and suggests that Ngn3 protein is required cell-autonomously for the down-regulation of Sox9 expression. Thus, Ngn3 and Sox9 are linked via a negative feedback loop: Sox9 positively controls Ngn3 expression, and Ngn3 negatively controls the expression of Sox9.
DISCUSSION
Endocrine progenitor cells control branching morphogenesis
A fundamental problem in organ morphogenesis is how the development of different tissue compartments is coordinated. The problem applies to compartments of distinct origins, for example epithelium versus blood vessels, as well as compartments that share a common origin, such as the endocrine and the exocrine pancreas. We have shown here that the endocrine compartment is a novel and important regulator of exocrine pancreas morphogenesis. In the absence of endocrine progenitor cells (but not their differentiated progeny), ducts become enlarged, and fewer branches are formed. Using genetic lineage tracing we found that in the absence of Ngn3 protein, epithelial cells activating the Ngn3 promoter are formed and survive, but fail to delaminate. This finding explains the thicker ducts observed in Ngn3−/− pancreata, particularly in central areas where Ngn3+ cells are normally abundant. According to this model, the control of duct morphology by endocrine progenitor cells simply reflects the number of cells that delaminate or are retained within the epithelium. Notably, mutant ducts were wider, not longer, which was reflected also by the overall normal size of Ngn3-deficient pancreata. Why Ngn3 deficiency causes enlargement of duct diameter and not length is an intriguing open question that merits further examination. Planar cell polarity signaling has been shown to control tube diameter versus length in other systems (Karner et al., 2009), and thus represents an interesting candidate mediator of the observed phenotype.
More surprisingly, Ngn3+ cells appear to positively control the formation of tip cells, the multi-potent progenitors that lead the way to new ductal branches, from which ductal, endocrine cells and acinar cells will emerge (Zhou et al., 2007). The control of tip formation by Ngn3+ cells cannot be readily explained by the number of delaminating or retained Ngn3+ cells. Rather, it appears that Ngn3+ cells communicate with adjacent trunk epithelial cells and enhance their likelihood of becoming tip cells. This scenario is consistent with the emerging view, based on genetic lineage tracing (Solar et al., 2009) and time-lapse studies (Puri and Hebrok, 2007), that pancreatic branches are formed laterally, from within trunks, rather than by splitting of existing tip cells. According to this model, the pancreas grows via cycles of trunk cells giving rise to lateral tip, which in turn leave behind new trunks. Our results establish Ngn3+ cells as important regulators of this cyclic process, controlling proper branching pattern and tissue balance of the pancreas.
The molecular basis for the control of branching by endocrine progenitor cells remains to be investigated. We propose that non-cell autonomous induction of Notch signaling in the ductal epithelium by Ngn3+ cells (see below) controls this process, by increasing the numbers of adjacent Nicd+ and Hes1+ cells. The role of Hes1 expression and Notch signaling in tip formation has not been directly studied before. However, consistent with the suggestion above, Hes1 expression was reported in distal tips (Apelqvist et al., 1999; Wang et al., 2005). Additionally, increased Notch activity was shown to inhibit acinar differentiation (Esni et al., 2004; Hald et al., 2003; Murtaugh et al., 2003), potentially biasing towards a multipotent tip phenotype.
One intriguing observation in our study is the augmentation of tip formation by GSI treatment. We showed that this drug impacted branching morphogenesis via its enhancing effect on the number of Ngn3+ cells (since Ngn3−/− explants are resistant to the effect). However, GSI is also thought to directly inhibit Notch activity, which according to our model should inhibit tip formation. How can these two seemingly contradictory functions of GSI be reconciled? We speculate that the discrepancy reflects the complexity of Notch signaling that includes GSI-resistant pathways. In support of this view, a recent study (Nakhai et al., 2008) has shown that development of the exocrine pancreas depends on the downstream Notch component Rbpj, but not on Notch1/2; this suggests that a non-canonical Notch signaling (which is GSI-independent, as we show here) controls exocrine pancreas development. More detailed genetic studies will be required to resolve this issue. An alternative explanation to the pro-tip/acinar effect of GSI is that it shifts Ngn3+ progenitors, cell autonomously, from endocrine to exocrine fate, as proposed recently (Cras-Meneur et al., 2009). Our results do not support such a model, since we found that GSI increased, rather than decreased, the number of Insulin+ cells in parallel to the enhanced formation of tips (Supplemental Fig. 7S). In addition, in lineage tracing experiments using the Ngn3-Cre BAC transgenic we did not observe augmentation of labeled acinar cells after GSI treatment (data not shown). One potentially important difference between our experiments and Cras-Meneur et al is the shorter duration of our protocol, which perhaps prevented indirect effects on the rate of proliferation of newly formed acinar cells. In summary, we prefer a model where GSI triggers the formation of Ngn3+ cells, which go on to become endocrine, and in parallel via lateral interactions boost the formation of tip cells that will become acinar.
Additionally, the documentation of epithelial cells that turned on the Ngn3 promoter but failed to accumulate Ngn3 protein merits some attention. These cells were instructed to become endocrine, and have started to express the key marker for endocrine differentiation. Previous studies have documented that no endocrine cells are formed in the absence of Ngn3 protein (Gradwohl et al., 2000), but the fate of these cells was rarely examined before (Cras-Meneur et al., 2009; Wang et al., 2010). Our results clearly show that Ngn3 protein is essential for delamination, potentially by activation of an expression module of epithelial to mesenchymal transition (Gouzi et al., 2011; Rukstalis and Habener, 2007). Remarkably, the bias of Ngn3-expressing cells towards an endocrine fate is reversible. Thus, in the absence of Ngn3 protein, these cells survive and remain within the epithelium, adopting what seems to be a normal ductal cell phenotype (based on general morphology and expression of the duct markers Hnf1beta and HNF6). As shown here, this has multiple consequences on pancreas morphogenesis. In our experiments using both Ngn3-Cre and Ngn3-CreER, the fate of Ngn3+ cells in Ngn3 mutants was primarily ductal. We propose that the occasional labeling seen in acinar cells (which was also reported by Wang et al, 2010, although to a larger extent) reflects events of lateral tip formation, branching and acinar differentiation from previously labeled trunk cells.
Notch-mediated lateral inhibition restricts endocrine pancreas development
A key finding of the current study is that Ngn3 expression in a proportion of epithelial cells is important to prevent the expression of Ngn3 in additional, adjacent epithelial cells. In fact, in the absence of Ngn3 protein, most duct cells activate the Ngn3 gene promoter at some point. This provides clear evidence for a process of lateral inhibition operating during pancreas endocrine development (Apelqvist et al., 1999; Wang et al., 2010). We note that our ability to observe this previously unrecognized phenomenon may be a result of the specific experimental system we employed. In our experiments we used genetic lineage tracing, which documents the cumulative number of Ngn3-expressing cells up to the time of observation. Examination of short-term Ngn3 promoter activity would have probably missed this phenotype, since lateral activation of Ngn3 is likely asynchronous, such that at any given time no significant enhancement of Ngn3 promoter activity is observed.
Lateral inhibition during neuronal development is mediated by Notch signaling. It is thought that downregulation of Notch signaling in epithelial cells derepresses proneural genes (homologs of Ngn3) and initiates a neuronal progenitor program that includes cellular delamination. At the same time, these progenitors upregulate Notch ligands which enhance the activity of Notch signaling in adjacent epithelial cells, preventing their differentiation and maintaining a self renewal state. Consequently, a mosaic pattern of neuronal differentiation and self renewal is generated (Bray, 2006; Fortini, 2009; Kopan and Ilagan, 2009). To test if pancreatic endocrine differentiation follows the neuronal paradigm, we examined the dependence of Notch signaling markers on Ngn3. Consistent with a Notch-mediated lateral inhibition model, Ngn3+ cells do not stain for Nicd and express minimal if any levels of the Notch pathway target Hes1 (Fig. 6 and Supplemental Fig. 10S); furthermore, either Ngn3 or Nicd/Hes1 expressing cells are not clustered but rather mosaic, as expected in a lateral inhibition setting. Most strikingly, we found that the presence of Ngn3 protein is critical for the expression of Hes1, as well as Nicd, in adjacent cells within the pancreatic ductal epithelium. Together, these observations suggest a plausible molecular mechanism for lateral inhibition during endocrine differentiation in the pancreas. According to this model, reduced Notch activity in a subset of duct cells derepresses Ngn3 expression and initiates endocrine development. Ngn3 protein, perhaps via a cell autonomous upregulation of Notch ligand activity (see below), causes the upregulation of Notch activity in adjacent epithelial cells (as reflected by staining for Notch intracellular domain and Hes1 expression). Hes1+; Nicd+ duct cells are biased against an endocrine fate, and remain in the epithelium (model, Fig. 7). These cells are likely responsible for expansion of the epithelium.
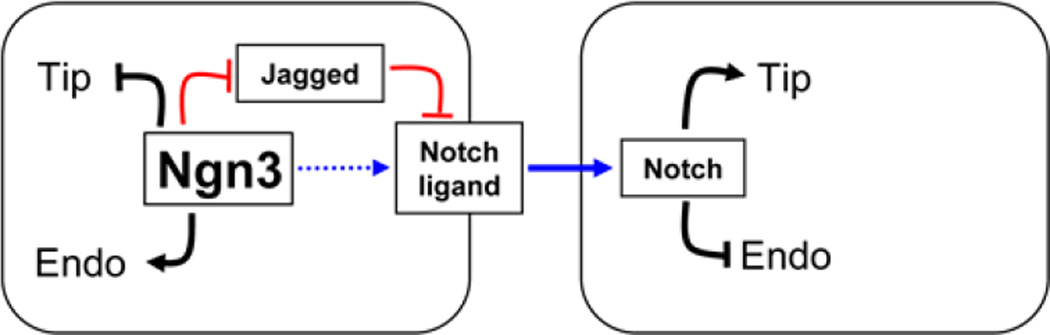
Figure 7. Model for the effects of Ngn3 protein on pancreatic epithelium.
The presence of Ngn3 protein biases epithelial cells cell autonomously towards an endocrine fate, and against a tip/branching fate. Activation of the Notch pathway in adjacent cells biases against an endocrine fate, manifesting lateral inhibition of endocrine differentiation. Instead, adjacent cells adopt a tip/branching fate. Ngn3-dependent downregulation of Jagged1 could support Notch activation in adjacent cells by potentiating the activity of Dll1 or another Notch ligand in Ngn3-expressing cells. Ngn3 may also directly increase the expression of Dll1 or other ligands. In mutants lacking Ngn3 protein, lateral inhibition of endocrine differentiation is absent, and all epithelial cells are directed to futile activation of the Ngn3 promoter. In parallel, the signal favoring tip/branching fate is absent, resulting in the formation of thick, unbranched tubes.
Recently, Wang and co-workers reported that Ngn3-expressing cells have higher Hes1 expression when Ngn3 gene dose is reduced (Wang et al., 2010). Superficially, this seems to contradict our results of reduced Hes1 expression in the ductal epithelium of Ngn3−/− mice. In fact, the results of the two studies are complementary and in agreement with our model. We propose that in wild type ducts, Ngn3+ cells have very low levels of Hes1, while adjacent Ngn3-negative cells have high levels. In the epithelium of Ngn3−/−embryos lateral interactions are attenuated; Ngn3+ cells do not downregulate Hes1, and adjacent Ngn3-negative cells do not upregulate Hes1. Thus the mutant epithelium has on average more Hes1 than wild type Ngn3+ cells (as shown by Wang et al), but less Hes1 than wild type Ngn3-negative cells (as suggested by our in-situ staining).
It is tempting to speculate that the non-cell autonomous, Ngn3-induced Notch activity is responsible not only for lateral inhibition of endocrine differentiation, but also for the Ngn3-dependency of tip formation and branching. Consistent with this idea, Hes1 was shown to be a tip marker (Apelqvist et al., 1999; Wang et al., 2005). However, further studies will be required to test how Notch controls tip formation.
It also remains unclear how Ngn3+ cells activate Notch signaling in adjacent cells. In this study and in a previous paper (Wang et al., 2010) we could not identify a reduction in the expression of any Notch ligand in cells that express the Ngn3 gene promoter but lack Ngn3 protein. A potential explanation comes from recent reports that Jagged1 mRNA is upregulated in Ngn3-deficient cells (Wang et al., 2010), and is under-represented in Ngn3+ cells (Soyer et al., 2010), and from our observation that expression of Jagged1 protein is reduced in Ngn3+ epithelial cells in wild type mice (Fig. 5). Given recent reports that Jagged1 inhibits Notch signaling during early pancreas morphogenesis (Golson et al., 2009) and in developing blood vessels (Benedito et al., 2009), it is possible that lateral inhibition in the pancreas is mediated by downregulation of inhibitory Jagged1 expression in Ngn3+ cells, permitting activation of Notch signaling in adjacent cells through other Delta-family ligands whose level of expression need not change (model, Fig. 7B). In support of this model, the expression of Manic Fringe, which is essential for the inhibitory effect of Jagged at the receiving cell, is restricted to Ngn3+ cells (Golson et al., 2009; Svensson et al., 2009; Xu et al., 2006). Thus, the control of Jagged1 and Manic Fringe expression by Ngn3 could be the basis for the lateral interactions controlling endocrine and tip fates. Further experiments will be required to test this possibility.
Conclusions
In summary, we propose that expression of Ngn3 cell-autonomously biases epithelial cells towards an endocrine fate, and against a tip fate (and consequently branching and acinar differentiation). At the same time, Ngn3 protein has an opposite non-cell autonomous effect: by triggering Notch activity in adjacent epithelial cells it biases these cells against an endocrine fate, and favors a tip/branching fate (Fig. 7). Thus, our results expose an unappreciated level of regulation, which coordinates the development of two seemingly unrelated compartments of the pancreas.
These findings may have practical implications for efforts to direct the differentiation of embryonic stem cells (ESC) towards transplantable insulin-producing beta cells. Recent progress in this area allows the efficient derivation of ESC-derived Pdx1+ pancreatic epithelial cells (Borowiak et al., 2009; Chen et al., 2009; D'Amour et al., 2005; D'Amour et al., 2006; Kroon et al., 2008). However, the yield of endocrine differentiation remains extremely low for unclear reasons. One theoretical possibility is that only a subset of pancreatic epithelial cells is competent to become endocrine, and that ESC culture conditions select against this competent population. Our results suggest that most if not all pancreatic epithelial cells residing in trunks are in principle capable of turning on Ngn3 expression and hence adopting an endocrine fate; in fact this seems to be the default fate of trunk cells, which is normally prevented by signals from adjacent Ngn3+ cells. We speculate that lateral inhibition could be one factor accounting for the low numbers of endocrine cells formed in ESC cultures. Interference with lateral inhibition communication might help improve the yield of pancreatic endocrine cells from ESC.
Supplementary Material
Highlights.
> The link between the endocrine pancreas and duct morphogenesis was examined. > Ngn3−/− mice have reduced branching and enlarged pancreatic ductlike structures. > Endocrine cell formation is controlled by Notch-mediated lateral inhibition. > All pancreatic epithelial cells in trunks are capable of turning on Ngn3 expression. >
ACKNOWLEDGEMENTS
We thank Abraham Fainsod, Ze’ev Paroush, Klaus Kaestner, Jan Jensen and Palle Serup for stimulating discussions. We thank Maike Sander, Chris Wright and Christoph Kellendonk for generous gifts of Ngn3, Pdx1 and Cre antibodies and Holger Russ for help with dissection of Pax6 mutant embryos. Supported by grants from JDRF, the Helmsley Foundation, European Union Seventh Framework Programme under grant agreement n°241883, ERC starting grant and the Dutch Friends of Hebrew University to YD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahnfelt-Ronne J, Hald J, Bodker A, Yassin H, Serup P, Hecksher-Sorensen J. Preservation of proliferating pancreatic progenitor cells by Delta-Notch signaling in the embryonic chicken pancreas. BMC Dev Biol. 2007a;7:63. doi: 10.1186/1471-213X-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahnfelt-Ronne J, Jorgensen MC, Hald J, Madsen OD, Serup P, Hecksher-Sorensen J. An improved method for three-dimensional reconstruction of protein expression patterns in intact mouse and chicken embryos and organs. J Histochem Cytochem. 2007b;55:925–930. doi: 10.1369/jhc.7A7226.2007. [DOI] [PubMed] [Google Scholar]
- Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Borowiak M, Maehr R, Chen S, Chen AE, Tang W, Fox JL, Schreiber SL, Melton DA. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell. 2009;4:348–358. doi: 10.1016/j.stem.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- Burlison JS, Long Q, Fujitani Y, Wright CV, Magnuson MA. Pdx-1 and Ptf1a concurrently determine fate specification of pancreatic multipotent progenitor cells. Dev Biol. 2008;316:74–86. doi: 10.1016/j.ydbio.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Borowiak M, Fox JL, Maehr R, Osafune K, Davidow L, Lam K, Peng LF, Schreiber SL, Rubin LL, Melton D. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol. 2009;5:258–265. doi: 10.1038/nchembio.154. [DOI] [PubMed] [Google Scholar]
- Cras-Meneur C, Li L, Kopan R, Permutt MA. Presenilins, Notch dose control the fate of pancreatic endocrine progenitors during a narrow developmental window. Genes Dev. 2009;23:2088–2101. doi: 10.1101/gad.1800209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- Duvillie B, Attali M, Bounacer A, Ravassard P, Basmaciogullari A, Scharfmann R. The mesenchyme controls the timing of pancreatic beta-cell differentiation. Diabetes. 2006;55:582–589. doi: 10.2337/diabetes.55.03.06.db05-0839. [DOI] [PubMed] [Google Scholar]
- Esni F, Ghosh B, Biankin AV, Lin JW, Albert MA, Yu X, MacDonald RJ, Civin CI, Real FX, Pack MA, Ball DW, Leach SD. Notch inhibits Ptf1 function and acinar cell differentiation in developing mouse and zebrafish pancreas. Development. 2004;131:4213–4224. doi: 10.1242/dev.01280. [DOI] [PubMed] [Google Scholar]
- Fortini ME. Notch signaling: the core pathway and its posttranslational regulation. Dev Cell. 2009;16:633–647. doi: 10.1016/j.devcel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Gao N, LeLay J, Vatamaniuk MZ, Rieck S, Friedman JR, Kaestner KH. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes Dev. 2008;22:3435–3448. doi: 10.1101/gad.1752608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasa R, Mrejen C, Leachman N, Otten M, Barnes M, Wang J, Chakrabarti S, Mirmira R, German M. Proendocrine genes coordinate the pancreatic islet differentiation program in vitro. Proc Natl Acad Sci U S A. 2004;101:13245–13250. doi: 10.1073/pnas.0405301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golson ML, Le Lay J, Gao N, Bramswig N, Loomes KM, Oakey R, May CL, White P, Kaestner KH. Jagged1 is a competitive inhibitor of Notch signaling in the embryonic pancreas. Mech Dev. 2009;126:687–699. doi: 10.1016/j.mod.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouzi M, Kim YH, Katsumoto K, Johansson K, Grapin-Botton A. Neurogenin3 initiates stepwise delamination of differentiating endocrine cells during pancreas development. Dev Dyn. 2011;240:589–604. doi: 10.1002/dvdy.22544. [DOI] [PubMed] [Google Scholar]
- Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood AL, Li S, Jones K, Melton DA. Notch signaling reveals developmental plasticity of Pax4(+) pancreatic endocrine progenitors and shunts them to a duct fate. Mech Dev. 2007;124:97–107. doi: 10.1016/j.mod.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- Hald J, Hjorth JP, German MS, Madsen OD, Serup P, Jensen J. Activated Notch1 prevents differentiation of pancreatic acinar cells and attenuate endocrine development. Dev Biol. 2003;260:426–437. doi: 10.1016/s0012-1606(03)00326-9. [DOI] [PubMed] [Google Scholar]
- Jensen J, Heller RS, Funder-Nielsen T, Pedersen EE, Lindsell C, Weinmaster G, Madsen OD, Serup P. Independent development of pancreatic alpha- and beta-cells from neurogenin3-expressing precursors: a role for the notch pathway in repression of premature differentiation. Diabetes. 2000a;49:163–176. doi: 10.2337/diabetes.49.2.163. [DOI] [PubMed] [Google Scholar]
- Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000b;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- Karner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, Carroll TJ. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet. 2009;41:793–799. doi: 10.1038/ng.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet. 2002;32:128–134. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- Kellendonk C, Tronche F, Casanova E, Anlag K, Opherk C, Schutz G. Inducible site-specific recombination in the brain. J Mol Biol. 1999;285:175–182. doi: 10.1006/jmbi.1998.2307. [DOI] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D’Amour KA, Carpenter MK, Baetge EE. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- Lee JC, Smith SB, Watada H, Lin J, Scheel D, Wang J, Mirmira RG, German MS. Regulation of the pancreatic pro-endocrine gene neurogenin3. Diabetes. 2001;50:928–936. doi: 10.2337/diabetes.50.5.928. [DOI] [PubMed] [Google Scholar]
- Lewis J. Notch signalling and the control of cell fate choices in vertebrates. Semin Cell Dev Biol. 1998;9:583–589. doi: 10.1006/scdb.1998.0266. [DOI] [PubMed] [Google Scholar]
- Martin M, Hauer V, Messmer M, Orvain C, Gradwohl G. Transcription factors in pancreatic development. Animal models. Endocr Dev. 2007;12:24–32. doi: 10.1159/000109602. [DOI] [PubMed] [Google Scholar]
- Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A. 2003;100:14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhai H, Siveke JT, Klein B, Mendoza-Torres L, Mazur PK, Algul H, Radtke F, Strobl L, Zimber-Strobl U, Schmid RM. Conditional ablation of Notch signaling in pancreatic development. Development. 2008;135:2757–2765. doi: 10.1242/dev.013722. [DOI] [PubMed] [Google Scholar]
- Puri S, Hebrok M. Dynamics of embryonic pancreas development using realtime imaging. Dev Biol. 2007;306:82–93. doi: 10.1016/j.ydbio.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukstalis JM, Habener JF. Snail2, a mediator of epithelial-mesenchymal transitions, expressed in progenitor cells of the developing endocrine pancreas. Gene Expr Patterns. 2007;7:471–479. doi: 10.1016/j.modgep.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonhoff SE, Giel-Moloney M, Leiter AB. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev Biol. 2004;270:443–454. doi: 10.1016/j.ydbio.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Seymour PA, Freude KK, Dubois CL, Shih HP, Patel NA, Sander M. A dosage-dependent requirement for Sox9 in pancreatic endocrine cell formation. Dev Biol. 2008;323:19–30. doi: 10.1016/j.ydbio.2008.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, Scherer G, Sander M. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci U S A. 2007;104:1865–1870. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipper M, Lewis J. Getting to the guts of enteroendocrine differentiation. Nat Genet. 2000;24:3–4. doi: 10.1038/71653. [DOI] [PubMed] [Google Scholar]
- Solar M, Cardalda C, Houbracken I, Martin M, Maestro MA, De Medts N, Xu X, Grau V, Heimberg H, Bouwens L, Ferrer J. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell. 2009;17:849–860. doi: 10.1016/j.devcel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature. 1997;386:399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- Soyer J, Flasse L, Raffelsberger W, Beucher A, Orvain C, Peers B, Ravassard P, Vermot J, Voz ML, Mellitzer G, Gradwohl G. Rfx6 is an Ngn3-dependent winged helix transcription factor required for pancreatic islet cell development. Development. 2010;137:203–212. doi: 10.1242/dev.041673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A, Gruss P. Pax6 is required for differentiation of glucagon-producing alpha-cells in mouse pancreas. Nature. 1997;387:406–409. doi: 10.1038/387406a0. [DOI] [PubMed] [Google Scholar]
- Svensson P, Bergqvist I, Norlin S, Edlund H. MFng is dispensable for mouse pancreas development and function. Mol Cell Biol. 2009;29:2129–2138. doi: 10.1128/MCB.01644-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treff NR, Vincent RK, Budde ML, Browning VL, Magliocca JF, Kapur V, Odorico JS. Differentiation of embryonic stem cells conditionally expressing neurogenin 3. Stem Cells. 2006;24:2529–2537. doi: 10.1634/stemcells.2006-0082. [DOI] [PubMed] [Google Scholar]
- Wang J, Kilic G, Aydin M, Burke Z, Oliver G, Sosa-Pineda B. Prox1 activity controls pancreas morphogenesis and participates in the production of "secondary transition" pancreatic endocrine cells. Dev Biol. 2005;286:182–194. doi: 10.1016/j.ydbio.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Wang S, Yan J, Anderson DA, Xu Y, Kanal MC, Cao Z, Wright CV, Gu G. Neurog3 gene dosage regulates allocation of endocrine and exocrine cell fates in the developing mouse pancreas. Dev Biol. 2010;339:26–37. doi: 10.1016/j.ydbio.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Wang S, Zhang J, Zhao A, Stanger BZ, Gu G. The fringe molecules induce endocrine differentiation in embryonic endoderm by activating cMyt1/cMyt3. Dev Biol. 2006;297:340–349. doi: 10.1016/j.ydbio.2006.04.456. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Law AC, Rajagopal J, Anderson WJ, Gray PA, Melton DA. A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell. 2007;13:103–114. doi: 10.1016/j.devcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Zong Y, Panikkar A, Xu J, Antoniou A, Raynaud P, Lemaigre F, Stanger BZ. Notch signaling controls liver development by regulating biliary differentiation. Development. 2009;136:1727–1739. doi: 10.1242/dev.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.