INTRODUCTION
Men who have sex with men (MSM) remain disproportionately burdened by the HIV epidemic. In the United States, an estimated 25,000 to 37,000 MSM were newly infected with HIV in 2006 [1] primarily through receptive anal intercourse [2–4]. Alcohol consumption among MSM is well-characterized [5] and has been implicated as a risk factor for HIV infection [6]. However, existing epidemiologic evidence of an effect of alcohol consumption on HIV seroconversion has been mixed [7, 8] and therefore inconclusive for supporting population-level alcohol interventions as a strategy for HIV prevention [9].
In particular, recent studies using multiple time-dependent measures of alcohol consumption and adjusting for potential time-dependent confounders provide mixed results with one [10] supporting an earlier finding of a harmful association [11] and two not supporting a harmful association [12, 13]. However, the standard statistical methods (e.g., regression, stratification) used in past studies may have failed to provide consistent estimates of the hypothesized detrimental effect of alcohol consumption because of inadequate control of time-dependent confounders [14, 15]. Recent work among injection drug users suggests that standard adjustment for time-dependent confounders (e.g., illicit drug use) may block indirect effects of alcohol consumption acting through such confounders and lead to biased estimates [16].
Here we applied marginal structural models to estimate the association between alcohol consumption and HIV seroconversion. Using this approach, we can account for measured time-dependent confounders affected by prior alcohol consumption. We analyzed data from MSM at risk of HIV seroconversion using prospective data from the Multicenter AIDS Cohort Study (MACS) collected between 1984 and 2008. Specifically, we examined the joint effects of alcohol consumption and unprotected receptive anal intercourse on the risk of HIV seroconversion. We hypothesized that both high levels of alcohol consumption and unprotected receptive anal intercourse increase the hazard of HIV seroconversion, and that this joint effect is greater than multiplicative.
METHODS
Study Sample
The study sample consisted of a subset of participants enrolled in the MACS, an ongoing study of the natural history of HIV infection among MSM in the US metropolitan areas of Baltimore, Maryland/Washington DC; Chicago, Illinois; Los Angeles, California; and Pittsburgh, Pennsylvania [17]. Enrolled were 6,972 men: 5,622 in 1984–1992 and 1,350 in 2001. Of these 6,972 men, 4,029 were sexually active, but HIV-seronegative at their baseline visit and were therefore eligible for this study. We analyzed data on the 3,725 HIV-seronegative men who completed at least one follow-up visit.
Participants are followed semiannually at study visits that involve a physical examination, blood draws, and standardized questionnaires including the Center for Epidemiologic Studies Depression (CES-D) scale [18]. The risk behavior portions of the questionnaire were interviewer administered initially and were converted to audio computer-assisted self-interview for newly enrolled participants beginning in October 2001 and at follow-up visits for all participants in October 2002. Institutional review boards approved protocols and written informed consent forms completed by all study participants. MACS design details and questionnaires are available at http://www.statepi.jhsph.edu/macs/macs.html.
Ascertainment of HIV Seroconversion
Participants were followed from their baseline visit until HIV seroconversion, death, loss to follow-up, or administrative censoring. HIV status was determined from blood specimens tested by enzyme-linked immunosorbent assay and was confirmed by Western blot. The midpoint between dates of the last seronegative and the first seropositive test was taken as the estimated date of HIV seroconversion; when this date was more than one year after the last seronegative test date (n = 33), participants were classified as lost to follow-up. Follow-up practices in the MACS cohort have been described previously [19]. Briefly, death information was obtained from death certificates, the Social Security Death Index, the National Death Index, autopsy records, and other notification sources. We censored follow-up for participants who failed to attend study visits for more than one year at the minimum of: their date of death (if applicable), one year after their last visit, or 1 January 2008 (date of administrative censoring). All remaining seronegative participants seen after 1 January 2007 were administratively censored on 1 January 2008.
Assessment of Alcohol Consumption
The typical number of drinks per week consumed by each participant was calculated as the product of participant-reported average number of drinking-days per week and average number of drinks per drinking-day (range: 0–84 drinks/week). A drink was defined explicitly as one 12-ounce beer (~355 mL), one 4–5-ounce glass of wine (~120–150 mL), or one mixed drink with 1.5 ounces (~44 mL) of 80-proof hard liquor. The few (<1%) reports of 10 or more drinks per drinking-day were classified as 12 drinks. In models, we considered three levels of drinking: nondrinkers, moderate drinkers (1–14 drinks/week), and heavy drinkers (>14 drinks/week) based on reports averaged over the prior two visits (approximately one year). This categorization reflects current public health recommendations that adult men consume no more than 2 drinks per day [20]. The exposure window was chosen to maximize stability of the alcohol assessment. We considered the impact of this choice of exposure window on our results by considering a range of empirical induction periods (≥2 years prior) shown in Supplementary Table 1. Trends were generally insensitive to the exposure window chosen although, as expected, the magnitude of the observed effect decreased as length of the exposure window increased.
Assessment of Sexual Risk Behavior
The number of partners with whom the participant was the receptive partner during unprotected receptive anal intercourse (hereafter, partners) was previously identified as a strong predictor of HIV seroconversion in the MACS [13, 21] and other cohorts [4]; we therefore consider this exposure a marker of overall sexual risk behavior. Participants self-report the number of partners they have had at each semiannual visit. The few (<1%) reports of more than six partners since the previous visit were reset to the median of those with more than six partners (10 partners). In models, we considered the number ofpartners asone or fewer partners or multiple partners. Similar to alcohol measures, we averaged the number of partners over the previous two visits. The reference group combined men with 1 partner and those who report no partners because participants with a single long-term partner may not be at increased risk of HIV seroconversion and MACS participants currently without a partner are not representative of MSM who do not have unprotected anal intercourse. The overall distribution of alcohol consumption and partner number is presented stratified by time in Supplementary Table 2.
Assessment of Covariates
Based on previously identified determinants of alcohol consumption [22, 23] and HIV risk factors [13], we considered several time-fixed and time-dependent covariates as confounders. The following variables were assessed at baseline: participant’s race and ethnicity (white non-Hispanic, white Hispanic, or black), age, enrollment city, and education (college graduate or not). Data on time-dependent confounders were recorded at each semiannual visit and included depressive symptoms indicated by a CES-D score >16; self-report of either gonorrhea or Chlamydial infection; cigarette smoking (current or not); and use of any of the following illicit drugs: cocaine, crack cocaine, marijuana/hash, or nitrite inhalants (i.e., poppers). Injection drug use was uncommon (<1%) as was methamphetamine use (4%), which furthermore was not captured consistently over follow-up. Just 7% of the cohort reported use of any other drugs, including heroin. We therefore considered use of cocaine, crack cocaine, marijuana/hash, or nitrite inhalants as confounders.
Baseline data on smoking, CES-D score and number of partners were missing for 6%, 6%, and 7% of participants, respectively. Data on all other variables were missing for <2% of participants. For the few values missing at baseline, we imputed the mode. For missing values over follow-up, the value from the previous visit was carried forward (smoking, 6%; CES-D score, 8%; number of partners, 9%; all others, <4%).
Statistical Analysis
We used a joint marginal structural Cox proportional hazards model to estimate the joint effects of alcohol consumption and partner number on HIV seroconversion [24]. The marginal structural model provides asymptotically consistent estimates of contrasts in potential outcomes under the assumptions of consistency, exchangeability, positivity, and correct model specification for each exposure and censoring. Details of the estimation of the joint marginal structural model are provided in Appendix A.
Cumulative incidence of HIV seroconversion curves accounting for time-dependent confounders are presented [27]. Effects were quantified using hazard ratios (HRs), and precision was assessed through 95% confidence limits (CL) based on robust variances. Departure from additivity was assessed using the relative excess risk due to interaction (RERI) [28]. We evaluated the contribution of the product term using a robust Wald χ2 test. No evidence of departure from proportional hazards for the exposures was observed in models that included exposure by time (P = 0.86) or exposure by log-time (P = 0.70) product terms, which allowed us to assume that the hazards of seroconversion remained constant over the 24 year follow-up period.
Alongside our weighted results, we present observed counts of HIV seroconversions and corresponding incidence rates calculated as number of HIV seroconversions divided by the number of person years of observation. We also present results from standard analyses, which adjust for the same time-dependent confounders by including lagged values of time-dependent covariates in a standard Cox model [29]. Results were similar to those observed when concurrent values of time-dependent covariates were included in the model. All analyses were conducted with SAS version 9.2 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Between 1984 and 2008, 3,725 men were followed for a median of 10.5 years (interquartile range (IQR): 4.7–11.7), during which 529 HIV seroconversions were observed. Eighty-three (2%) participants died during follow-up, 311 (8%) were lost to follow-up, and 2,802 (75%) were administratively censored.
Participants were mostly white non-Hispanic (82%) and college graduates (59%). At baseline, members of this sexually active population reported a median of 1 (IQR: 0–2) partner in the prior two years. Illicit drug use was common (77%), as was smoking (51%) and most participants consumed alcohol (9% nondrinkers) but at a generally moderate level: median 8 drinks/week (IQR: 2–16) (Table 1). Over follow-up, HIV risk behaviors were less prevalent: participants reported no partners at 78% of follow-up visits; illicit drug use was reported at 48% of follow-up visits, and median alcohol consumption was 4 drinks/week (IQR: 2–12) over follow-up, each measured in the prior six months.
Table 1.
Enrollment characteristics of 529 Multicenter AIDS Cohort Study HIV seroconverters and 3,196 HIV-seronegative participants
| Seroconverters (n = 529) |
Seronegative Participants (n = 3,196) |
Total (n = 3,725) |
||||
|---|---|---|---|---|---|---|
| Characteristic | n | % | n | % | n | % |
| Median age at baseline in years (IQR) |
30.8 (26.3, 36.4) | 33.7 (28.4, 40.0) | 33.4 (28.0, 39.6) | |||
| Race/ethnicity: | ||||||
| White non-Hispanic | 440 | 83.2 | 2,623 | 82.1 | 3,063 | 82.2 |
| White Hispanic | 38 | 7.2 | 166 | 5.2 | 204 | 5.5 |
| Black | 51 | 9.6 | 407 | 12.7 | 458 | 12.3 |
| College graduate | 274 | 51.8 | 1921 | 60.1 | 2195 | 58.9 |
| US city: | ||||||
| Baltimore, Maryland | 134 | 25.3 | 882 | 27.6 | 1,016 | 27.3 |
| Chicago, Illinois | 123 | 23.3 | 687 | 21.5 | 810 | 21.7 |
| Los Angeles, California | 118 | 22.3 | 874 | 27.3 | 992 | 26.6 |
| Pittsburgh, Pennsylvania |
154 | 29.1 | 753 | 23.6 | 907 | 24.3 |
| Median alcohol consumptiona in drinks/week (IQR) |
8 (4, 16) | 8 (2, 14) | 8 (2, 16) | |||
| 0 | 29 | 5.5 | 286 | 8.9 | 315 | 8.5 |
| 1–14 | 327 | 61.8 | 2,119 | 66.3 | 2,446 | 65.7 |
| >14 | 173 | 32.7 | 791 | 24.7 | 964 | 25.9 |
| Smokera | 292 | 55.2 | 1591 | 49.8 | 1883 | 50.6 |
| Depressive symptomsa,b | 88 | 16.6 | 588 | 18.4 | 676 | 18.1 |
| Illicit drug usea,c | 466 | 88.1 | 2,387 | 74.7 | 2,853 | 76.6 |
| Number of URAI partnersa |
||||||
| 0 −1 | 225 | 42.5 | 2,380 | 74.5 | 2,605 | 69.9 |
| >1 | 304 | 57.5 | 816 | 25.5 | 1,120 | 30.1 |
| Sexually transmitted infectionsa,d |
63 | 11.9 | 215 | 6.7 | 278 | 7.5 |
Abbreviations: IQR, interquartile range; URAI, unprotected receptive anal intercourse.
Prior two years.
Center for Epidemiologic Studies Depressions (CES-D) >16.
Marijuana/hash, cocaine/crack cocaine, or poppers.
Chlamydia or gonorrhea.
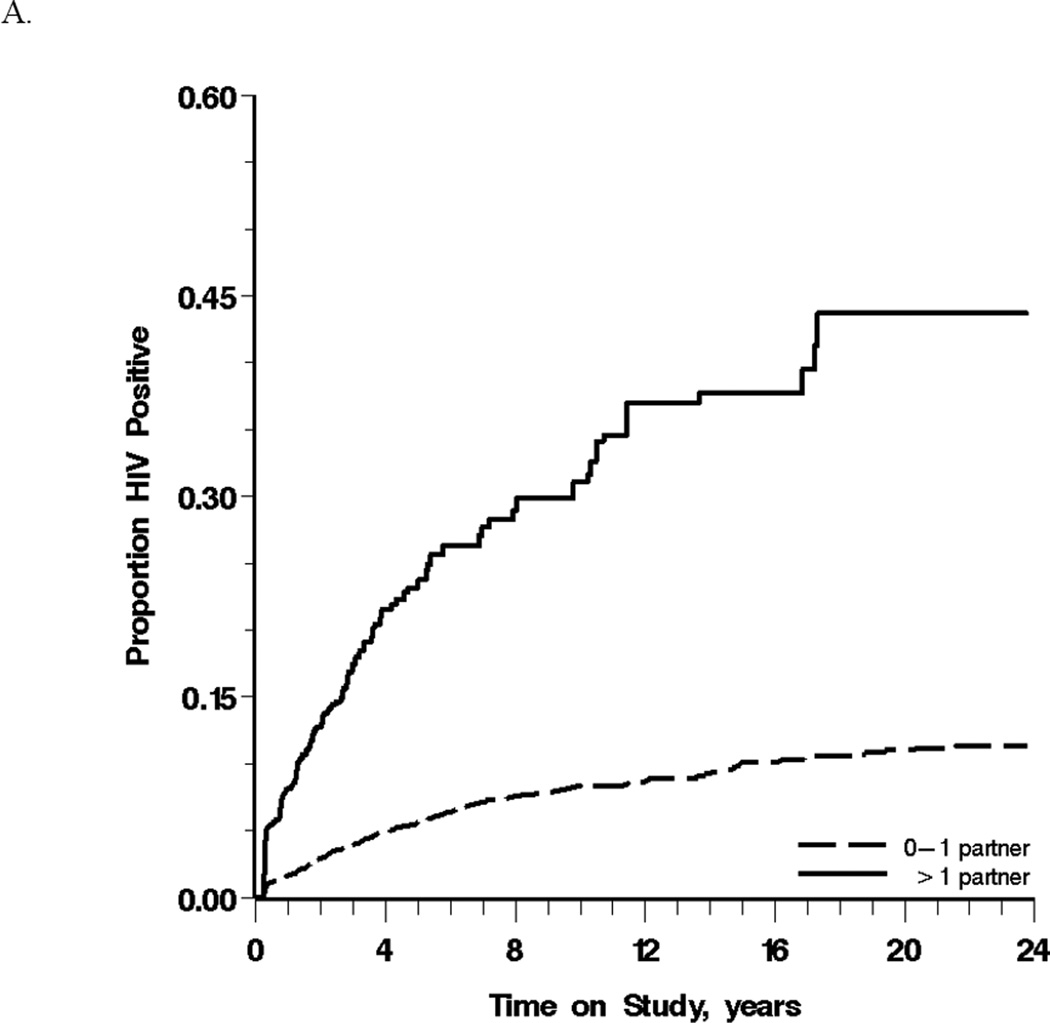
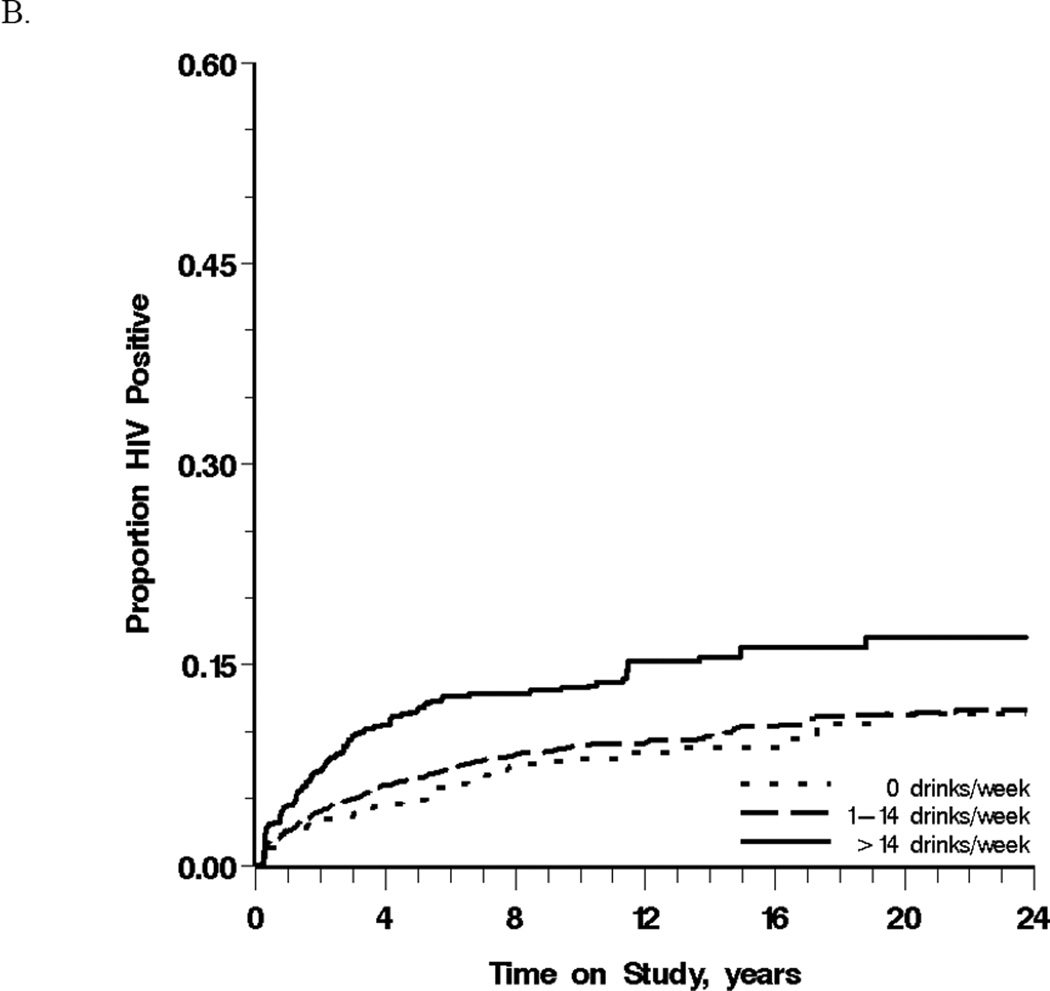
Reports of heavy drinking were most common from white, non-Hispanics (91%) compared to white Hispanics and Black, illicit drug users (73%) compared to non-users, and men reporting multiple partners (13%) compared to those reporting 0–1 partners. Men reporting 0–1 or >1 partner on average over the prior year experienced crude HIV incidence rates of 9 (95% CL: 8, 10) and 76 (95% CL: 66, 86) cases per 1,000 person-years, respectively. Figure 1A depicts this cumulative incidence for these two groups in the weighted population. For nondrinkers, moderate drinkers and heavy drinkers over the prior year the crude incidence of HIV seroconversion were 10 (95% CL: 7, 13), 13 (95% CL: 12, 15), and 26 (95% CL: 22, 31) cases per 1,000 person-years, respectively (Table 2). Heavy drinkers were most likely to HIV seroconvert over follow-up in the weighted population (χ2 Wald trend test P <0.001) (Figure 1B).
Figure 1.
Cumulative incidence of HIV seroconversion between 1984 and 2007 among 3,725 men who have sex with men, stratified by (A) the number of sexual partners with whom the participant was the receptive anal intercourse partner and (B) alcohol consumption, in the prior year.
Table 2.
Effect of alcohol consumptiona on HIV seroconversion among 3,725 men in the Multicenter AIDS Cohort Study between 1984 and 2007.
| Unadjusted |
Adjustedb |
Weightedc |
||||||
|---|---|---|---|---|---|---|---|---|
| Number of Seroconversions |
PY | HR | 95% CL | HR | 95% CL | HR | 95% CL | |
| Nondrinker | 40 | 4,062.98 | 1. | 1. | 1. | |||
| Moderate Drinker | 340 | 26,084.75 | 1.11 | 0.80, 1.54 | 0.91 | 0.65, 1.27 | 1.10 | 0.78, 1.54 |
| Heavy Drinker | 149 | 5,722.04 | 1.52 | 1.07, 2.16 | 1.19 | 0.83, 1.70 | 1.61 | 1.12, 2.29 |
Abbreviations: CL, confidence limits; HR, hazard ratio; PY, person-years
Alcohol consumption in the prior year: nondrinker (0 drinks/week), moderate drinker (0–14 drinks/week), heavy drinker (>14 drinks/week).
Cox proportional hazards model adjusted for baseline (age (spline), race, ethnicity, education) covariates and time-dependent (illicit drug use, cigarette smoking, depression, sexually transmitted infection, and multiple unprotected receptive anal intercourse partners) covariates lagged one visit.
Cox proportional hazards model weighted to account for confounding and selection bias by time-dependent covariates lagged one visit and adjusted for baseline covariates.
Table 2 presents the effects of alcohol consumption on HIV seroconversion from models that average over partners. In unadjusted, adjusted, and weighted models, the hazard for moderate drinkers was similar to that for nondrinkers, whereas the hazard for heavy drinkers was elevated. Compared to the unadjusted HR for heavy drinkers of 1.52 (95% CL: 1.07, 2.16), adjustment for age, race, ethnicity, study site, depression symptoms, college graduation, smoking, illicit drug use, number of partners, and sexually transmitted infection using standard methods produced an attenuated HR of 1.19 (95% CL: 0.83, 1.70). After accounting for the same variables using marginal structural models, the hazard of HIV seroconversion for heavy drinkers in the past year was 1.61 (95% CL: 1.12, 2.29) times that of nondrinkers.
Table 3 presents the joint effects of alcohol consumption and partners in the prior year on HIV seroconversion from models that include a product term. We represent these joint effects in two ways: (1) by examining the effect of alcohol consumption within strata of partners; and (2) by presenting HRs relative to a common referent: nondrinkers without multiple partners. In the weighted model, the association between heavy drinking and HIV seroconversion appeared stronger among men with multiple partners (HR = 1.96; 95% CL: 1.03, 3.72) versus without (HR = 1.37; 95% CL: 0.88, 2.16), although this difference was imprecise (P = 0.42). The observed HR for moderate drinkers with multiple partners compared to nondrinkers without multiple partners (HR = 4.48) was similar to those expected under a multiplicative model (expected HR = 4.04). The observed HR for heavy drinkers with multiple partners compared to nondrinkers without multiple partners was larger than expected under a multiplicative model: HR = 7.40 and expected HR = 5.18, although again this departure was imprecise (Table 3). With respect to interaction on the additive scale, the RERI suggests departure from additivity for heavy drinkers with multiple partners (RERI = 3.25; 95% CL: 0.32, 6.17) but not moderate drinkers with multiple partners (RERI = 0.63; 95% CL: −1.81, 3.07).
Table 3.
Effects of alcohol consumptiona and risky sexual behavior in the prior year on HIV seroconversion among 3,725 men in the Multicenter AIDS Cohort Study between 1984 and 2007.
| ≤1 URAI Partner |
>1 URAI Partner |
||||||
|---|---|---|---|---|---|---|---|
| HRb | 95% CL | HRb | 95% CL | HRc | 95% CL | P- valued |
|
| Unadjusted | 0.50 | ||||||
| Nondrinker | 1. | 4.12 | 2.04, 8.32 | 1. | |||
| Moderate drinker | 1.07 | 0.73, 1.58 | 5.04 | 3.33, 7.63 | 1.22 | 0.66, 2.27 | |
| Heavy drinker | 1.31 | 0.84, 2.05 | 7.62 | 4.97, 11.70 | 1.85 | 0.99, 3.46 | |
| Adjustede | 0.28 | ||||||
| Nondrinker | 1. | 3.21 | 1.57, 6.60 | 1. | |||
| Moderate drinker | 0.86 | 0.58, 1.28 | 3.31 | 2.15, 5.10 | 1.03 | 0.55, 1.94 | |
| Heavy drinker | 0.97 | 0.61, 1.52 | 4.97 | 3.18, 7.77 | 1.55 | 0.81, 2.94 | |
| Weightedf | 0.42 | ||||||
| Nondrinker | 1. | 3.78 | 1.82, 7.82 | 1. | |||
| Moderate drinker | 1.07 | 0.72, 1.59 | 4.48 | 2.91, 6.88 | 1.19 | 0.63, 2.24 | |
| Heavy drinker | 1.37 | 0.88, 2.16 | 7.40 | 4.74, 11.54 | 1.96 | 1.03, 3.72 | |
Abbreviations: CL, confidence limits; HR, hazard ratio; URAI, unprotected receptive anal intercourse.
Alcohol consumption in the prior year: non-drinker (0 drinks/week), moderate drinker (0–14 drinks/week), heavy drinker (>14 drinks/week).
Nondrinkers with ≤ 1 URAI partner as referent.
Nondrinkers with >1 URAI partner as referent.
Joint robust Wald χ2 test.
Adjusted for baseline (age (spline), race, ethnicity, education) covariates and time-dependent (illicit drug use, cigarette smoking, depression, sexually transmitted infection) covariates lagged one visit.
Weighted to account for confounding and selection bias by time-dependent covariates lagged one visit and adjusted for baseline covariates.
Again, compared to results from the standard Cox model, results from the weighted model suggested a stronger effect. For example, when heavy drinkers with multiple partners were compared to nondrinkers without multiple partners, the HR from the marginal structural model was 7.40 (95% CL: 4.74, 11.54) and the analogous HR from the standard adjusted model was 4.97 (95% CL: 3.18, 7.77) (Table 3). Similar to the weighted analysis, no statistically significant evidence was found for greater-than-multiplicative interaction in the standard analysis (P = 0.28) (Table 3).
DISCUSSION
We reported the effect of alcohol consumption and risky sexual behavior on HIV seroconversion in prospective data on 3,725 MSM followed in 1984–2008. Heavy alcohol consumption was associated with 1.61 times the hazard of HIV seroconversion compared to no consumption. We furthermore presented results from models that use traditional adjustment approaches alongside results from weighted models showing that traditional approaches produced attenuated estimates, although confidence intervals dod overlap. Without results from the weighted models, we might have erroneously concluded that there was no independent association between alcohol consumption and HIV seroconversion.
The unadjusted HR of 1.52 we reported for heavy alcohol consumption versus nondrinking is similar to unadjusted HRs reported in previous studies of MSM populations [4, 10–13, 30] although it is noteworthy that these studies defined alcohol consumption differently and are therefore not directly comparable. We reported an attenuated HR of 1.19 when adjusted for time-dependent confounders using traditional adjustment. This attenuation mirrors that reported by the San Francisco Men’s Health Study[12] and in an earlier report of MACS data [13]. The latter study reported an unadjusted HR of 2.05 (95% CL: 1.53, 2.74) for heavy versus less-than-heavy drinking but an HR of 1.13 (95% CL: 0.81, 1.56) after adjustment for time-dependent confounders. A statistically significant adjusted association between heavy alcohol consumption and HIV seroconversion persisted in only the EXPLORE cohort (HR= 1.97; 95% CL: 1.32, 2.96) [10]. Our findings and the majority of previous studies suggest that standard adjustment removes part of the indirect effect of alcohol consumption acting through other time-dependent HIV risk factors for which authors have previously adjusted (e.g., illicit drug use).
Changes in behaviors and expectancy that accompany alcohol consumption may be responsible for increased sexual risk behaviors and for subsequent HIV seroconversion observed among heavy drinkers [31, 32]. Alcohol consumption is associated with higher numbers of sexual partners, higher numbers of unprotected anal sex acts, and condom failure [33, 34]. Acute alcohol consumption has also been linked to suppression of both the innate and adaptive immune response and increased susceptibility to numerous infections, including HIV [32, 35–37].
A limitation of the present data is collection of alcohol consumption measures that require recall over a 6-month period. As researchers have stated previously, global measures of alcohol consumption do not allow investigation of specific contextual modifiers of the relationships between alcohol consumption and sexual risk behaviors such as partner type and partner’s alcohol consumption [6, 38, 39]. These contextual factors in turn may explain why we did not see a dramatic departure from multiplicative combination between the effects of alcohol consumption and sexual risk behavior on HIV seroconversion. For example, researchers have found that MSM are more likely to use condoms with casual partners while drinking but less likely to use condoms with steady partners in the same setting [40–42].
Self-reported alcohol consumption may also be an inadequate proxy for alcohol-induced responses. The behavioral and physiologic affects of alcohol may be person-specific, dependent on genetic background, body mass composition and diet. Future research applying biomarkers of alcohol consumption to evaluate the reliability or accuracy of self-report data is needed in studies of sexual health.
As with any analysis, the validity of our inferences is limited by the degree to which we met our assumptions. First, we assumed no unmeasured confounding and no informative censoring due to unmeasured factors. However, there are likely unmeasured behavioral factors confounding the observed association. Moreover, we acknowledge that measurement of the confounders we included is imperfect (e.g., self-reported as opposed to directly assessed sexually transmitted infections) and may result in residual confounding. Second, we assumed that heavy drinkers who seroconverted during the study period are representative of those who seroconverted prior to study entry. Men who seroconverted prior to study entry may have engaged in more concomitant high-risk behaviors than those whose seroconversions were observed, leading us to observe a weaker association between alcohol consumption and HIV seroconversion. Third, we assumed that mode of exposure is irrelevant to the observed outcome [43]. However, the behavioral mechanisms described above suggest that ignoring type of alcohol consumed and timing of its consumption with respect to HIV exposure may not be reasonable and may limit our ability to prescribe generalizable interventions based on our findings. Nevertheless, the demonstrable effect of alcohol consumption, measured broadly, is valuable in that it supports research to identify the particular means of exposure relevant for interventions.
This study has several important strengths. First, it used information from a large prospective cohort of sexually active MSM followed for over 2 decades. Second, a large number of HIV seroconversions were observed, including a sizable portion among men previously reporting heavy drinking. Finally, using state-of-the-art quantitative methods, this study more fully captures the direct and indirect effects of alcohol consumption on HIV seroconversion-- specifically, both the direct effect of alcohol on HIV susceptibility and the indirect effects mediated through HIV risk behaviors, such as illicit drug use that are also affected by prior alcohol consumption.
The potential for linking alcohol interventions with HIV prevention activities was described more than a decade ago [44], and randomized interventions that explicitly address alcohol’s contribution to HIV have been tested in Africa [45–47]. However, such interventions have lagged behind for US adult MSM [39]. We have reported an effect of alcohol consumption on HIV seroconversion among MSM of similar magnitude to illicit drugs such as methamphetamine, cocaine, and ecstasy [13]. Under the above-stated assumptions, our results support the conclusion that 16% of HIV seroconversions among heavy drinkers could be prevented if half of these drinkers reduced their drinking to moderate levels; 21% could be prevented if two-thirds reduced their drinking to moderate levels [48]. If replicated, our findings renew the call for population-level HIV interventions among US MSM that explicitly address heavy alcohol consumption.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Chanelle Howe, PhD and Myron Cohen, MD for expert advice and Ms. Debby Anderson for editorial assistance.
This work was supported by the National Institute on Alcohol Abuse and Alcoholism [grant number R01-AA-01759 to S.R.C.]. Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS) with centers (Principal Investigators) at The Johns Hopkins Bloomberg School of Public Health (Joseph B. Margolick, Lisa P. Jacobson), Howard Brown Health Center, Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services (John P. Phair, Steven M. Wolinsky), University of California, Los Angeles (Roger Detels), and University of Pittsburgh (Charles R. Rinaldo). The MACS is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute [grant numbers UO1-AI-35042, 5-MO1-RR-00052 (GCRC), UO1-AI-35043, UO1-AI-35039, UO1-AI-35040, UO1-AI-35041]. Website located at http://www.statepi.jhsph.edu/macs/macs.html.
Footnotes
Conflicts of interest and source of funding: All authors declare no conflict of interest.
Author contributions: Dr. Sander had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Individual authorship roles were as follows: study concept and design: Sander, Cole, Jacobson, Ostrow; acquisition of data: Cole, Jacobson; analysis and interpretation of data: Sander, Cole, Stall, Eron, Napravnik, Gaynes, Jacobson, Johnson-Hill, Bolan, Ostrow; drafting of the manuscript: Sander; Critical revision of the manuscript for important intellectual content: Sander, Cole, Stall, Eron, Napravnik, Gaynes, Jacobson, Johnson-Hill, Bolan, Ostrow; statistical analysis: Sander, Cole; Obtained funding: Cole, Jacobson, Ostrow; administrative, technical, or material support: Stall, Jacobson, Johnson-Hill, Bolan, Ostrow; supervision: Cole, Eron, Napravnik, Gaynes.
REFERENCES
- 1.Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300:520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kingsley LA, Detels R, Kaslow R, et al. factors for seroconversion to human immunodeficiency virus among male homosexuals. Results from the Multicenter AIDS Cohort Study. Lancet. 1987;1:345–349. doi: 10.1016/s0140-6736(87)91725-9. [DOI] [PubMed] [Google Scholar]
- 3.Detels R, English P, Visscher BR, et al. Seroconversion, sexual activity, and condom use among 2915 HIV seronegative men followed for up to 2 years. J Acquir Immune Defic Syndr. 1989;2:77–83. [PubMed] [Google Scholar]
- 4.Page-Shafer K, Veugelers PJ, Moss AR, Strathdee S, Kaldor JM, van Griensven GJ. Sexual risk behavior and risk factors for HIV-1 seroconversion in homosexual men participating in the Tricontinental Seroconverter Study, 1982–1994. Am J Epidemiol. 1997;146:531–542. doi: 10.1093/oxfordjournals.aje.a009311. [DOI] [PubMed] [Google Scholar]
- 5.Stall R, Paul JP, Greenwood G, et al. Alcohol use, drug use and alcohol-related problems among men who have sex with men: the Urban Men's Health Study. Addiction. 2001;96:1589–1601. doi: 10.1046/j.1360-0443.2001.961115896.x. [DOI] [PubMed] [Google Scholar]
- 6.Woolf SE, Maisto SA. Alcohol use and risk of HIV infection among men who have sex with men. AIDS Behav. 2009;13:757–782. doi: 10.1007/s10461-007-9354-0. [DOI] [PubMed] [Google Scholar]
- 7.Howe CJ, Sander PM, Plankey MW, Cole SR. Effects of time-varying exposures adjusting for time-varying confounders: the case of alcohol consumption and risk of incident human immunodeficiency virus infection. Int J Public Health. 2010;55:227–228. doi: 10.1007/s00038-010-0120-0. [DOI] [PubMed] [Google Scholar]
- 8.Baliunas D, Rehm J, Irving H, Shuper P. Alcohol consumption and risk of incident human immunodeficiency virus infection: a meta-analysis. Int J Public Health. 2010;55:159–166. doi: 10.1007/s00038-009-0095-x. [DOI] [PubMed] [Google Scholar]
- 9.Shuper PA, Neuman M, Kanteres F, Baliunas D, Joharchi N, Rehm J. Causal considerations on alcohol and HIV/AIDS--a systematic review. Alcohol Alcohol. 2010;45:159–166. doi: 10.1093/alcalc/agp091. [DOI] [PubMed] [Google Scholar]
- 10.Koblin BA, Husnik MJ, Colfax G, et al. Risk factors for HIV infection among men who have sex with men. AIDS. 2006;20:731–739. doi: 10.1097/01.aids.0000216374.61442.55. [DOI] [PubMed] [Google Scholar]
- 11.Penkower L, Dew MA, Kingsley L, et al. Behavioral, health and psychosocial factors and risk for HIV infection among sexually active homosexual men: the Multicenter AIDS Cohort Study. Am J Public Health. 1991;81:194–196. doi: 10.2105/ajph.81.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chesney MA, Barrett DC, Stall R. Histories of substance use and risk behavior: precursors to HIV seroconversion in homosexual men. Am J Public Health. 1998;88:113–116. doi: 10.2105/ajph.88.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plankey MW, Ostrow DG, Stall R, et al. The relationship between methamphetamine and popper use and risk of HIV seroconversion in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2007;45:85–92. doi: 10.1097/QAI.0b013e3180417c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Howe CJ, Cole SR, Ostrow DG, Mehta SH, Kirk GD. A prospective study of alcohol consumption and HIV acquisition among injection drug users. AIDS. 2011;25:221–228. doi: 10.1097/QAD.0b013e328340fee2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126:310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 18.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 19.Dudley J, Jin S, Hoover D, Metz S, Thackeray R, Chmiel J. The Multicenter AIDS Cohort Study: retention after 9 1/2 years. Am J Epidemiol. 1995;142:323–330. doi: 10.1093/oxfordjournals.aje.a117638. [DOI] [PubMed] [Google Scholar]
- 20.Department of Agriculture, Department of Health and Human Services (US) Dietary guidelines for Americans,2010. 7th ed. ed. Washington, DC: USDA; 2011. [Google Scholar]
- 21.Ostrow DG, Plankey MW, Cox C, et al. Specific sex drug combinations contribute to the majority of recent HIV seroconversions among MSM in the MACS. J Acquir Immune Defic Syndr. 2009;51:349–55. doi: 10.1097/QAI.0b013e3181a24b20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sander PM, Cole SR, Ostrow DG, et al. 43rd Annual Meeting of the Society for Epidemiologic Research. supplement 11. Vol. 171. Seattle, WA: American Journal of Epidemiology; 2010. Predictors of alcohol consumption among U.S. men who have sex with men (abstract #607-S) p. pS151. [Google Scholar]
- 23.Sander PM, Cole SR, Ostrow DG, Mehta SH, Kirk GD. Determinants of alcohol consumption in HIV-uninfected injection drug users. Drug Alcohol Depend. 2010;111:173–176. doi: 10.1016/j.drugalcdep.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the joint causal effect of nonrandomized treatments. J Am Stat Assoc. 2001;96:440–448. [Google Scholar]
- 25.D'Agostino RB, Lee ML, Belanger AJ, Cupples LA, Anderson K, Kannel WB. Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study. Stat Med. 1990;9:1501–1515. doi: 10.1002/sim.4780091214. [DOI] [PubMed] [Google Scholar]
- 26.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cole SR, Hernan MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75:45–49. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Li R, Chambless L. Test for additive interaction in proportional hazards models. Ann Epidemiol. 2007;17:227–236. doi: 10.1016/j.annepidem.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Cox DR. Regression models and life tables (with discussion) J R Stat Soc (B) 1972;24:187–220. [Google Scholar]
- 30.Kippax S, Campbell D, Van de Ven P, et al. Cultures of sexual adventurism as markers of HIV seroconversion: a case control study in a cohort of Sydney gay men. AIDS Care. 1998;10:677–88. doi: 10.1080/09540129848307. [DOI] [PubMed] [Google Scholar]
- 31.Steele CM, Josephs RA. Alcohol myopia. Its prized and dangerous effects. Am Psychol. 1990;45:921–933. doi: 10.1037//0003-066x.45.8.921. [DOI] [PubMed] [Google Scholar]
- 32.Dingle GA, Oei TP. Is alcohol a cofactor of HIV and AIDS? Evidence from immunological and behavioral studies. Psychol Bull. 1997;122:56–71. doi: 10.1037/0033-2909.122.1.56. [DOI] [PubMed] [Google Scholar]
- 33.Greenwood GL, White EW, Page-Shafer K, et al. Correlates of heavy substance use among young gay and bisexual men: The San Francisco Young Men’s Health Study. Drug Alcohol Depend. 2001;61:105–112. doi: 10.1016/s0376-8716(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 34.Stone E, Heagerty P, Vittinghoff E, et al. Correlates of condom failure in a sexually active cohort of men who have sex with men. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:495–501. doi: 10.1097/00042560-199904150-00013. [DOI] [PubMed] [Google Scholar]
- 35.Bagasra O, Kajdacsy-Balla A, Lischner HW, Pomerantz RJ. Alcohol intake increases human immunodeficiency virus type 1 replication in human peripheral blood mononuclear cells. J Infect Dis. 1993;167:789–797. doi: 10.1093/infdis/167.4.789. [DOI] [PubMed] [Google Scholar]
- 36.Cook RT. Alcohol abuse, alcoholism, and damage to the immune system--a review. Alcohol Clin Exp Res. 1998;22:1927–1942. [PubMed] [Google Scholar]
- 37.Balla AK, Lischner HW, Pomerantz RJ, Bagasra O. Human studies on alcohol and susceptibility to HIV infection. Alcohol. 1994;11:99–103. doi: 10.1016/0741-8329(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 38.Leigh BC, Stall R. Substance use risky sexual behavior for exposure to HIV Issues in methodology, interpretation, and prevention. Am Psychol. 1993;48:1035–1045. doi: 10.1037//0003-066x.48.10.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heath J, Lanoy A, Maisto S. The role of alcohol and substance use in risky sexual behavior among older men who have sex with men: a review and critique of the current literature. AIDS Behav. 2011 doi: 10.1007/s10461-011-9921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanable PA, McKirnan DJ, Buchbinder SP, et al. Alcohol use and high-risk sexual behavior among men who have sex with men: the effects of consumption level and partner type. Health Psychol. 2004;23:525–532. doi: 10.1037/0278-6133.23.5.525. [DOI] [PubMed] [Google Scholar]
- 41.Seage GR, 3rd, Mayer KH, Wold C, et al. The social context of drinking, drug use, and unsafe sex in the Boston Young Men Study. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:368–375. doi: 10.1097/00042560-199804010-00012. [DOI] [PubMed] [Google Scholar]
- 42.Stueve A, O'Donnell L, Duran R, San Doval A, Geier J. Being high and taking sexual risks: findings from a multisite survey of urban young men who have sex with men. AIDS Educ Prev. 2002;14:482–495. doi: 10.1521/aeap.14.8.482.24108. [DOI] [PubMed] [Google Scholar]
- 43.Cole SR, Frangakis CE. The consistency statement in causal inference: a definition or an assumption? Epidemiology. 2009;20:3–5. doi: 10.1097/EDE.0b013e31818ef366. [DOI] [PubMed] [Google Scholar]
- 44.Shoptaw S, Frosch D. Substance abuse treatment as HIV prevention for men who have sex with men. AIDS Behav. 2000;4:193–203. [Google Scholar]
- 45.Kalichman SC, Simbayi LC, Vermaak R, Cain D, Jooste S, Peltzer K. HIV/AIDS risk reduction counseling for alcohol using sexually transmitted infections clinic patients in Cape Town, South Africa. J Acquir Immune Defic Syndr. 2007;44:594–600. doi: 10.1097/QAI.0b013e3180415e07. [DOI] [PubMed] [Google Scholar]
- 46.Kalichman SC, Simbayi LC, Vermaak R, et al. Randomized trial of a community-based alcohol-related HIV risk-reduction intervention for men and women in Cape Town South Africa. Ann Behav Med. 2008;36:270–279. doi: 10.1007/s12160-008-9067-2. [DOI] [PubMed] [Google Scholar]
- 47.Stanton BF, Li X, Kahihuata J, et al. Increased protected sex and abstinence among Namibian youth following a HIV risk-reduction intervention: a randomized, longitudinal study. AIDS. 1998;12:2473–80. doi: 10.1097/00002030-199818000-00017. [DOI] [PubMed] [Google Scholar]
- 48.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.