Summary
Despite enormous data collection and analysis efforts, the genetic influences on common epilepsies remain mostly unknown. We propose that reasons for the lack of progress can be traced to three factors: (1) A reluctance to consider fine-grained phenotype definitions based on extensive and carefully collected clinical data; (2) the pursuit of genetic analysis methods that are popular but poorly conceived and are inadequate to the task of resolving the problems inherent in common disease studies; (3) preconceived ideas about the genetic mechanisms that cause epilepsy (which we have discussed elsewhere). We propose a paradigm for finding epilepsy-related loci and alleles that has proven successful in other common diseases.
Keywords: Exome, Genome-wide association, Linkage analysis, Phenotype, Single nucleotide polymorphisms, Whole genome sequencing
The initial optimism for finding common epilepsy genes when the field was younger has given way to doubt. Yet the optimism was justified. Considering the problems in identifying common disease genes in general, genetic studies of epilepsy have distinct advantages. (1) Seizures are unambiguous event-based traits, unlike, for example, neuropsychiatric disorders (e.g., depression), whose symptoms overlap with normal behavior. (2) The epilepsies with the strongest genetic influence (e.g., idiopathic generalized epilepsy [IGE], benign rolandic epilepsy) show strong familiality and no known environmental risk factors. (3) The phenotypes (seizure types and patterns), although overlapping, appear to have differing genetic influences, making it possible to differentiate those influences (Durner et al., 2001; Winawer et al., 2005). (4) Patient-related subclinical markers (e.g., abnormal electroencephalography [EEG] patterns and neuropsychological traits) occur in family members unaffected by seizures (e.g., Tsuboi & Christian, 1973; Durner et al., 1991; Bali et al., 2007; Pal et al., 2010), which increases the power to find the underlying genetic influences. (5) The existing evidence suggests that the IGEs are caused by a small number of genes (an oligogenic model of inheritance) (Greenberg et al., 1992; Durner et al., 2001; Winawer et al., 2002).
Despite these apparent advantages, progress in understanding genetics of the common epilepsies has been disappointing. Part of the reason for this, we have asserted (Greenberg & Subaran, 2011), has been an undue focus on the idea that “epilepsy” is a “channelopathy.” However, there are other, perhaps more important factors that we discuss in this article, specifically: (1) The issue of phenotype definition and (2) the use and misuse of the existing genetic methods.
Phenotype
It is perplexing that many epilepsy genetic studies persist in treating IGE as though it were a unitary phenotype. The range of phenotypic syndromes that fall under the classification of IGE is notable—childhood absence, juvenile absence, juvenile myoclonic epilepsy (JME), epilepsy with generalized tonic–clonic seizures (and variations) (Greenberg et al., 1995), and awakening grand mal, photomyoclonic, or photoconvulsive seizures, to name a few. The assumption that these diverse phenotypes share the same genotype is similar to assuming, for example, that all “mental retardation” draws on the same narrow set of genes and that, by lumping them together, some of those genes will “show up.” That belief has been enhanced over the last decade as genetic analysis methods abandoned concerns about actual inheritance and focused on “risk,” that is, focused on detecting association of single nucleotide polymorphisms (SNPs) with disease. These approaches yielded many loci of often miniscule odds ratios (i.e., small contributions to the difference between cases and controls), but “statistically significant” effect. Because these methods emphasized the “many minor contributions” to disease model—essentially a polygenic model—the idea of a strong genetic “cause,” rather than “risk,” seems to have been lost and the new focus became improving risk prediction.
This vagueness in phenotype definition can devastate positive findings in a genetic study because it leads to heterogeneity. If one is aware of the effects of heterogeneity and accepts that multiple genotypes can cause similar-looking phenotypes, then an effective data collection approach will (1) focus on narrowing the phenotype definition to be used for data collection so that all subjects are as clinically identical as possible and (2) collect sufficient clinical data to test for the existence of heterogeneity even after data are collected. For example, dividing a sample into separate phenotypic groups allowed observation of a genetic signal in an IGE group—a signal that was masked when all groups were analyzed together (Durner et al., 1999, 2001) (see Fig. 1).
Figure 1.
Effect of heterogeneity on locus detection. Linkage data includes JME and non-JME IGE families. (A) All IGE treated as one phenotype. (B) JME families analyzed alone. (C) Non-JME families analyzed alone. Analysis of all data together and using heterogeneity lod scores (HLOD [not shown]) did not show significant evidence of linkage but treating the non-JME families separately gave significant results. (Blue lines show analysis including only subjects with seizures. Red lines include family members without seizures but with abnormal EEG studies) [Adapted from Durner et al., 1999].
Fine-grained phenotype definitions obviously make data collection a more difficult task because it becomes harder to reach a critical mass for any one of the forms. Furthermore, one must also obtain a careful clinical history and be willing to eliminate patients from the study if they do not meet the exacting criteria. Nonetheless, such efforts are essential if the data collection is not to be a wasted effort. Unfortunately, diagnoses just for treatment have all too often proven inadequate for genetic studies. Here is an example of why:
History: The patient was neurologically well until the seizures began in his late teens. His seizures are generalized tonic–clonic [emphasis added], without warning. Video monitoring documented EEG consistent with juvenile myoclonic epilepsy. The patient is seizure-free on his current medication.
Complaint: Right hand shakes while using a tool. Hand tremors secondary to muscle fatigue.
Procedures: Video-EEG monitoring [Several days of EEG monitoring off antiseizure medication without any seizures or abnormal EEG patterns]. One year later, EEG monitoring showed no abnormalities.
This subject was referred as a patient with JME because of the presence of 4–6 Hz spikes and waves on EEG; no myoclonus was reported. This case is easier to reject than many others because there were no documented myoclonias, and the patient denied them except for the hand shaking while using a tool, a symptom identified as secondary to muscle fatigue and unrelated to epilepsy. Therefore, successful treatment based on a specific diagnosis does not automatically make the subject appropriate for genetic studies. But many other cases are not simple and lead to questions such as these: Should myoclonias that do not occur on awakening be counted as JME? How about when onset of seizures is as early as 5 or 6 years of age (Asconape & Penry, 1984; Panayiotopoulos et al., 1994)? Should such patients be assumed to have the same underlying genetics as a case in which the onset age was 14? Should a case with a history of myoclonic absence (nonsyndromic) be classified as a separate entity? The point is, although a misdiagnosis in the above case, and many other cases observed over the years, did not interfere with successful treatment, the patient does not meet the definition of JME for genetic studies. Inclusion of the paient in a study focused on JME would likely degrade any genetic evidence. And these are questions just about JME. How varied are other IGE forms? Yet the temptation to “keep the numbers up” by including questionable patients is a pressure most investigators have experienced.
The recent tendency has been to believe and act as though data quantity is more important than data quality. Even if one just gets a large enough sample (particularly in case–control association studies) to detect something that affects disease expression, that “something” is not likely to be causal and will only slightly increase risk, even if the effect detected is real.
The epilepsy genetics literature is filled with examples of studies looking for genes that cause IGE. In the older literature, there was often an attempt to break down the phenotypes further, but recently the tendency is to lump them, perhaps again acting on the notion that if the sample size is large enough, something will be found. Two examples of such lumping: Klassen et al. (2011) sequenced 237 channel genes (influenced by the assumption that epilepsy is a “channelopathy” Greenberg & Subaran, 2011) in 152 cases of IGE and 139 controls. The selection of IGE patients was based on the following:
“Cases meeting the accepted criteria for either idiopathic or cryptogenic epilepsy (Commission on Classification and Terminology of the International League Against Epilepsy, 1989) were recruited in accordance with the approved study protocol. In the proposed new classification, the terms “idiopathic” and “cryptogenic” have been revised in favor of the term “genetic epilepsy” when describing the underlying etiology of the disorder (Berg et al., 2010), and includes seizures occurring in epilepsy patients with presumed genetic origin.”
Therefore, patients with epilepsy of unknown origin, presumed to be genetic but of diverse phenotypes, were subjected to exonic sequencing. There are a number of interesting features and findings in this excellent work, but a better understanding of what causes IGE is only questionably one of them. Also interesting is mention of the new proposed classification of changing IGE to GGE (genetic generalized epilepsy) in the absence of any understanding of those genetics, except in rare syndromes.
A second example is from Leu et al. (2012), in which a linkage genome scan meta-analysis was performed on data from the EPICURE (Functional Genomics and Neurobiology of Epilepsy) consortium. (We note that this analysis appears to be a joint rather than meta-analysis; data from the different centers are analyzed together.) The only two phenotypic categories that are analyzed separately are patients with myoclonic jerks and those without. As we noted above, there are many different ways of classifying IGEs as separate syndromes. Not to use this large dataset to try to resolve some possible subtypes to generate hypotheses makes inadequate use of the information. In addition to the phenotypic issues, data from different sources were combined, and there is also the question of possible ethnic differences, regional differences, and so on that remain unresolved.
Investigators must stop treating IGE as though it were a single diagnostic and genetic entity, or the field will find itself in the same position as psychiatry with regard to the Axis I disorders—30 years of genetic studies and little to show for them. The clinical entities classified as IGE display a number of differentiable symptoms that may reflect differing underlying genetic influences. These can be fruitfully explored if the clinical data are clear, consistent, and homogenous. As we have shown in previous studies of IGE (Durner et al., 2001; Pal et al., 2003; Greenberg et al., 2005), and in rolandic epilepsy (Bali et al., 2007; Strug et al., 2009), a modest sample with careful clinical diagnosis, and a willingness to discard data that vary from rigorous definitions, can prove the existence of genes with a strong influence on epilepsy expression.
Genetic Methods
Herein we discuss advantages and disadvantages of three basic approaches to genetic analysis: association analysis, linkage analysis, and whole exome/genome sequencing.
Association
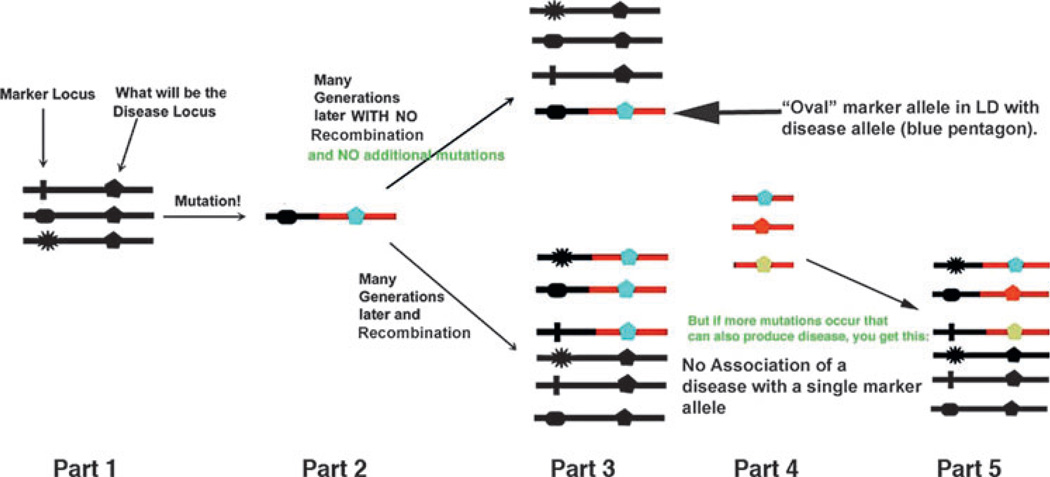
Association studies look for marker allele frequency differences between cases and controls. Such a difference indicates that there may be a disease allele at some locus assumed to be in linkage disequilibrium (LD) with the marker allele. There are advantages to this approach. (1) Association analysis is sensitive and can detect even minor influences on disease expression, but, as we shall see, major influences for common diseases are often missed. (2) This type of analysis requires collecting only cases and controls, not families. However, these advantages can also be disadvantages. (1) Is a discovered effect worth pursuing? Working to understand how any locus affects disease expression is resource intensive. Will expending those resources on a locus of unknown importance ultimately yield useful information? (2) Looking only at cases and controls yields no direct information about inheritance; such information requires family data. (3) Association analysis usually assumes the existence of LD between a single marker allele and a single disease-related allele. If there are multiple disease-related alleles at a disease-related locus, then it becomes more difficult to find association with any one of the disease alleles. The usual assumption is that there is no allelic heterogeneity, that is, there is only one disease allele at a locus (see Fig. 2). Therefore, important effects of a locus (such as the cumulative effect of several disease alleles) can easily be missed in an association analysis.
Figure 2.
How LD works. Part 1: the marker locus with multiple alleles is “next to” a coding locus with only one allele (the pentagon). Part 2: a mutation in a single person occurs at what is now becomes the disease locus. The “oval” allele happens to be the marker allele on the DNA strand with the now mutated allele at the disease locus. Part 3: after generations, if there is no recombination between the marker and disease loci, the mutation remains on the same DNA strand with the “oval” allele. The two alleles are “associated.” The alternative is that there is recombination between the loci, and the “oval” allele is no longer the exclusive neighbor of the disease allele, and there is no association. Part 4: other mutations occur at the disease locus. These occur independently of what the allele at the marker locus happens to be, thus degrading any association between that “oval” allele and the disease. Part 5: whether due to recombination or to multiple disease mutations, there is then no association between marker and disease.
Candidate gene studies are a form of association analysis in which one guesses which kinds of genes may be involved in the mechanisms that produce a disease, and then looks for disease-associated alleles at each of those loci. The candidate genes are usually chosen by guessing at the mechanism and then pursuing genes based on that guess. Any positive results are then used to justify the presumed mechanism and pursue more candidates based on the same mechanism.
The most extreme version of association studies is genome-wide association studies (GWAS), which test 105–106 or more polymorphic SNP loci over the genome. In order to correct for the vast number of statistical tests, significant p-values must be incredibly small, according to current opinion. Cutoffs in the range of 10−7 to 10−8 are common. This stringent criterion also necessitates that the sample sizes of patients and controls be in the thousands or tens of thousands. Almost always, something is detected, usually with odds ratios a little over 1.0, meaning, in what is almost certainly a data set with multiple disease etiologies, that the gene detected has minimal influence on disease expression. If we assume that such associations reflect an actual influence on the disease, that is, not the result of an artifact such as population stratification or a random false positive, then there are two problems. (1) Which gene is actually involved? An association only tells us that the associated marker SNP is in linkage disequilibrium with a disease-related allele at the disease-related locus. The associated SNP might be in the disease locus or it might be some distance away (Enattah et al., 2002). (2) More important, is it worth the years of effort to find out what gene is the right gene, given that the right gene may have minimal influence on disease expression— and it is unclear which aspect of the phenotype, or which disease, it is influencing?
Therefore, the problem with GWAS and candidate gene studies is not that they do not yield results: it is that the results, although statistically significant, can lack strong biologic significance.
Exploiting the results of GWAS is, again, also hampered by a lack of detailed clinical information that would be necessary to actually determine what symptoms or clusters of clinical characteristics correlate with the presence of the marker. The cost of collecting detailed information on the sample sizes necessary would usually be prohibitive.
Nonetheless, association analysis, when used in the correct context, is an essential tool for genetic studies (see below). Once we know that an important gene exists in a specific area of the genome, association analysis and/or sequencing is a next logical step. What we need are the locations of those genes truly important for disease expression, some “pillars of disease,” that we know are worth pursuing, causes around which we can build a full understanding of epilepsy susceptibility. The best way to do that is to collect rigorous phenotypic data and use a genetic analysis method that finds loci of major effect: linkage analysis.
Linkage analysis
Linkage analysis works by looking for cosegregation of disease with marker alleles within families. The basic unit of data in linkage analysis is the family, and linkage yields information about inheritance, about the movement of alleles from one generation to the next, and how that movement correlates with the presence of disease. Furthermore, the underlying biologic process that drives linkage is recombination between loci. Unlike association analysis, the results of linkage analysis are unaffected by allelic heterogeneity. This also means that linkage analysis can yield information about genetic variables that are often beyond the reach of association: mode of inheritance, penetrance, gene–gene interaction, and locus heterogeneity.
Linkage has been the first step in finding loci for more identified and confirmed genes than any other technique. Its greatest successes have been for Mendelian diseases, but also for some common diseases, including type 1 diabetes mellitus, inflammatory bowel disease, autoimmune thyroid disease, and even some epilepsy loci. Nonetheless, there are problematic factors that can lead to failure to find true disease-influencing loci. Some of those failures originate in the way that data have been collected and the way linkage analysis has been used (Greenberg, 2011). The most problematic factors are, again, heterogeneity and incorrect phenotype definition—confounders affecting both linkage and association analysis—but the wealth of information contained in family data can help us resolve those confounders.
A characteristic of linkage analysis is that it is relatively insensitive to loci that are not major contributors to disease expression (Greenberg, 1993). This has both disadvantages and advantages.
One disadvantage is that loci with alleles of “minor” effect cannot be easily detected. However, as noted, if there are several such alleles at a locus, the cumulative effect on disease transmission may make the locus easier to detect using linkage analysis, unlike association analysis in which allelic heterogeneity can destroy a signal. Of interest, a recent finding proves that for multipoint linkage, increasing the sample size actually decreases the probability of a false positive for a fixed cutoff value. This means that, for any chosen statistical cutoff, the probability of falsely detecting linkage decreases with sample size. One implication of this is that even if the “lod” score is, say 1.0 (which would not usually be considered statistically significant or even interesting) with a sufficient sample size, that cutoff could become statistically significant (Hodge et al., 2008). Therefore, if properly exploited, this finding could greatly increase the range of loci detectable by linkage analysis. Again, that means identifying loci that are contributing to the inheritance (trait) of the disease not merely to risk (state).
However, viewed another way, the “inability” to detect loci of minor effect means that any loci that linkage does detect can be expected to be necessary, or at least important, for disease expression and, therefore, well worth the effort it will take to find and characterize the disease gene at the detected locus. Part of the approach we are advocating is, as a first step, to find loci that are major contributors to epilepsy susceptibility, loci that will give us clues to what are likely varied mechanisms that must occur together for epilepsy to manifest.
Perhaps the chief disadvantage of linkage analysis is its imprecision in identifying the actual gene; localization for non-Mendelian and reduced penetrance loci is greater than about 5 Mb, even being as high as 20 Mb. The regions are relatively large because linkage depends on recombination to “separate” marker loci that are distant from the disease locus. As a thought experiment, if there were no recombination at all, linkage analysis could only identify the chromosome that contained a disease locus because, near or far, all marker loci on the same chromosome would be linked to the disease locus. Recall that linkage uses data from families, and the recombinations that occur from one generation to the next. Once the probability of a recombination becomes unlikely, alleles at nearby loci are more probably inherited together in the next generation. The larger that sample, the more likely that at least one recombinant will appear, and one reliable recombinant in one family is all it takes to narrow the region. Furthermore, new techniques that use dense multipoint data and LD information now make it possible to achieve narrower regions (Stewart et al., 2010, 2011; Vieland et al., 2011).
An important advantage of linkage analysis is that one can take account of (locus) heterogeneity, unlike association methods for which no such technique exists. This method, called heterogeneity lod scores, or HLOD, is a relatively limited, not necessarily powerful, approach (see Fig. 1), but it can make the difference between detecting statistically significant linkage evidence and missing such evidence, although the estimates of the amount of heterogeneity can be biased (Pal & Greenberg, 2002; Vieland & Logue, 2002). Nonetheless, it can be useful, not only for identifying the existence of heterogeneity (Greenberg et al., 2000; Hodge et al., 2006; Tomer et al., 2007), but also because the information can lead to the identification of gene–gene interaction (Hodge et al., 2006). In addition, the wealth of potential information in family data yields critical information about heterogeneity, gene expression, phenotypic range, and the relationship between genes and expression.
Therefore, linkage analysis can tell us of the very existence of genes important for disease expression and where they are located, and can give us information about the inheritance, genetic variables, and heterogeneity. In addition, the family data can be used to better understand possible variable expression of the disease in families.
Whole exome and whole genome sequencing (WGS)
The justification behind whole exome sequencing can be summarized as follows: The assumed reality justifying GWAS was that “the (single) common disease-related variant” contributing to a common disease would be in linkage disequilibrium with a common marker allele (“common disease, common variant”). Because that assumed reality did not lead to the identification of many important disease loci, the idea transformed into “common disease, rare variant,” that is, that there may not be a single, common disease-related variant but many disease-related variants in the exons of a gene. That is, common diseases are frequent because exons of a disease gene contain many infrequent mutations, each mutation capable of producing disease (i.e., allelic heterogeneity). If this view of the origin of common disease is true, then we need only sequence all the exons in the genome to identify which gene(s) is causing the disease. It is clear that such an approach may well work for fully penetrant Mendelian diseases caused by exonic mutations because the mutations can be followed as they segregate within families. However, the usual problems with common diseases quickly come into play: reduced penetrance, heterogeneity, misdiagnosis, and so on. Furthermore, the whole notion of multiple mutations in exons has not been shown to be a workable hypothesis, especially taking into account that 98% of the genome is not exonic and that exons are highly evolutionarily conserved. Ignored in this belief is that the control regions of the genes, which occur in noncoding portions of the DNA, are highly likely to be involved in the mis-expression of the gene that leads to disease, and these areas are not necessarily evolutionarily conserved. Indeed, why should we assume that the “mutation” is an SNP variant rather than of a group of SNPs, a repeat, or some other variant in DNA structure?
The obvious problem with WGS is: how does one analyze 3 billion base pairs and separate normal variation from disease-related variation? There is, as yet, no answer to this question. Although some analyses of rare Mendelian conditions allow the use of single family data to identify disease-related SNPs, the problems of trying to use the same approach with common disease data are the same as those encountered in other approaches. One could use WGS data in a linkage analysis, but since linkage analysis gains its location information from recombination, only a fraction of the sequence data would be useful. If GWAS yielded mostly ambiguity and minor disease contributions using only 106 SNPs, imagine the ambiguity that can be achieved with 3×109 bp.
How Should We Pursue The Genetic Contribution To The Common Epilepsies?
First, it is absolutely critical that the phenotype issue be addressed; this is admittedly not an easy problem. For example, one of the few universally acknowledged IGE syndromes that is considered separate from other forms of IGE is JME, but it was not until 1957 that Janz & Christian (1957) first described impulsiv petit mal (what came to be known as JME) and it was not until the 1980s that the diagnosis came to be widely recognized (Delgado-Escueta & Enrile-Bacsal, 1984). The identification, using linkage analysis, of several loci (Durner et al., 2001) for different IGE syndromes, and the identification and replication of candidate genes in two regions via association analysis (Pal et al., 2003; Greenberg et al., 2005; Cavalleri et al., 2007; Lucarelli et al., 2007) emphasize that extreme care in phenotype classification, including ethnic homogeneity, is crucial to finding verifiable genetic signals. It only hampers progress to believe that all IGEs are caused by the same gene(s). Lumping different genetic diatheses together will destroy, not enhance, genetic signals, especially if one is then unwilling to test the “lumps” separately.
Therefore, it is equally important to have the willingness to explore data and not remain fixated on certain definitions. As emphasized earlier, it must be accepted and acknowledged at the outset of any study that there will likely be locus (and allelic) heterogeneity in the data and that resolution of heterogeneity lay in exploring phenotypic definitions. But there seems to be a reluctance to explore data one has collected for fear of being accused of multiple testing. The problem is best stated by paraphrasing the Bard:
“We would rather bear those hypotheses we have,
Than test others that we know not of.
Thus the fear of multiple testing does make Cowards of us all,
And thus the Native hue of Science is sicklied o’er, With the pale cast of Statistics.”
(with apologies to Shakespeare)
In other words, investigators have learned to fear correction procedures for multiple tests because this usually makes some or all of their results statistically not significant and, therefore, difficult to publish. A few courageous investigators (and journals) realize that, in order to advance the field, one must posit reasonable hypotheses, even if the corresponding p-values are not significant after correction. However, to attain the ultimate goal of actually finding major genetic mechanisms for the IGEs, we must integrate exploratory efforts with detailed phenotype information.
Second, we should be collecting as much carefully obtained family data as possible, including clinical interviews with as many family members and, ideally, EEG and possibly other clinical data on at least first-degree relatives, affected or unaffected. Collecting and analyzing family data fell out of fashion with the advent of GWAS, but it is only family data that will tell us about the passage of genes from one generation to the next (i.e., trait) as opposed to differences in alleles between case and control populations (i.e., state). In addition, family data yield information about subclinical markers (e.g., EEG) that can be used to increase information not only about how the disease is transmitted and the location of the genes influencing the disease but also about variation of the phenotype (Pal et al., 2010), and possible genotypic differences in, for example, family members with the subclinical marker but without seizures (penetrance).
Third, linkage analysis should be the first step in analyzing the family data. One can effectively get reasonable linkage information from about only 6,000, approximately equally spaced, SNPs across the genome, although perhaps the minimum nowadays is 150,000, and 106 is not unusual. Although one cannot effectively get a million pieces of independent linkage information from a million SNPs (because of linkage disequilibrium), the advantage of such a high density scan is one can use the same data to look for association in regions showing significant evidence of linkage. Taken together with the clinical observations, such data are extremely rich in genetic information, allowing one to test hypotheses of different phenotypes, disease definitions, modes of inheritance, and possible gene–gene interaction, once there is strong evidence of linkage. (Sequencing of the linkage regions is also an approach, but knowing what variants one is looking for is then important.) One of the most important uses of linkage is that one can use the results to aid in identifying heterogeneity and use that information to point to genetically independent subtypes of IGE, especially when coordinated with the clinical information. We used this to great effect in previous linkage studies, not only of epilepsy (Durner et al., 1999, 2001; Greenberg et al., 2000), but also effective in studies of autoimmune thyroid disease (Tomer et al., 2007; Vieland et al., 2008).
Summary
The genomic technology available to us today has an almost futuristic quality. Many investigators are of the firm opinion that being able to genotype almost any number of SNPs and being able to get exome and whole genome sequences relatively inexpensively should lead to heretofore unimagined breakthroughs. In some areas, such as population genetics and evolutionary biology, that has been the case. However, in identifying the causes and mechanisms of common diseases, these same expectations have accompanied each of the genetic technology advances over the past 30 years, and usually, these expectations have not been met. GWAS proved a disappointment because the “common disease, common variant” mantra on which it was based was naive and simplistic. We seem to have jumped into “common disease, rare variant” paradigm under the belief that the relevant variants will be in exons, ignoring the genetic control regions and the remaining 98% of the genome. Whole genome sequencing is providing us with enormous amounts of information that no one has yet figured out how to process or to filter or to use for common disease. Even linkage analysis, when it was inappropriately applied based on naive beliefs and inadequate phenotyping, was similarly disappointing (Greenberg, 2011). Somehow, in all this technology, we seem to have lost sight of the basic question, “What is it, exactly, that we are studying?” The current beliefs about how things work are accepted as facts and we appear to be reluctant to reconsider them, but only by such reconsideration can we make any progress (Spence et al., 2003; Greenberg & Subaran, 2011).
Acknowledgments
This work supported in part by NIH grants NS061829, DK067555, NS070323 and by the New York State Psychiatric Institute. We thank Dr. Susan E. Hodge for critiquing the manuscript.
Footnotes
Disclosures
The authors have no conflicts of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Asconape J, Penry JK. Some clinical and EEG aspects of benign juvenile myoclonic epilepsy. Epilepsia. 1984;25:108–114. doi: 10.1111/j.1528-1157.1984.tb04163.x. [DOI] [PubMed] [Google Scholar]
- Bali B, Kull LL, Strug LJ, Clarke T, Murphy PL, Akman CI, Greenberg DA, Pal DK. Autosomal dominant inheritance of centrotemporal sharp waves in rolandic epilepsy families. Epilepsia. 2007;48:2266–2272. doi: 10.1111/j.1528-1167.2007.01221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, Engel J, French J, Glauser TA, Mathern GW, Moshe SL, Nordli D, Plouin P, Scheffer IE. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- Cavalleri GL, Walley NM, Soranzo N, Mulley J, Doherty CP, Kapoor A, Depondt C, Lynch JM, Scheffer IE, Heils A, Gehrmann A, Kinirons P, Gandhi S, Satishchandra P, Wood NW, Anand A, Sander T, Berkovic SF, Delanty N, Goldstein DB, Sisodiya SM. A multicenter study of BRD2 as a risk factor for juvenile myoclonic epilepsy. Epilepsia. 2007;48:706–712. doi: 10.1111/j.1528-1167.2007.00977.x. [DOI] [PubMed] [Google Scholar]
- Delgado-Escueta AV, Enrile-Bacsal F. Juvenile myoclonic epilepsy of Janz. Neurology. 1984;34:285–294. doi: 10.1212/wnl.34.3.285. [DOI] [PubMed] [Google Scholar]
- Durner M, Sander T, Greenberg DA, Johnson K, Beck-Mannagetta G, Janz D. Localization of idiopathic generalized epilepsy on chromosome 6p in families of juvenile myoclonic epilepsy patients. Neurology. 1991;41:1651–1655. doi: 10.1212/wnl.41.10.1651. [DOI] [PubMed] [Google Scholar]
- Durner M, Zhou G, Fu D, Abreu P, Shinnar S, Resor SR, Moshe SL, Rosenbaum D, Cohen J, Harden C, Kang H, Wallace S, Luciano D, Ballaban-Gil K, Klotz I, Dicker E, Greenberg DA. Evidence for linkage of adolescent-onset idiopathic generalized epilepsies to chromosome 8-and genetic heterogeneity. Am J Hum Genet. 1999;64:1411–1419. doi: 10.1086/302371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durner M, Keddache MA, Tomasini L, Shinnar S, Resor SR, Cohen J, Harden C, Moshe SL, Rosenbaum D, Kang H, Ballaban-Gil K, Hertz S, Labar DR, Luciano D, Wallace S, Yohai D, Klotz I, Dicker E, Greenberg DA. Genome scan of idiopathic generalized epilepsy: evidence for major susceptibility gene and modifying genes influencing the seizure type. Ann Neurol. 2001;49:328–335. [PubMed] [Google Scholar]
- Enattah NS, Sahi T, Savilahti E, Terwilliger JD, Peltonen L, Jarvela I. Identification of a variant associated with adult-type hypolactasia. Nat Genet. 2002;30:233–237. doi: 10.1038/ng826. [DOI] [PubMed] [Google Scholar]
- Greenberg DA. Linkage analysis of “necessary” disease loci versus “susceptibility” loci. Am J Hum Genet. 1993;52:135–143. [PMC free article] [PubMed] [Google Scholar]
- Greenberg DA. Computer simulation is an undervalued tool for genetic analysis: a historical view and presentation of SHIMSHON – a Web-based genetic simulation package. Hum Hered. 2011;72:247–257. doi: 10.1159/000330633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DA, Subaran R. Blinders, phenotype, and fashionable genetic analysis: a critical examination of the current state of epilepsy genetic studies. Epilepsia. 2011;52:1–9. doi: 10.1111/j.1528-1167.2010.02904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DA, Durner M, Delgado-Escueta AV. Evidence for multiple gene loci in the expression of the common generalized epilepsies. Neurology. 1992;42:56–62. [PubMed] [Google Scholar]
- Greenberg DA, Durner M, Resor S, Rosenbaum D, Shinnar S. The genetics of idiopathic generalized epilepsies of adolescent onset: differences between juvenile myoclonic epilepsy and epilepsy with random grand mal and with awakening grand mal. Neurology. 1995;45:942–946. doi: 10.1212/wnl.45.5.942. [DOI] [PubMed] [Google Scholar]
- Greenberg DA, Durner M, Keddache M, Shinnar S, Resor SR, Moshe SL, Rosenbaum D, Cohen J, Harden C, Kang H, Wallace S, Luciano D, Ballaban-Gil K, Tomasini L, Zhou G, Klotz I, Dicker E. Reproducibility and complications in gene searches: linkage on chromosome 6, heterogeneity, association and maternal inheritance in juvenile myoclonic epilepsy. Am J Hum Genet. 2000;66:508–516. doi: 10.1086/302763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DA, Cayanis E, Strug L, Marathe S, Durner M, Pal DK, Alvin GB, Klotz I, Dicker E, Shinnar S, Bromfield EB, Resor S, Cohen J, Moshe SL, Harden C, Kang H. Malic enzyme 2 may underlie susceptibility to adolescent-onset idiopathic generalized epilepsy. Am J Hum Genet. 2005;76:139–146. doi: 10.1086/426735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge SE, Ban Y, Strug LJ, Greenberg DA, Davies TF, Concepcion ES, Villanueva R, Tomer Y. Possible interaction between HLADRbeta1 and thyroglobulin variants in Graves’ disease. Thyroid. 2006;16:351–355. doi: 10.1089/thy.2006.16.351. [DOI] [PubMed] [Google Scholar]
- Hodge SE, Rodriguez-Murillo L, Strug LJ, Greenberg DA. Multipoint lods provide reliable linkage evidence despite unknown limiting distribution: type I error probabilities decrease with sample size for multipoint lods and mods. Genet Epidemiol. 2008;32:800–815. doi: 10.1002/gepi.20350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz D, Christian W. Impulsiv-petit mal. Dtsch Z Nervenheilk. 1957;176:348–386. [Google Scholar]
- Klassen T, Davis C, Goldman A, Burgess D, Chen T, Wheeler D, McPherson J, Bourquin T, Lewis L, Villasana D, Morgan M, Muzny D, Gibbs R, Noebels J. Exome sequencing of ion channel genes reveals complex profiles confounding personal risk assessment in epilepsy. Cell. 2011;145:1036–1048. doi: 10.1016/j.cell.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leu C, de Kovel CG, Zara F, Striano P, Pezzella M, Robbiano A, Bianchi A, Bisulli F, Coppola A, Giallonardo AT, Beccaria F, Trenite DK, Lindhout D, Gaus V, Schmitz B, Janz D, Weber YG, Becker F, Lerche H, Kleefuss-Lie AA, Hallman K, Kunz WS, Elger CE, Muhle H, Stephani U, Moller RS, Hjalgrim H, Mullen S, Scheffer IE, Berkovic SF, Everett KV, Gardiner MR, Marini C, Guerrini R, Lehesjoki AE, Siren A, Nabbout R, Baulac S, Leguern E, Serratosa JM, Rosenow F, Feucht M, Unterberger I, Covanis A, Suls A, Weckhuysen S, Kaneva R, Caglayan H, Turkdogan D, Baykan B, Bebek N, Ozbek U, Hempelmann A, Schulz H, Ruschendorf F, Trucks H, Nurnberg P, Avanzini G, Koeleman BP, Sander T. Genome-wide linkage meta-analysis identifies susceptibility loci at 2q34 and 13q31.3 for genetic generalized epilepsies. Epilepsia. 2012;53:308–318. doi: 10.1111/j.1528-1167.2011.03379.x. [DOI] [PubMed] [Google Scholar]
- Lucarelli P, Rizzo R, Gagliano A, Palmarino M, Volzone A, Arpino C, Curatolo P. Association between D18S474 locus on chromosome 18q12 and idiopathic generalized epilepsy. Brain Dev. 2007;29:9–12. doi: 10.1016/j.braindev.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Pal DK, Greenberg DA. Evaluating genetic heterogeneity in complex disorders. Hum Hered. 2002;53:216–226. doi: 10.1159/000066195. [DOI] [PubMed] [Google Scholar]
- Pal DK, Evgrafov OV, Tabares P, Zhang F, Durner M, Greenberg DA. BRD2 (RING3) is a probable major susceptibility gene for common juvenile myoclonic epilepsy. Am J Hum Genet. 2003;73:261–270. doi: 10.1086/377006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal DK, Li W, Clarke T, Lieberman P, Strug LJ. Pleiotropic effects of the 11p13 locus on developmental verbal dyspraxia and EEG centrotemporal sharp waves. Genes Brain Behav. 2010;9:1004–1012. doi: 10.1111/j.1601-183X.2010.00648.x. [DOI] [PubMed] [Google Scholar]
- Panayiotopoulos CP, Obeid T, Tahan AR. Juvenile myoclonic epilepsy: a 5-year prospective study. Epilepsia. 1994;35:285–296. doi: 10.1111/j.1528-1157.1994.tb02432.x. [DOI] [PubMed] [Google Scholar]
- Spence MA, Greenberg DA, Hodge SE, Vieland VJ. The emperor’s new methods. Am J Hum Genet. 2003;72:1084–1087. doi: 10.1086/374826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart WC, Peljto AL, Greenberg DA. Multiple subsampling of dense SNP data localizes disease genes with increased precision. Hum Hered. 2010;69:152–159. doi: 10.1159/000267995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart WC, Drill EN, Greenberg DA. Finding disease genes: a fast and flexible approach for analyzing high-throughput data. Eur J Hum Genet. 2011;19:1090–1094. doi: 10.1038/ejhg.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strug LJ, Clarke T, Chiang T, Chien M, Baskurt Z, Li W, Dorfman R, Bali B, Wirrell E, Kugler SL, Mandelbaum DE, Wolf SM, McGoldrick P, Hardison H, Novotny EJ, Ju J, Greenberg DA, Russo JJ, Pal DK. Centrotemporal sharp wave EEG trait in rolandic epilepsy maps to elongator protein complex 4 (ELP4) Eur J Hum Genet. 2009;17:1171–1181. doi: 10.1038/ejhg.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomer Y, Menconi F, Davies TF, Barbesino G, Rocchi R, Pinchera A, Concepcion E, Greenberg DA. Dissecting genetic heterogeneity in autoimmune thyroid diseases by subset analysis. J Autoimmun. 2007;29:69–77. doi: 10.1016/j.jaut.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Tsuboi T, Christian W. On the genetics of primary generalized epilepsy with sporadic myoclonias of impulsive petit mal. A clinical and electroencephalographic study of 399 probands. Humangenetik. 1973;19:155–182. doi: 10.1007/BF00282192. [DOI] [PubMed] [Google Scholar]
- Vieland VJ, Logue M. HLODs, trait models, and ascertainment: implications of admixture for parameter estimation and linkage detection. Hum Hered. 2002;53:23–35. doi: 10.1159/000048601. [DOI] [PubMed] [Google Scholar]
- Vieland VJ, Huang Y, Bartlett C, Davies TF, Tomer Y. A multilocus model of the genetic architecture of autoimmune thyroid disorder, with clinical implications. Am J Hum Genet. 2008;82:1349–1356. doi: 10.1016/j.ajhg.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieland VJ, Huang Y, Seok SC, Burian J, Catalyurek U, O’Connell J, Segre A, Valentine-Cooper W. KELVIN: a software package for rigorous measurement of statistical evidence in human genetics. Hum Hered. 2011;72:276–288. doi: 10.1159/000330634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winawer M, Rabinowitz D, Ottman R. Distinct genetic influences on myoclonic and absence seizures. Epilepsia. 2002;43:125. doi: 10.1212/wnl.61.11.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winawer MR, Marini C, Grinton BE, Rabinowitz D, Berkovic SF, Scheffer IE, Ottman R. Familial clustering of seizure types within the idiopathic generalized epilepsies. Neurology. 2005;65:523–528. doi: 10.1212/01.wnl.0000172920.34994.63. [DOI] [PMC free article] [PubMed] [Google Scholar]