Abstract
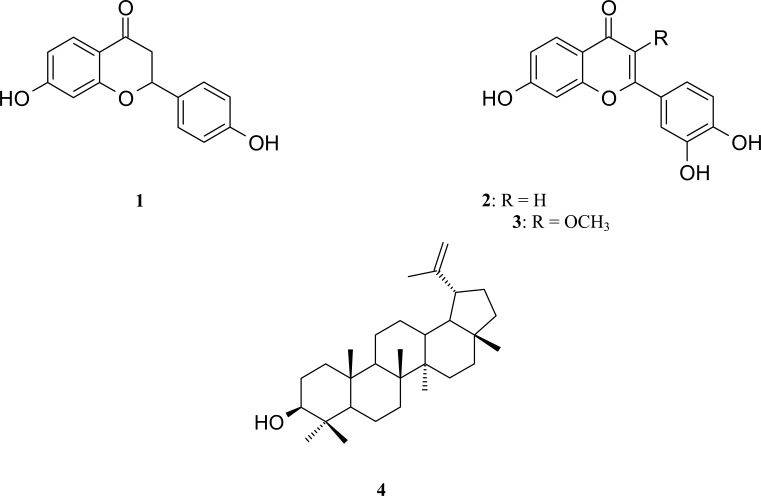
Three flavonoids were isolated for the first time from the Sudanese medicinal plants Albizia zygia. Compounds 1–3 were identified by interpretation of ESI mass data, 1H, 13C and 2D NMR as well as by comparison with published data as 4′,7-dihydroxyflavanone (1) 3′,4′,7-trihydroxyflavone (2), 3-O-methylfisetin (3′,4′,7-trihydroxy-3-methoxyflavone, 3). All flavonoids were tested against Plasmodium falciparum, and only compound 2 showed high antimalarial activity (IC50 0.078 µg/ml).
Keywords: Albizia zygia, Mimosoideae, flavonoids, antimalarial activity
Introduction
Albizia zygia (DC.) J.F. Macbr. (Leguminosae subfamily Mimosoideae) is a gum producing tree widely found in West Africa (Hutchinson et al., 1972). In previous phytochemical studies of A. zygia, lupen-20(30)-en-3β-ol and its glycoside, stigmast-5-en-3β-ol, and 5α-stigmasta-7,22-dien-3β-ol were isolated (Schoppa and Pachaly, 1981) as well as albiziaprenol and phytol were reported (Pachaly et al., 1983). Also, the gum of the plant has been widely investigated for its chemical and physical properties (e.g. as thickening agent) in comparison with other mucilages (Ashton et al., 1975; Mital et al., 1979). In traditional medicine, the powdered bark of A. zygia is used alone or as a decoction in southern Sudan as an antimalarial and antiparasitic drug. The methanolic extract of the stem bark exhibited antiprotozoal activity (IC50 1.0 µg/ml) against Plasmodium falciparum strain K1, the protozoa responsible for malaria, and Trypanosoma brucei rhodesiense (IC50 0.2 µg/ml), which causes African trypanosomiasis (Ndjakou et al., 2007). It is interesting to note that lupeol, which was isolated previously from this plant has been found to inhibit the growth of P. falciparum by 45% at 25 µg/ml (De Almeida Alves et al., 1997), which could account at least in part for the antiprotozoal activity observed for the methanol extract of A. zygia (Ndjakou et al., 2007).
Material and Methods
The bark of A. zygia was collected from Shambat, Sudan, in June 2006. The plant material was authenticated by Mr. Wail El Saddig (National Research Centre, Sudan) based on comparison with voucher specimen deposited in the herbarium of the Institute of the Medicinal and Aromatic Plants, Khartoum, Sudan.
An air-dried sample (1.0 kg) of the bark of A. zygia was powdered and extracted (3×) with 50% CH2Cl2/MeOH. The extracts were pooled and evaporated under reduced pressure to give a dark brown residue (148.6 g). This extract was partitioned between ethyl acetate and water, which delivered 7.3 g of crude product after evaporation of the organic phase, and 139.5 g from the water phase. Fractionation of the organic extract was carried out using silica gel flash chromatography (230–400 mesh) with a cyclohexane/EtOAc gradient. According to the TLC profiles three major fractions were obtained. Fraction FI was further purified on Sephadex LH-20 (Lipophilic Sephadex, Amersham Biosciences Ltd.; purchased from Sigma-Aldrich Chemie, Steinheim, Germany) using CH2Cl2/MeOH (6:4) followed by gradient chromatography on LiChroprep RP-18 (Merck KGaA, Darmstadt, Germany) eluted with MeOH/H2O (10:90 to 50:50), which afforded compound 1 (1.0 mg). Fraction FII was also separated on LiChroprep RP-18 eluted with MeOH/H2O (10:90 to 50:50) to give compounds 2 (2.7 mg) and 3 (1.7 mg).
The gum from the water phase was triturated with MeOH and the soluble part, after evaporation to dryness, was subjected to gradient chromatography on silica gel eluted with EtOAc/MeOH. This afforded compound 4 (2.9 mg).
Results and Discussion
Compounds 1–4 were unambiguously identified by interpretation of ESI mass data, 1H, 13C and 2D NMR spectra as well as by comparison with published data as 4′,7-dihydroxyflavanone (1) (Umehara et al., 2009), 3′,4′,7-trihydroxyflavone (2) (Wu et al., 2008), 3-O-methylfisetin (3′,4′,7-trihydroxy-3-methoxyflavone, 3) (Wu et al., 2008), and lup-20(29)-en-3-ol (4) (Blair et al., 1970).
The genus Albizia consists of over 150 species. The occurrence of albiziasaponins A, B, C (Pal, et al., 1995), macrocyclic alkaloids budmunchiamines L4, L5, and L6 (Ajay and Laxmi, 1997), and albizinin (Ma, 1997) have been reported from Albizia lebbeck. Kaempferol and quercetin were isolated from A. julibrissin (Lau et al., 2007) and A. lebbeck (El-Mousallamy, 1998). Prenylated flavonoids such as sophoflavescenol and kurarinone were also known from A. lebbeck (Jung et al., 2004). The unusual biflavonoids, eucaediflavone and albiproflavone were delivered from A. procera (Yadav and Bhadoria, 2004). Other flavonoids were reported from A. amara and A. adianthifolia (Arthur et al., 1978); however, this is the first report of flavonoids from the species A. zygia.
In our study compounds 1–3 were tested against P. falciparum K1: compound 2 exhibited high antimalarial activity (IC50 0.078 µg/ml), but displayed unfortunately also cytotoxic effects (IC50 0.405 µg/ml) against a cell line L6.
The results of this phytochemical study of the bark of A. zygia are in agreement with previous data reported for other Albizia species Arthur et al., 1978).
Acknowledgements
The authors would like to thank Dr. H. Frauendorf and Mr. R. Machinek for the mass and NMR spectra, F. Lissy and A. Kohl for technical assistance and Marcel Kaiser in Swiss Tropical Institute, Parasite Chemotherapy for determining the antimalarial activity. MAA thanks the German Academic Exchange Service (DAAD) for a Ph.D. grant.
References
- 1.Ajay KD, Laxmi NM. Macrocyclic budmunchiamine alkaloids from Albizia lebbek. J Nat Prod. 1997;60:1036–1037. [Google Scholar]
- 2.Arthur CH, Brookes KB, Bull JR, McGarry EJ, McGarry JM. Flavonoids of Albizia adianthifolia. Phytochemistry. 1978;17:1681–1682. [Google Scholar]
- 3.Ashton WR, Jefferies M, Morley RG, Pass G, Phillips GO, Power DM. Physical properties and applications of aqueous solutions of African Albizia zygia gum. J Sci Food Agric. 1975;26:697–704. [Google Scholar]
- 4.Blair JA, Ongley PA, Chiswell J, Griffiths MHG. Isolation of lupeol from the bark of Heritiera utilis (Tarrietia utilis) Phytochemistry. 1970;9:671. [Google Scholar]
- 5.De Almeida Alves TM, Nagem TJ, de Carvalho LZ, Krettli AU, Zani CL. Anti-plasmodial triterpene from Vernonia brasiliana. Planta Med. 1997;63:554–555. doi: 10.1055/s-2006-957764. [DOI] [PubMed] [Google Scholar]
- 6.El-Mousallamy AMD. Leaf flavonoids of Albizia lebbeck. Phytochemistry. 1998;48:759–761. doi: 10.1016/s0031-9422(97)01117-5. [DOI] [PubMed] [Google Scholar]
- 7.Hutchinson J, Dalziel JM, Keay RWJ. Flora of West Tropical Africa. Virginia: University of Virginia Press; 1972. [Google Scholar]
- 8.Jung MJ, Kang SS, Jung HA, Kim GJ, Choi JS. Isolation of flavonoids and a cerebroside from the stem bark of Albizzia julibrissin. Arch Pharm Res. 2004;27:593–599. doi: 10.1007/BF02980155. [DOI] [PubMed] [Google Scholar]
- 9.Lau CS, Carrier DJ, Beitle RR, Bransby DI, Howard LR, Lay JO, Jr, Liyanage R, Clausen EC. Identification and quantification of glycoside flavonoids in the energy crop Albizia julibrissin. Bioresource Technol. 2007;98:429–435. doi: 10.1016/j.biortech.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Ma YT. Tannins from Albizia lobbek. Phytochemistry. 1997;46:1451–1452. [Google Scholar]
- 11.Mital HC, Ocran J, Fokuo YD. Studies on Albizia zygia gum. Acta Pharm Technol. 1979;25:93–100. [Google Scholar]
- 12.Ndjakou LB, Vonthron-Senecheau C, Fongang Soh R, Tantangmo F, Ngouela S, Kaiser M, Tsamo E, Anton R, Weniger B. In vitro antiprotozoal activities and cytotoxicity of some selected Cameroonian medicinal plants. J Ethnopharmacol. 2007;111:8–12. doi: 10.1016/j.jep.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 13.Pachaly P, Redeker F, Schoppa T. Inhaltsstoffe von Albizzia zygia, 2. Arch Pharm. 1983;316:651–652. [Google Scholar]
- 14.Pal BC, Achari B, Yoshikawa K, Arihara S. Saponins from Albizia lebbeck. Phytochemistry. 1995;38:1287–1291. doi: 10.1016/0031-9422(94)00796-v. [DOI] [PubMed] [Google Scholar]
- 15.Schoppa T, Pachaly P. Inhaltsstoffe von Albizzia zygia. Archiv der Pharmazie. 1981;314:18–25. [Google Scholar]
- 16.Umehara K, Nemoto K, Matsushita A, Terada E, Monthakantirat O, De-Eknamkul W, Miyase T, Warashina T, Degawa M, Noguchi H. Flavonoids from the heartwood of the Thai medicinal plant Dalbergia parviflora and their effects on estrogenic-responsive human breast cancer cells. J Nat Prod. 2009;72:2163–2168. doi: 10.1021/np900676y. [DOI] [PubMed] [Google Scholar]
- 17.Wu J-H, Tung Y-T, Chien S-C, Wang S-Y, Kuo Y-H, Shyur L-F, Chang S-T. Effect of phytocompounds from the heartwood of Acacia confusa on inflammatory mediator production. J Agric Food Chem. 2008;56:1567–1573. doi: 10.1021/jf072922s. [DOI] [PubMed] [Google Scholar]
- 18.Yadav S, Bhadoria BK. Novel biflavonoids from the leaves of Leucaena diversifolia and Albizia procera and their protein binding efficiency. J Indian Chem Soc. 2004;81:392–394. [Google Scholar]