Abstract
The present study aimed to examine the effect of pumpkin (Cucurbita pepo L.) seeds supplementation on atherogenic diet-induced atherosclerosis. Rat were divided into two main groups , normal control and atherogenic control rats , each group composed of three subgroups one of them supplemented with 2% arginine in drinking water and the other supplemented with pumpkin seeds in diet at a concentration equivalent to 2% arginine. Supplementation continued for 37 days. Atherogenic rats supplemented with pumpkin seeds showed a significant decrease (p<0.001) in their serum concentrations of total cholesterol and LDL — C as they dropped from 4.89 mmol / L to 2.55 mmol /L and from 3.33 mmol / L to 0.70 mmol / L respectively. Serum concentrations of HDL-C were also significantly elevated in the same group. Although, atherogenic rats supplemented with 2% arginine showed significant increase in serum concentration of HDL-C, no significant changes were observed in their serum concentrations of total cholesterol and LDL-C. Our results showed that treatment of atherogenic rats with pumpkin seeds significantly decreased serum concentrations of TC and LDL-C. Our findings suggest that pumpkin seeds supplementation has a protective effect against atherogenic rats and this protective effect was not attributed to the high arginine concentrations in pumpkin seeds.
Keywords: arginine, low density lipoprotein, cholesterol, atherosclerosis, nitric oxide, C-reactive protein
Introduction
Cardiovascular disease (CVD) continues to be one of the most important health problems and the leading cause of death in many countries all over the world (McDonnell et al., 2009). Several studies have reported that low-density lipoprotein (LDL) oxidation plays a principal role in the pathogenesis of atherosclerosis, and lowering LDL-cholesterol reduces the risk of the disease and also reduces morbidity and mortality (Chong and Bachenheimer , 2000). Tremendous interest has been focused in finding natural substances present in foods or medicinal plants that decrease the risk of cardiovascular and degenerative diseases by reduction of oxidative stress and counteraction of macromolecular oxidation (Magalhaes et al., 2009).
Pumpkin seeds is a rich source of proteins, polyunsaturated fatty acids (Applequist et al., 2006 ; Sabudak, 2007 ), phytosterols (Phillips et al ., 2005 ; Ryan et al., 2007) , antioxidant vitamins, such as carotenoids and tocopherol (Stevenson et al ., 2007 ) and trace elements, such as selenium and zinc (Glew et al ., 2006). Pumpkin seeds have many health benefits, as pumpkin seed oil can retard the progression of hypertension and reduce hypercholesterolemia (Al-Zuhair et al ., 1997), protect against arthritis (Fahim et al., 1995 ) and possess a hypoglycemic activity (Caili et al ., 2006). Treatment of hypertensive rats with felodipine or captopril separately or combined with pumpkin seed oil resulted in improvement of free radical scavengers in the heart and kidney tissues (Al-Zuhair et al., 2000). Diets rich in pumpkin seeds were also reported to be associated with lower levels of gastric, breast, lung, and colorectal cancer (Huang et al., 2004). Pumpkin seeds are capable of causing the evacuation of parasitic intestinal worms (Applequist et al., 2006; Caili et al., 2006). Several investigations showed that L- arginine has an efficient effect in reducing lesion size, aortic intimal thickening, coronary intimal plaques, reduced homocysteine levels, and inhibit lipid peroxidation (Dhawan et al ., 2005; West et al ., 2005; Rasmusen et al ., 2007). Although, it has been reported that pumpkin seeds are rich in arginine (Glew et al ., 2006) however, the present work attempts to study the effect of pumpkin seeds administration on serum lipid concentrations in atherogenic rats.
Materials and Methods
Materials
Pumpkin (Cucurbita sp.) seeds were purchased from local market. Atherogenic diet (for atherogenesis induction) was purchased from ICN pharmaceuticals (Costa Mesa, CA-USA). Kits for the assay of total cholesterol (TC), low density lipoprotein-cholesterol (LDL-C), high density lipoprotein-cholesterol (HDL-C), triglycerides (TG), and glucose were purchased from Human GmbH (Wiesbaden , Germany). C reactive protein (CRP) kit was obtained from R&D Systems, Inc. (Minneapolis, MN, USA). Nitric oxide (NO) kit was purchased from BioVendor - Laboratorni Medicina a.s.(Modrice , Czech Republic). All other chemicals used were ACS grade.
Animals
Male Wistar rats (110–160 g) were obtained from the animal house, Faculty of Pharmacy, King Saud University, Riyadh, Saudi Arabia. Rats were divided into 6 groups. Group 1 consist of rats that were kept on standard laboratory diet; this group was used as normal controls (NC). Group 2 consist of normal rats that were kept on 2% arginine in drinking water (NC+Arg). Group 3 consist of normal rats that pumpkin seeds (PS) were mixed with the standard laboratory diet (57 g Standard laboratory diet + 43 g P.S ), this group designated as ( NC +PS ). Group 4 consisted of rats that were kept only on atherogenic diet (AT). Group 5 consisted of rats that were kept on atherogenic diet and treated with 2% arginine in drinking water (AT+Arg). Group 6 consisted of rats in which pumpkin seeds (PS) were mixed with the atherogenic diet (57 g atherogenic diet + 43 g P.S ), this group was designated as ( AT+PS ). Animals were housed in normal conditions (Temperature 22 ± 2 °C, a minimum relative humidity of 40%, 12 h light/dark cycle). All diets were given for 37 days with free access to water. Body weight and food intake of controls and treated rats were recorded daily. All animal experiments were carried out in accordance with King Saud University Ethical Committee Acts. At the end of the experimental period and after 12 h of fasting, aliquots of blood samples from all groups were collected in plain tubes. Serum samples were separated from cells by centrifugation at 5000g for 10 min in a refrigerated centrifuge and were kept at − 80 °C until analysis.
Methods
Measurement of serum concentrations of lipids and glucose
Glucose, total cholesterol (TC), triglycerides (TG), high density lipoprotein-cholesterol( HDL-C), and low density lipoprotein-cholesterol (LDL-C) were estimated in serum samples by standard enzymatic methods using Human kits (Barham and Trinder, 1972; Richmond 1973; Schettler and Nussel, 1975; Izawa et al., 1997; Okada et al .,1998) respectively . The concentrations of these parameters were measured in a Humalyzer model 3000.
Measurement of serum concentration of C reactive protein
C reactive protein (CRP) levels in serum were determined by the sandwich ELISA method using a commercially available kit from R&D Systems, Inc. (Minneapolis, MN, USA). Appropriate controls and standards as specified by the kit manufacturer were used. Serum CRP concentrations were expressed as microgram per milliliter serum.
Measurement of total nitrates and nitrites
Nitric oxide (NO) concentrations in serum were determined by measuring accumulation of nitrates and nitrites based on Griess reaction (Moshage et al ., 1995) according to the procedures of a commercially available kit from BioVendor - Laboratorni Medicina a.s.(Modrice , Czech Republic) concentrations were expressed as µmol / L .
Measurement of serum arginine concentrations
Serum arginine concentrations were determined using the method of (Bacchus and London, 1971) with modifications. To a 0.02 ml serum sample, 0.2 ml NaOH 2 mol / L, 0.8 ml 0.01g % 8- hydoxyquinoline and 0.4 ml 0.1g % N-bromosuccinimide were added in that order. The solution was thoroughly mixed and absorbance read at 500 nm after 2 min incubation. Absorbance readings at 500 nm were compared with an arginine standard curve to estimate concentrations. A 2.5 mmol/L L-arginine solution was used as standard.
Measurement of arginine and protein concentrations in pumpkin seeds
100 g of dry pumpkin seeds powder were extracted twice with petroleum ether overnight. Filtrates collected by centrifugation at 15000 rpm for 10 min were discarded and the residues were divided into 2 parts and their weights were recorded. The first part was resuspended in distilled water and the final volume was recorded. Appropriate serial dilutions of 5, 10, 25, 50 and 100 were made. The protein content of diluted samples was assayed by Biuret method (Gornall et al., 1949). 6 N HCl was added to the second part of the residue and the suspension was left overnight , filtrates were collected and serial dilutions were made as above. Arginine content of diluted samples was determined by the same protocol as mentioned earlier .
Data analysis
The data presented in this investigation are expressed as means ± standard error of the mean (SEM) and comparison between experimental and corresponding control rats was made by using Student's t-tests. The probability value of <0.05 was considered significant.
Results
Animal weights, food intake, and water ingestion
Although, all studied groups showed a significant increase in their final body weights, however, group 2 (NC + arg) and group 6 (AT + PS) that were treated with the same concentration of arginine (2%) exhibited the most significant increase in their final body weights ( p<0.001 ) when compared to their matched groups. Food intake was significantly decreased (p<0.001) in control rats treated with either 2% arginine or pumpkin seeds. On the other hand, no significant change was observed in food intake of atherogenic rats treated with pumpkin seeds (Table 1). Ingested water was significantly increased in rats kept on atherogenic diet (AT) as compared to normal control rats (p<0.001).
Table 1.
General characteristics of control and atherogenic rats treated with arginine and pumpkin seeds
| Characteristics | St.Lab.diet | St.Lab.diet + 2% Arg. |
St.Lab.diet + P.S |
AT. Diet | AT. diet + 2% Arg. |
AT. diet + P.S |
| Initial B.Wt. ( g ) | 112.0 ±1.02 ( 10 ) |
110.3 ±0.69 ( 11 ) |
114 ±0.6 ( 14 ) |
117 ±0.67 ( 14 ) |
115.6 ±0.64 ( 13 ) |
116.14 ±1.09 ( 12 ) |
| Final B.Wt. ( g ) | 266.9 ±1.07 ( 10 ) |
251.33 ±1.33† ( 11 ) |
261.6 ± 1.07** ( 14 ) |
274.3 ±1.38 * ( 14 ) |
281.7 ±0.96 * ( 13 ) |
307.1 ±1.24† ( 12 ) |
| Food Intake ( g / day ) |
20.04 ±0.39 ( 10 ) |
16.83 ±0.62† ( 11 ) |
16.52 ±0.94† ( 14 ) |
18.29 ±0.45† ( 14 ) |
21.44 ±0.35† ( 13 ) |
19.33 ±0.55 ( 12 ) |
| Water consumption ( ml / day ) |
34.97 ±1.27 ( 10 ) |
35.71 ±1.36 ( 11 ) |
35.64 ±1.09 ( 14 ) |
43.59 ±1.10† ( 14 ) |
50.86 ±1.22† ( 13 ) |
42.0 ± 1.31 ( 12 ) |
Data are expressed as mean±SEM, with number of rats given in parentheses. As compared with matched groups,
p<0.01 ;
p< 0.02 ;
p<0.001(student t test).
Protein and arginine concentrations in pumpkin seeds
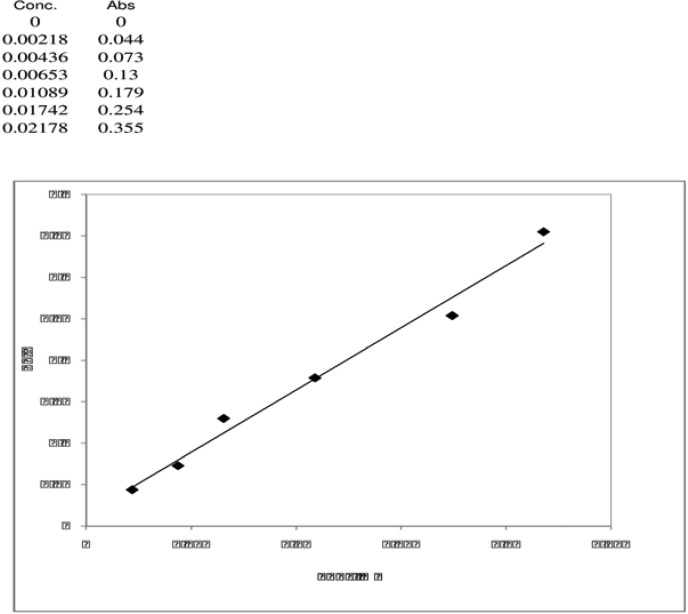
The protein concentration in pumpkin seeds was estimated to be 39.5g / 100 g PS using Biuret method and BSA as standard at a concentration of 5 mg / ml. Arginine concentration in pumpkin seeds was estimated to be 4.7 g / 100 g PS using the method in Materials and Methods Section and standard arginine at a concentration of 2.5 mmol / L (Figure 1).
Figure 1.
Standard curve of L-arginine. Working standard concentration is 2.5 mmol / L
Changes in serum lipid concentrations
Both of TC and LDL-C concentrations were significantly decreased in atherogenic rats treated with pumpkin seeds, as total cholesterol concentrations decreased by 48% from 4.89 mmol / L to 2.55 mmol /L ( P< 0.001 ), whereas LDL -C concentrations amazingly decreased by 79% from 3.33 mmol / L to 0.70 mmol / L (Table 2). No significant changes were observed in serum TG concentrations when atherogenic rats treated with either 2% arginine or pumpkin seeds, in contrast, a significant increase was noticed in the same parameter with control rats treated with pumpkin seeds (p<0.001) . A significant increase (p<0.001) was observed in serum HDL-C concentrations of atherogenic rats supplemented with either 2% arginine or pumpkin seeds.
Table 2.
Serum concentrations of total cholesterol (TC), triglycerides (TG), low-density lipoprotein-cholesterol (LDL-C), and high-density lipoprotein-cholesterol (HDL-C), in control and atherogenic rats treated with arginine and pumpkin seeds
| Parameters | St.Lab.diet | St.Lab.diet + 2% Arg. |
St.Lab.diet + P.S |
AT. Diet | AT. diet + 2% Arg. |
AT. diet + P.S |
| Total cholesterol ( mmol / L ) |
1.43 ±0.07 ( 16 ) |
1.70 ±0.05 ( 15 ) |
1.88 ±0.04 * ( 15 ) |
4.89 ±0.14 † ( 12 ) |
5.66 ±0.12 ( 13 ) |
2.55 ±0.06 † ( 13 ) |
| Triglycerides ( mmol / L ) |
1.04 ±0.03 ( 16 ) |
0.91 ±0.02 ( 15 ) |
1.96 ±0.06 † ( 14 ) |
1.72 ±0.08 † ( 12 ) |
1.42 ±0.04 ( 15 ) |
1.85 ±0.06 ( 13 ) |
| LDL — C ( mmol / L ) |
0.60 ±0.03 ( 14 ) |
0.52 ±0.02 ( 13 ) |
0.49 ±0.02 ( 10 ) |
3.33 ±0.07 † ( 11 ) |
2.71 ±0.06 ( 15 ) |
0.70 ±0.03 † ( 14 ) |
| HDL — C ( mmol / L ) |
0.44 ± 0.02 ( 13 ) |
0.48 ±0.02 ( 15 ) |
0.47 ±0.01 ( 14 ) |
0.43 ±0.01 ( 12 ) |
0.96 ±0.03 † ( 15 ) |
0.89±0.03 † ( 15 ) |
Data are expressed as mean±SEM, with number of rats given in parentheses. As compared with matched groups,
p<0.05 ;
p<0.001 (student t test).
Changes in serum concentrations of glucose, arginine, CRP and NO
Serum glucose concentrations in atherogenic rats were not significantly different from that of their matched control rats. Although no changes were observed in serum glucose concentrations of atherogenic rats supplemented with either 2% arginine or pumpkin seeds, however, control rats supplemented with pumpkin seeds showed a significant increase in serum glucose concentrations (p<0.02) (Table 2).
Atherogenic rats showed a significant increase (p<0.001) in serum arginine levels compared to their matched control rats (16.54 and 9.23 mg / dl respectively). Control rats supplemented with either 2% arginine or pumpkin seeds showed a significant decrease (p<0.001) in serum arginine concentrations, conversely, no significant changes were noticed in serum arginine levels of treated atherogenic rats. Serum levels of CRP in atherogenic rats were not significantly different from that in control rats. Supplementation with either 2%arginine or pumpkin seeds had no significant effect on serum CRP levels of treated groups when compared to their matched controls (Table 3). Serum concentrations of nitric oxide were markedly decreases in atherogenic rats (p< 0.005) compared to their matched control rats (24.52 and 38.79 µmol / L respectively). Control rats supplemented with either 2% arginine or pumpkin seeds showed no changes in serum NO concentrations, however, atherogenic rats treated with 2% arginine showed a significant increase in serum NO levels as it increased from 24.52 to 30.28 µmol / L. On the other hand, atherogenic rats supplemented with pumpkin seeds showed a markedly decrease in serum NO concentrations (Table 3).
Table 3.
Serum concentrations of glucose , arginine , C- reactive protein (CRP) ,and nitric oxide (NO) in control and atherogenic rats treated with arginine and pumpkin seeds
| Parameters | St.Lab.diet | St.Lab.diet + 2% Arg. |
St.Lab.diet + P.S |
AT. Diet | AT. diet + 2% Arg. |
AT. diet + P.S |
| Glucose ( mmol / L ) |
6.19± 0.18 (14) |
6.83± 0.14 (15) |
7.03± 0.20 * (14) |
6.58± 0.12 (10) |
6.63± 0.17 (15) |
6.95± 0.14 (12) |
| Arginine (mg / dL ) |
9.23± 0.29 (12) |
4.69± 0.13 † (10) |
5.83± 0.16 † (10) | 16.54± 0.30 † (10) | 16.07± 0.26 (11) |
15.59± 0.33 (12) |
| CRP (µg / ml ) |
488.24± 23.85 (13) |
477.26± 20.20 (12) |
483.67± 18.37 (11) |
498.26± 19.16 (9) |
474.10± 21.37 (11) |
479.61± 15.36 (12) |
| NO µmol / L | 38.79± 2.26 (7) |
40.43± 2.42 (7) |
41.91± 2.66 (7) |
24.52± 1.64 ‡ (7) |
30.28± 1.75 * (7) |
14.58± 0.84 † (7) |
Data are expressed as mean±SEM, with number of rats given in parentheses. As compared with matched groups,
p<0.02 ;
p<0.005 ;
p<0.001 (student t test).
Discussion
Results of the present study showed that atherogenic rats supplemented with pumpkin seeds showed a significant decrease in their serum concentrations of TC and LDL-C, this decrease was 48% and 79% respectively (p< 0.001). Although, our first assumption for the role of L-arginine in the prevention of atherogenesis progress was based on the premise that arginine exerts its effect through facilitating increase of endothelial NO formation as reported by several investigators (Cooke et al., 1992; Brandes et al., 2000), however, our findings showed that supplementation of L-arginine at a concentration of 2% in drinking water did not significantly change the serum concentrations of TC and LDL-C in atherogenic rats. Lam et al., (2008) reported that unsaturated fatty acids inhibit the activity of intestinal acyl coenzyme A: cholesterol acyltransferase (ACAT), the rate-limiting step in cholesterol absorption, from this view, and as a rich source of poly unsaturated fatty acids, the effect of pumpkin seeds on the serum concentrations of TC in atherogenic rats could be explained.
Our finding that pumpkin seeds markedly decreased the serum concentrations of LDL-C in atherogenic rats is in a full agreement with that of Makni et al. (2008) who reported that flax and pumpkin seed mixture significantly decreased LDL/HDL ratio and had anti-atherogenic and hepatoprotective effects. In addition, the serum arginine levels were significantly increased in atherogenic rats compared to normal control rats (16.54 and 9.23 mg / dl respectively). This increase in serum arginine levels in atherogenic rats could be to due to the attenuation of arginine uptake through cationic amino acid transporter-1 as reported by Schwartz et al. (2007). Both groups of atherogenic rats supplemented with 2% arginine and pumpkin seeds showed no significant changes in their serum arginine concentrations, this also could be explained by the attenuation of arginine uptake through cationic amino acid transporter-1. Our results showed no association between serum concentrations of arginine and CRP in all groups of study , this finding contrasts the report by (Wells et al., 2005) which concluded the relationship between arginine intake and CRP levels in humans, this difference could be due to species differences .
Although, serum arginine levels were significantly increased in atherogenic rats compared to control rats (p<0.001), serum concentrations of nitric oxide significantly decreased (Table 3), this decrease in NO may be due to the increased concentrations of endogenous asymmetric dimethylarginine (ADMA), an inhibitor of endothelial nitric oxide synthase (eNOS). Based on this fact, our findings coincide with the uncoupling mechanism of eNOS in atherosclerotic endothelial dysfunction reported by (Yang and Ming, 2006). Unexpectedly, atherogenic rats supplemented with pumpkin seeds showed a significant decrease in serum NO concentrations as it dropped by 41% from 24.52 to 14.58 µmol / L (p<0.001), a rational explanation could be that pumpkin seeds consist of palmitic acid and linoleic acid at a concentration of 38.6 and 92.0 mg / g dry weight respectively (Glew et al ., 2006 ), and these fatty acids are capable to inducing dephosphorylation and/or inactivation of eNOS on Ser1177 leading to a decrease of the enzyme functional activity and thereafter reducing the level of NO (Guo et al ., 2008).
In conclusion, the current study showed that a pumpkin seed has a beneficial effect on atherogenic rats as it caused a significant reduction in serum concentrations of TC and LDL-C and increased the serum levels of HDL-C. Further studies are needed to elucidate the mechanism by which a pumpkin seed has such hypocholesterolaemic effect. Our future work will be focused on separation of active ingredients and recognition of their specific activities, and will target the effect of pumpkin seeds on 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase.
Acknowledgment
The authors would like to thank King Abdulaziz City for Science and Technology (KACST) for their generous grant (No AT-17 -97). The authors extend their sincerest gratitude to the Research Centre, College of Science for supporting this study (No ST/Bio/ 2008/ 37).
References
- 1.Al-Zuhair H, Abdel-Fattah A A, Abd el Latif H A. Efficacy of simvastatin and pumpkin-seed oil in the management of dietary-induced hypercholesterolemia. Pharmacol Res. 1997;35:403–408. doi: 10.1006/phrs.1997.0148. [DOI] [PubMed] [Google Scholar]
- 2.Al-Zuhair H, Abdel-Fattah A A, El-Sayed M I. Pumpkin-seed oil modulates the effect of felodipine and captopril in spontaneously hypertensive rats. Pharmacol Res. 2000;41:555–563. doi: 10.1006/phrs.1999.0622. [DOI] [PubMed] [Google Scholar]
- 3.Applequist W L, Avula B, Schaneberg BT, Wang Y H, Khan I A. Comparative fatty acid content of seeds of four Cucurbita species grown in a common (shared) garden. J Food Composition and Analysis. 2006;19:606–611. [Google Scholar]
- 4.Bacchus RA, London DR. The measurement of arginine in plasma. Clin Chim Acta. 1971;33:479–482. doi: 10.1016/0009-8981(71)90515-8. [DOI] [PubMed] [Google Scholar]
- 5.Barham D, Trinder P. An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst. 1972;97:142–145. doi: 10.1039/an9729700142. [DOI] [PubMed] [Google Scholar]
- 6.Brandes RP, Brandes S, Böger RH, Bode-Böger SM, Mügge A. L-arginine supplementation in hypercholesterolemic rabbits normalizes leukocyte adhesion to non-endothelial matrix. Life Sci. 2000;66:1519–1524. doi: 10.1016/s0024-3205(00)00469-0. [DOI] [PubMed] [Google Scholar]
- 7.Caili F, Huan S, Quanhong L. A review on pharmacological activities and utilization technologies of pumpkin. Plant Foods for Human Nutrition. 2006;61:73–80. doi: 10.1007/s11130-006-0016-6. [DOI] [PubMed] [Google Scholar]
- 8.Chong PH, Bachenheimer BS. Current, new and future treatments in dyslipidaemia and atherosclerosis. Drugs. 2000;60:55–93. doi: 10.2165/00003495-200060010-00005. [DOI] [PubMed] [Google Scholar]
- 9.Cooke JP, Singer AH, Tsao P, Zera P, Rowan RA, Billingham ME. Antiatherogenic effects of L-arginine in the hypercholesterolemic rabbit. J Clin Invest. 1992;90:1168–1172. doi: 10.1172/JCI115937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhawan V, Handu SS, Nain CK, Ganguly NK. Chronic L-arginine supplementation improves endothelial cell vasoactive functions in hypercholesterolemic and atherosclerotic monkeys. Mol Cell Biochem. 2005;269:1–11. doi: 10.1007/s11010-005-1810-4. [DOI] [PubMed] [Google Scholar]
- 11.Fahim AT, Abdel-Fattah AA, Agha AM, Gad MZ. Effect of pumpkin seed oil on the level of free radical scavengers induced during adjuvant- arthritis in rats. Pharmacol Res. 1995;31:73–79. doi: 10.1016/1043-6618(95)80051-4. [DOI] [PubMed] [Google Scholar]
- 12.Glew RH, Glew RS, Chuang LT, Huang YS, Millson M, Constans D. Amino acid, mineral and fatty acid content of pumpkin seeds (Cucurbita spp) and Cyperus esculentus nuts in the Republic of Niger. Plant Foods for Human Nutrition. 2006;61:51–56. doi: 10.1007/s11130-006-0010-z. [DOI] [PubMed] [Google Scholar]
- 13.Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- 14.Guo WX, Yang OD, Liu YH, Xie XY, Wang-Miao Niu RC. Palmitic and Linoleic Acids Impair Endothelial Progenitor Cells by Inhibition of Akt/eNOS Pathway. Arch Med Res. 2008;39:434–442. doi: 10.1016/j.arcmed.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Huang XE, Hirose K, Wakai K, Matsuo K, Ito H, Xiang J. Comparison of lifestyle risk factors by family history for gastric, breast, lung and colorectal cancer. Asian Pacific Journal of Cancer Prevention. 2004;5:419–427. [PubMed] [Google Scholar]
- 16.Izawa S, Okada M, Matsui H, Horita Y. A new direct method for measuring HDL-cholesterol which does not produce any biased values. J Med and Pharm Sci. 1997;37:1385–1388. [Google Scholar]
- 17.Lam CK, Chen J, Cao Y, Yang L, Wong YM, Yeung SY, Yao X, Huang Y, Chen ZY. Conjugated and non-conjugated octadecaenoic acids affect differently intestinal acyl coenzyme A: cholesterol acyltransferase activity. Atherosclerosis. 2008;198:85–93. doi: 10.1016/j.atherosclerosis.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Magalhaes AS, Silva BM, Pereira JA, Andrade PB, Valentao P, Carvalho M. Protective effect of quince (Cydonia oblonga Miller) fruit against oxidative hemolysis of human erythrocytes. Food Chem Toxicol. 2009;47:1372–1377. doi: 10.1016/j.fct.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Makni M, Fetoui H, Gargouri NK, Garoui M, Jaber H, Makni J, Boudawara T, Zeghal N. Hypolipidemic and hepatoprotective effects of flax and pumpkin seed mixture rich in omega-3 and omega-6 fatty acids in hypercholesterolemic rats. Food Chem Toxicol. 2008;46:3714–3720. doi: 10.1016/j.fct.2008.09.057. [DOI] [PubMed] [Google Scholar]
- 20.McDonnell B, Hearty S, Leonard P, O'Kennedy R. Cardiac biomarkers and the case for point-of-care testing. Clin Biochem. 2009;42:549–561. doi: 10.1016/j.clinbiochem.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 21.Moshage H, Kok B, Huizenga J, Jansen P. Nitrate and nitrite determination in plasma: a critical evaluation. Clin Chem. 1995;41:892–896. [PubMed] [Google Scholar]
- 22.Okada M, Matsui H, Ito Y, Fujiwara A, Inano K. Low-density lipoprotein cholesterol can be chemically measured: a new superior method. J Lab Clin Med. 1998;132:195–201. doi: 10.1016/s0022-2143(98)90168-8. [DOI] [PubMed] [Google Scholar]
- 23.Phillips K M, Ruggio DM, Ashraf-Khorassani M. Phytosterol composition of nuts and seeds commonly consumed in the United States. J Agr and Food Chem. 2005;53:9436–9445. doi: 10.1021/jf051505h. [DOI] [PubMed] [Google Scholar]
- 24.Rasmusen C, Moinard C, Martin C, Tricottet V, Cynober L, Couderc R. L-arginine plus atorvastatin for prevention of atheroma formation in genetically hypercholesterolaemic rabbits. Br J Nutr. 2007;97:1083–1089. doi: 10.1017/S0007114507659066. [DOI] [PubMed] [Google Scholar]
- 25.Richmond W. Preparation and properties of a cholesterol oxidase from Nocardia sp. and its application to the enzymatic assay of total cholesterol in serum. Clin Chem. 1973;19:1350–1356. [PubMed] [Google Scholar]
- 26.Ryan E, Galvin K, O'Connor T P, Maguire A R, O'Brien N M. Phytosterol, squalene, tocopherol content and fatty acid profile of selected seeds, grains, and legumes. Plant Foods for Human Nutrition. 2007;62:85–91. doi: 10.1007/s11130-007-0046-8. [DOI] [PubMed] [Google Scholar]
- 27.Sabudak T. Fatty acid composition of seed and leaf oils of pumpkin, walnut, almond, maize, sunflower and melon. Chemistry of Natural Compounds. 2007;43:465–467. [Google Scholar]
- 28.Schettler G, Nussel E. Method for triglycerides. Aeb Med Soz Med Prav Med. 1975;10:25–29. [Google Scholar]
- 29.Schwartz IF, Ingbir M, Chernichovski T, Reshef R, Chernin G, Litvak A, Weinstein T, Levo Y, Schwartz D. Arginine uptake is attenuated, through post-translational regulation of cationic amino acid transporter-1, in hyperlipidemic rats. Atherosclerosis. 2007;194:357–363. doi: 10.1016/j.atherosclerosis.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 30.Stevenson D G, Eller FJ, Wang L, Jane J L, Wang T, Inglett G E. Oil and tocopherol content and composition of pumpkin seed oil in 12 cultivars. J Agr and Food Chem. 2007;55:4005–4013. doi: 10.1021/jf0706979. [DOI] [PubMed] [Google Scholar]
- 31.Wells BJ, Mainous AG, Everett CJ. Association between dietary arginine and C-reactive protein. Nutrition. 2005;21:125–130. doi: 10.1016/j.nut.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 32.West SG, Likos-Krick A, Brown P, Mariotti F. Oral L-arginine improves hemodynamic responses to stress and reduces plasma homocysteine in hypercholesterolemic men. J Nutr. 2005;135:212–217. doi: 10.1093/jn/135.2.212. [DOI] [PubMed] [Google Scholar]
- 33.Yang Z, Ming XF. Recent advances in understanding endothelial dysfunction in atherosclerosis. Clin Med Res. 2006;4:53–65. doi: 10.3121/cmr.4.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]