Abstract
Strychnos nux vomica Linn.(Loganaceae) commonly known as Nux vomica (Kupeelu), is a poisonous plant and its seeds are used widely in Ayurvedic system of medicine since time immemorial. Ayurveda advocates that nux vomica seeds are to be administered in therapeutics only after going through certain purificatory measures (Shodhana). There are more than six media: cow's urine (Go mutra), cow's milk (Go dugdha), cow's ghee (Go ghrita), Kanji (thin gruel), castor oil (Eranda taila) and fresh ginger juice (Ardraka swarasa) etc., which have been reported in different classical texts of Ayurveda for proper processing of nux vomica seeds. In this study, an attempt has been made to purify the seeds by using three different methods as described in ancient treatise by using cow's urine and cow's milk as media alone and together. This study revealed that all the methods studied reduced the toxicity of strychnine and brucine contents in comparison to the raw seeds as determined by HPTLC. Out of these three methods maximum reduction in strychnine and brucine contents was found when the seeds were purified by keeping them in cow's urine for seven days followed by boiling in cow's milk for three hrs.
Keywords: Kupeelu, Strychnos nuxvomica, Shodhana, strychnine, Ayurveda, brucine, Cow's milk, Cow's urine
Introduction
Kupeelu (Strychnos nuxvomica Linn.) is a therapeutically potent plant in Ayurveda and its seeds are being used extensively in different classical formulations (Gogte, 2000). The plant is reported under the poisonous group (Sharma et al., 2000) and the major chemical constituents of the nux vomica i.e strychnine and brucine have been reported for their adverse effects (Nadkarni, 1976). 16 alkaloids have been separated and identified from the crude nux vomica and 80% of them are strychnine and brucine, and their derivatives, such as isostrychnine and brucine N-oxide (Cai et al., 1990). The seeds also contain chlorogenic acid, a glycoside (loganin), and about 3% of fixed oil. Strychnine (C21H22O2N2; m.p. 286 to 288° C) and brucine (C23H26O4N2; m.p. 178° C) are the most important and strongly toxic alkaloids present in this, besides other minor alkaloidal constituents. These alkaoids occur not only in the seed but also in the roots, bark, leaves, fruit-pulp, and the hard fruit-shells (Anonymous, 1998) and twenty-two identified alkaloids have been isolated from the root bark and leaves of S. nux vomica (Baser Kemal et al., 1982).
In Ayurveda, different methods are in practice for the specific Shodhana (purificatory measures) procedures of nux vomica seeds (Sharma et al., 2000; Trikamji, 2008; Anonymous, 1978). The concept of Shodhana in Ayurveda is not only a process of detoxification but also a process to enhance the potency and efficacy of the drug (Shastri, 2009). It was reported that aconite (Vatsanabha) purified by cow's urine is converted to cardiac stimulant, whereas raw aconite is cardiac depressant (Singh et al., 1985).
The seeds of purified nux vomica are mainly used as aphrodisiac, appetizer, anti periodic, digestive, purgative, and stimulant. They are also used in anaemia, asthma, bronchitis, intermittent malarial fever and in weakness of extremities (Sabnis, 2006). Purified Kupeelu is specially recommended during senility as Rasayana (antioxidant) since it is considered to be potent drug in countering old age problems on account of growing age in old persons facing degeneration and natural decay of cells and tissues in senility (Pandey, 2002). Detoxified seeds are also found to be more hepatoprotective than raw seeds when examined in albino rats (Gopikrishna et al. 2010).
Nux vomica is a powerful poison in large doses, producing tetanic convulsions and eventually death and in lesser doses it may manifest mental derangement (Anonymous, 2005). Akbar et al. 2010 reported the reduction in strychnine content by following the detoxification of nux vomica seeds as mentioned in Unani system of medicine. Shodhana (Purificatory measures) of nux vomica by using cow's milk and urine has been mentioned in Ayurveda, which is yet to be evaluated.
Media used for the proper processing in both Unani and Ayurveda system may be the same but the processes are entirely different. Therefore, in the present study, we compared the effect of three different methods of processing mentioned in the ancient Ayurveda scriptures (i.e. with cow's urine, cow's milk and both) on the reduction of strychnine and brucine content in nux vomica seeds.
Materials and methods
Fully matured Kupeelu (Strychnos nuxvomica Linn.) (voucher 8009) fruits were collected from the field of Bankura district, West Bengal in India during the month of December and were botanically authenticated by pharmacognosists and sample specimens were kept in the museum for future reference. Seeds were taken out from the fruit pulp, thoroughly washed in tap water and shade dried. Cow's urine (Go mutra) and milk (Go dugdha) were collected from the local cow shed in the morning at 6 A.M. and were used for purificatory measures of the seeds. The dry seeds were first dropped in a beaker containing water. The seeds which floated on the surface of water or found broken, black in color were rejected and the seeds which settled at the bottom of the beaker were selected for proper processing after drying in air and were considered as raw drug (KR) (Gopalkrishna et al., 2010).
Materials for Shodhana (Purificatory measures)
Stainless steel tray (48 cm × 30 cm × 7 cm) having capacity of 3 L, stainless steel vessel (20 cm × 30 cm) having capacity of 7 L, stainless steel rod (28 cm.), cotton thread 30 cm., measuring mug (capacity of 1 L), muslin cloth (45 cm × 45 cm), stainless steel spatula (length 30 cm), digital weighing machine, pyrometer, digital induction cooker.
Materials for HPTLC
A CAMAG (Switzerland) HPTLC system equipped with a sample applicator Linomat V sample applicator was used for application of samples. CAMAG TLC Scanner 3, Reprostar and Wincats 4.02 was used for scanning the plates. CAMAG twin through glass chamber was used for developing the plates.
Chemicals
Pure strychnine and brucine were obtained from Sigma Aldrich, U.S.A and precoated silica gel 60 F254 TLC aluminium plates (10×10 cms, 0.2mm thick), AR grade tolune, ethyl acetate, diethyl amine, methanol and chloroform were obtained from M/S Merck Ltd. Mumbai, India.
Purificatory measures of Kupeelu (Strychnos nux vomica Linn.) in different media
Each purificatory measures was carried out in three different batches by using two different media (cow's urine and cow's milk) either individually (Sharma et al., 2000) or together step by step in a single method (Trikamji, 2008).
Purificatory measures of nuxvomica seeds using cow's urine as media
The clean and dried raw seeds (KR) of 100 g were kept in 1 liter of cow's urine in a stainless steel tray for 7 days. Every day at 7 A.M. the cow's urine was replaced by fresh one. On the eighth day, the seeds were taken out and washed with lukewarm water (40–45°C). The KGD seeds were then made into powder and kept in an air tight glass container as ‘KGD powder for further study. From KGM seeds sample, some seeds were taken out, their seed coat and embryo were removed with a knife and cotyledons were pulverized and kept in an air tight glass container as ‘KGM powder’ for further use.
Purificatory measures of nuxvomica seeds using cow's milk
100 g of raw seeds of Kupeelu (KR) were kept in a muslin cloth and a bundle was made. The bundle was then hanged in a stainless steel vessel and cow's milk was poured in the vessel to completely immerse the bundle. It was then boiled on an induction cooker for three hrs at 100°C throughout the experiment. 6 liters of cow's milk was utilized throughout the process. After boiling for three hours, the seeds were taken out from bundle and washed with lukewarm water. The seed coat and embryo were removed with a knife; the cotyledons were dried and kept in an air tight container marked as ‘KGD seeds' for further study. KGD seeds were then made into powder and kept in an air tight glass container as ‘KGD powder’.
Purificatory measures of nux vomica seeds by immersion in cow's urine followed by boiling in cow's milk
KGM seeds (obtained from method I) were kept in a muslin cloth and a bundle was made. The bundle was then hanged in a stainless steel vessel and cow's milk was poured in to the vessel to completely immerse the bundle. It was then boiled on an induction cooker for three hrs at 100°C throughout the procedure. 6 liters of cow's milk was required for this purpose. After boiling for three hrs, the seeds were taken out from bundle and washed with lukewarm water. The seed coat and embryo were removed with a knife; the cotyledons were dried and kept in an air tight container marked as ‘KGMD seeds’. KGMD seeds were then pulverized and kept in an air tight glass container for further study as ‘KGMD powder’.
HPTLC method for estimation of strychnine and brucine
Preparation of standard strychnine and brucine solution
Strychnine standard (10 mg) and brucine standard (10 mg) were accurately weighed and dissolved in methanol in two standard flasks and final volumes were adjusted to 10 ml with methanol (1 µg/µl)
Calibration curve for strychnine and brucine
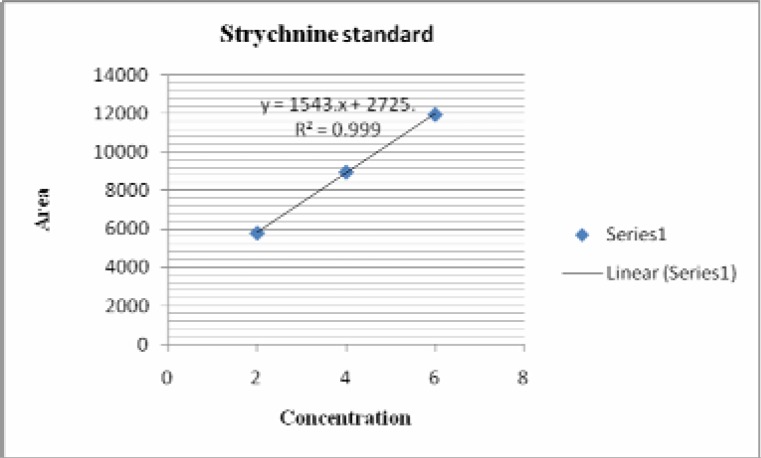
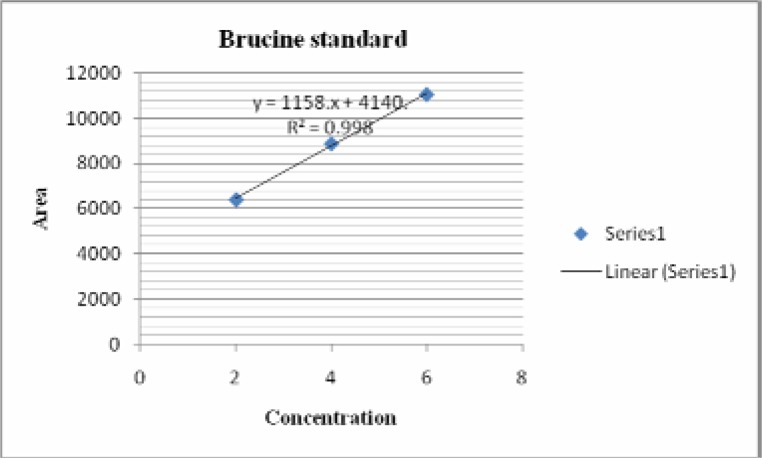
The standard solutions corresponding to 2 µg to 6 µg of standard strychnine and brucine were applied on TLC plates (10cm× 10cm), precoated with silica gel as 6 mm bands by using CAMAG Linomat IV sample applicator. The plate was developed in a solvent system of Toluene: Ethyl acetate: Diethyl amine (7: 2:1, v/v) in a CAMAG twin through chamber up to a distance of 7.5 cm at a temperature of 30 ± 2° C. The plates were air dried and scanned at a wavelength of 254 nm using CAMAG TLC scanner and CATS V 4.06 software. The peak area of strychnine and brucine were recorded for each concentration. The calibration curves of strychnine and brucine were obtained by plotting the graphs of peak areas vs. concentrations of strychnine and brucine.
Preparation of sample solutions for estimation of strychnine and brucine
All the purified samples (2g each) were defatted individually with petroleum ether. Defatted samples were then mixed with 10% ammonia and finally extracted with 25 ml methanol for 1 hr. under reflux. The methanol extracts were filtered and concentrated to 5 ml and used as test solutions. 5 µl of each test solution was spotted along with 2 to 6 µl standard solutions of strychnine and brucine. The plates were developed in mobile phase of Toluene: Ethyl acetate: Diethyl amine (7: 2:1, v/v) and scanned at 254 nm for strychnine and brucine. Peak areas were noted and quantity of strychnine and brucine were calculated by comparing the areas of standard solutions from calibration curve.
Results and Discussion
65.23% and 69.26% of processed Kupeelu were obtained after proper processing in Cow's urine and Cow's milk respectively, whereas 69.60% final product was obtained in the third method. The intensely bitter raw Kupeelu became salty bitter or sweetish bitter after processing and grayish powder of raw seeds turned into whitish, whitish gray or grayish white in colour after processing (Tables 1–3). Changes in these organoleptic characters might be due to the contact of the seeds with the media like cow's urine and milk which have specific colour, odour and taste and the chemical constituents present in the cow's urine and milk ultimately led to some chemical changes in the seeds during processing; resulting in changes in taste, odour and colour. However, more studies are required to explain the exact mechanism occurring during the processing of the seeds. The moisture content in Kupeelu was increased after processing but the water soluble and alcohol soluble extractive values were decreased in all the samples after proper processing. Water and alcohol soluble extractive values in purified seeds in cow's urine were comparatively less than that of milk purified samples (Table 4). The cow's urine being alkaline in nature may facilitate the extraction of alkaloids like strychnine and brucine from nux vomica. So, it may be concluded that cow's urine is a better extraction media than milk.
Table 1.
Effect of Shodhana (Purificatory measures) by cow's urine on yield of final products and organoleptic characters
| Batch | Weight (g) of the KGM seeds | Organoleptic characters of KGM powder | |||||
| Initial | After soaking |
After removing seed coat & embryo |
After drying |
Color | Odor | Taste | |
| KGM-1 | 100 | 176.60 | 106.00 | 64.50 | Whitish gray |
Characteristic of urine |
Salty bitter |
| KGM-2 | 100 | 180.50 | 108.60 | 66.00 | Whitish gray |
Characteristic of urine |
Salty bitter |
| KGM-3 | 100 | 178.80 | 107.20 | 65.20 | Whitish gray |
Characteristic of urine |
Salty bitter |
| Average | 100 | 178.63 | 107.26 | 65.23 | |||
Table 3.
Effect of Shodhana (Purificatory measures) with cow's urine & milk on yield of final products and organoleptic characters
| Batch | Weight (g) of KGMD seeds | Organoleptic characters of KGMD powder | |||||
| Initial | After soaking and boiling |
After removing seed coat & embryo |
After drying |
Color | Odor | Taste | |
| B01KGMD | 100 | 196.00 | 126.90 | 70.30 | Grayish white |
Characteristic | Bitter |
| B02KGMD | 100 | 195.20 | 122.20 | 69.50 | Grayish white |
Characteristic | Bitter |
| B03KGMD | 100 | 192.70 | 119.10 | 69.00 | Grayish white |
Characteristic | Bitter |
| Average | 100 | 194.63 | 122.73 | 69.60 | |||
Table 4.
Physicochemical parameters of raw and properly processed seeds
| Parameters | Samples | |||
| Raw Kupeelu | Processed by cow's urine |
Processed by cow's milk |
Processed by cow's urine and milk |
|
| Loss on drying | 3.39 % w/w | 3.67 % w/w | 5.03 % w/w | 6.28 % w/w |
| Ash value | 1.11% w/w | 1.58 % w/w | 1.36 % w/w | 1.21% w/w |
| Water soluble extractive | 37.83 % w/w | 21.49 % w/w | 34.20 % w/w | 33.59 % w/w |
| Methanol soluble extractive | 3.89 % w/w | 0.83 % w/w | 1.67 % w/w | 1.04 % w/w |
Table 2.
Effect of Shodhana (Purificatory measures) by cow's milk on yield of final products and organoleptic characters
| Batch | Weight (g) of the KGD seeds | Organoleptic characters of KGD powder | |||||
| Initial | After boiling |
After removing seed coat & embryo |
After drying |
Color | Odor | Taste | |
| KGD-1 | 100 | 187.60 | 117.90 | 70.30 | White | Characteristic of milk |
Sweetish bitter |
| KGD-2 | 100 | 186.50 | 116.20 | 68.50 | White | Characteristic of milk |
Sweetish bitter |
| KGD-3 | 100 | 187.10 | 117.10 | 69.00 | White | Characteristic of milk |
Sweetish bitter |
| Average | 100 | 187.06 | 117.06 | 69.26 | |||
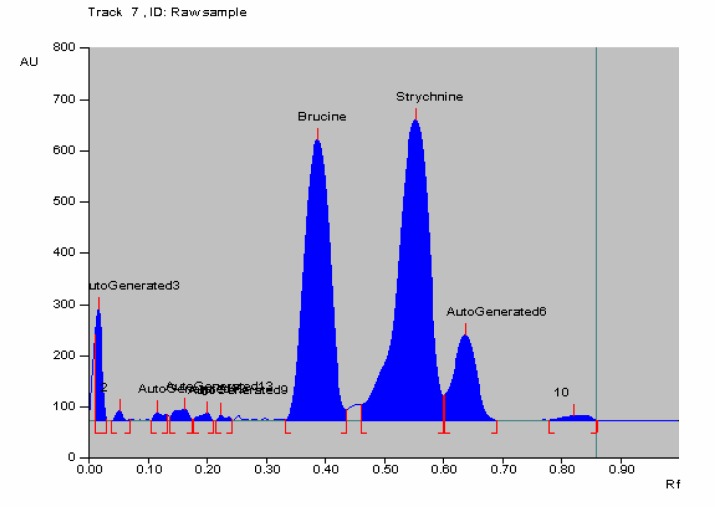
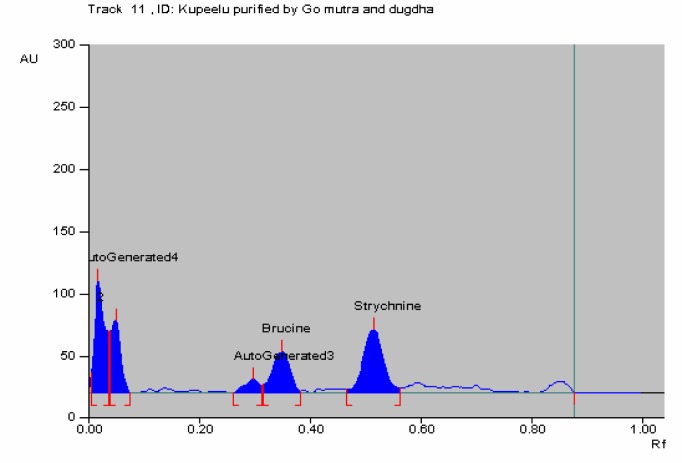
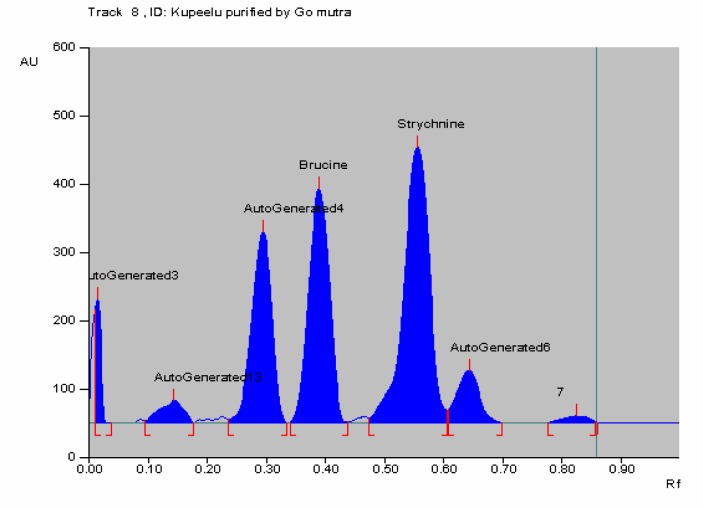
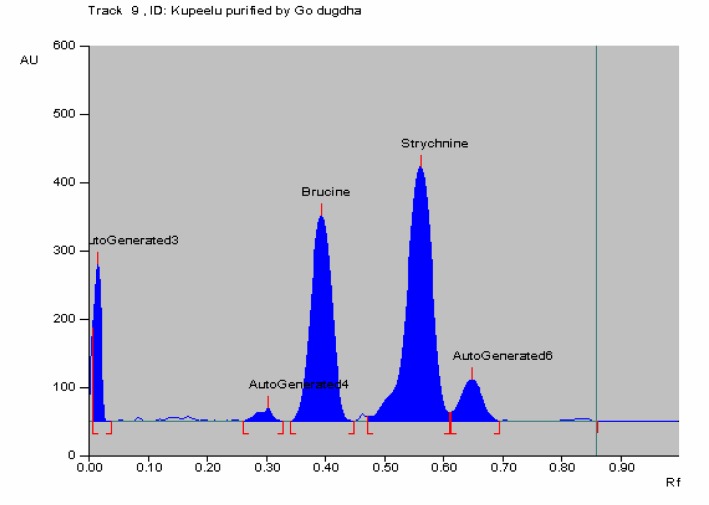
In HPTLC chromatogram, UV spectrum at 254 nm of standard strychnine (Rf 0.54) and standard brucine (Rf 0.34) were shown in Figures 1–2 and peak areas of strychnine and brucine in all the samples were exposed in Figures 3–6. Calibration curves of strychnine and brucine were prepared by plotting concentrations of strychnine and brucine in the range of 2–6 µg/spot versus average area of the peak. The responses for concentrations of standard strychnine and brucine were found to be linear (Figures 7 – 8). The amount of strychnine and brucine in raw and purified samples were computed from the calibration curves which suggested that the highest reduction of strychnine and brucine was found in the third method of proper processing (Table 5). It may be due to the combined effect of the two media in the process of proper processing. First, alkalinity of cow's urine initiated the extraction process and further it was potentiated by milk as boiling in milk converted the strychnine into less toxic isostrychnine (Cai et al., 1990).
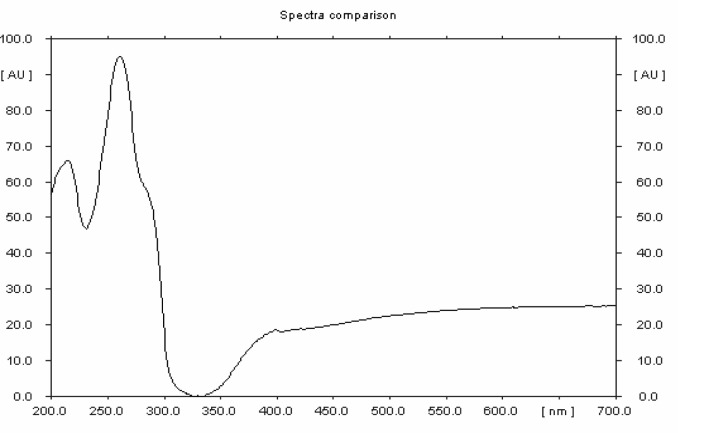
Figure 1.
HPTLC profile of standard strychnine
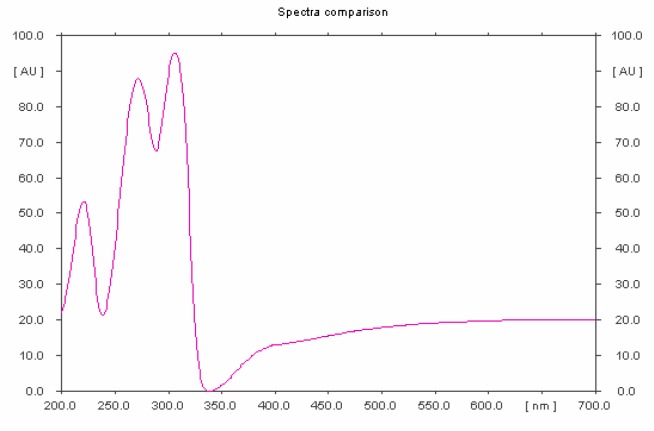
Figure 2.
HPTLC profile of standard brucine
Figure 3.
HPTLC of raw Kupeelu showing peak area of strychnine and brucine
Figure 6.
HPTLC of Kupeelu processed by cow's urine followed by cow's milk (Go mutra & dugdha) showing peak area of strychnine and brucine
Figure 7.
Calibration curve of strychnine
Figure 8.
Calibration curve of brucine
Table 5.
Results of estimation of strychnine and brucine in raw and processed samples of Kupeelu by HPTLC
| Samples | Amount of strychnine found (% w/w) |
Amount of brucine found (% w/w) |
| Raw Kupeelu (KR) | 1.442 | 0.659 |
|
Kupeelu processed by cow's urine (KGM) |
0.449 | 0.314 |
|
Kupeelu processed by cow's milk (KGD) |
0.289 | 0.201 |
|
Kupeelu processed by cow's urine and milk (KGMD) |
0.131 | 0.079 |
Figure 4.
HPTLC of Kupeelu processed by cow's urine (Go mutra) showing peak area of strychnine and brucine
Figure 5.
HPTLC of Kupeelu processed by cow's milk (Go dugdha) showing peak area of strychnine and brucine
Conclusion
It may be concluded that the processing with simultaneous cow's urine followed by milk may be regarded as the best method of proper processing to reduce the toxic alkaloids up to therapeutic effective dose form. These findings strongly confirm the claims of ancient classics of Ayurveda that Shodhana (Purificatory measures) successfully reduces the toxic effects of the drugs. Further studies are suggested to prove the therapeutic efficacy of properly processed seeds of nux vomica advocating the present methods.
Acknowledgement
The authors are very much thankful to Director of I.P.G.T & R.A, G.A.U, Jamnagar, Gujarat, India for providing all necessary facilities in carrying out this research work.
References
- 1.Trikamji Acharya Jadavji. Siddhayog Samgraha. 13th edition. Allahabad, Shree Baidyanath Ayurved Bhavan Ltd.; 2008. p. 163. [Google Scholar]
- 2.Akbar S, Khan SA, Akbar M, Iqbal M. Use of strychnos nuxvomica (Azraqi) seeds in Unani System Of Medicine: Role of detoxification. Afr J Tradit Complement Altern Med. 2010;7(4):286–290. doi: 10.4314/ajtcam.v7i4.56689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Ayurvedic Formulary Of India. 1st edition. New Delhi: Govt. of India; 1978. p. 172. Anonymous. [Google Scholar]
- 4.The Wealth Of India. Vol. 10. New Delhi: National Institute of Science Communication, Council Of Scientific & Industrial Research; 1998. p. 62. Anonymous. [Google Scholar]
- 5.Database of Indian Medicinal Plants. Vol. 5. New Delhi: Documentation and Publication Division CCRAS; 2005. p. 139. Anonymous. [Google Scholar]
- 6.Baser Kemal HC, Bisset Norman G. Alkaloids of Sri Lankan Strychnos nux-vomica. Phytochemistry. 1982;21(6):1423–1429. [Google Scholar]
- 7.Cai BC, Hattori M, Namba T. Processing of nux vomica. II. Changes in alkaloid composition of the seeds of Strychnos nux-vomica on traditional drug-processing. Chem Pharm Bull[Internet] 1990;38(5):1295–1298. doi: 10.1248/cpb.38.1295. [cited 2010 June 3] Available from: http://www.ncbi.nlm.nih.gov/pubmed/2393954. [DOI] [PubMed] [Google Scholar]
- 8.Gogte VM. Ayurvedic Pharmacology & Therapeutic Uses of Medicinal Plants. 1st edition. Mumbai: Bharatiya Vidya Bhavan; 2000. pp. 345–347. [Google Scholar]
- 9.Gopalkrishna SV, Lakshmi narasu M, Ramachandra Setty S. Hepatoprotective activity of detoxified seeds of nux vomica against CCl4 induced hepatic injury in albino rats. Pharmacologyonline. 2010;1:803–815. [Google Scholar]
- 10.Nadkarni KM. Indian Materia Medica. 13th edition. Vol. 1. Mumbai: Popular Prakashan; 1976. pp. 1175–1181. [Google Scholar]
- 11.Gyanendra Pandey. Anti-Aging Herbal Drugs Of India. 1st edition. Delhi: Sri Satguru Publication; 2002. p. 248. [Google Scholar]
- 12.Mukund Sabnis. Chemistry & Pharmacology of Ayurvedic Medicinal Plants. 1st edition. Varanasi: Chaukhamba Amarabharati Prakashan; 2006. pp. 455–456. [Google Scholar]
- 13.Sharma SN, Shastri KN. Rasa Tarangini. 11th edition. New Delhi: Motilal Banarasidas Publication; 2000. pp. 163–164. [Google Scholar]
- 14.Shastri JLN. Dravyaguna Vijnana. 1st edition. Vol. 1. Varanasi: Choukhamba Orientalia; 2009. p. 320. [Google Scholar]
- 15.Singh LB, Singh RS, Bose R, Sen SP. Studies on the pharmacological action of Aconite in the form used in Indian Medicine. Bull Med Ethnobot Res. 1985;6:115–123. [Google Scholar]