Abstract
Xylene has been generally used as a clearing and deparaffinizing agent in histology. Because of the potential toxic and flammable nature of xylene, its substitutes have been introduced into some laboratories. In this study, we introduced a novel, non-toxic xylene substitute (SBO), which was generated through a mixture of 86% of white oil No.2 and 14% of N-heptane. SBO had a high boiling point (188°C) and flash point (144°C) coupled with a scentless and decreased volatility. To compare the effectiveness of SBO and xylene in histology, a wide range of tissue samples from rats and human beings were processed in parallel in SBO and xylene, subjected to various staining procedures. Similar to the xylene-processed paraffin blocks, the SBO-processed counterparts were easy to section without any evidence of cell shrinkage. Assessment of the SBO-treated sections stained with hematoxylin-eosin revealed a good maintenance of cell morphology and structure, and a clear definition of the cytoplasm and the nucleus. Moreover, comparable good results were achieved between the SBO- and xylene-processed tissues in other histochemical and immunohistochemical stainings. Six-month clinical applications at one department of pathology supported the potentials of SBO as a xylene substitute. In conclusion, we suggest that SBO is a safe and efficient substitute of xylene and may probably replace xylene without losing valuable diagnostic information.
Keywords: SBO, clearing agent, xylene, histology, toxicity
Introduction
Clearing is an important step in the preparation of histological sections, aiming to remove alcohol and other dehydrants from tissues prior to infiltration of the embedding material (usually paraffin wax). In the past dozens of years, xylene with excellent compatibility of alcohol and paraffin wax has been widely used as a clearing agent (Matthews, 1981; Pollard et al., 1987). Laboratory-grade xylene consists of m-xylene (40–65%), p-xylene (20%), o-xylene (20%) and ethyl benzene (6-20%) and traces of toluene, trimethyl benzene, phenol, thiophene, pyridine and hydrogen sulfide. A large number of animal studies have demonstrated that being excessively exposed to xylene can cause toxicity to multiple tissues such as the nervous system, the liver, the skin, and the lung (Gamberale et al., 1978; Hass et al., 1995; Kum et al., 2007a; Chatterjee et al., 2005; Sandikci et al., 2009). The cell toxicity of xylene has been linked to the induction of mitochondrial uncoupling and oxidative stress (Piotrowska et al., 2002; Revilla et al., 2007). Considering the serious adverse effects of xylene, many attempts have been made to replace this agent with safer alternatives. In recent years, numerous xylene substitutes have been commercially developed, among which are aromatic derivatives of terpene and others hydrocarbons (Buesa, 1997). Since their costs and effectiveness are still unsatisfactory, it makes the development of more novel, more effective and safer substitutes increasingly necessary. Vegetable oils (e.g. olive oil and coconut oil) have been documented to have potentials of being used as clearing agents; however, for unknown reasons, they are less effective in some cases of histological specimens compared to xylene (Piotrowska et al., 2002).
SBO is a non-toxic substitute of xylene generated through a mixture of 86% of white oil No.2 and 14% of N-heptane. It has new features of being colorless, scentless and virtually non-volatile. Compared with xylene, SBO has much higher flash point, boiling point and ignition point, so it can greatly reduce the pollution and increase the safety and reliability when being used. At the same time, it maintains the characteristics of being soluble in alcohol and paraffin. In this study, to improve the efficiency of SBO in tissue processing, the SBO and xylene were used in parallel in the preparation of a broad spectrum of tissue sections, and their effectiveness on staining results was investigated. The aim of the study was to assess the potential of SBO as a xylene substitute for histology.
Materials and Methods
Reagents
SBO was produced by Kunming Laidun Technology Co., Ltd. (Kunming, China), which was obtained through hydrogenation of a mixture of white oil and N-heptane. The detailed information provided by the producer indicates that SBO neither irritates nor sensitizes normal skin. It is safe and non- toxic. The SBO reagent was a colorless liquid at room temperature. Anti-carcinoembryonic antigen (CEA) and anti- high-molecular-weight cytokeratins were purchased from santa cruz biotechnology, inc. (Santa Cruz, CA, USA). 3′-diaminobenzidine tetrahydrochloride were purchased from Sigma Chemical Co (St. Louis, Mo. USA). Other reagents were of analytical-reagent grade and were purchased from Kunming Chemical Reagent Co. (Kunming, China), unless otherwise stated. The physicochemical properties of xylene and SBO are shown in Table 1.
Table 1.
Physicochemical properties of xylene and SBO
| Compound | Molecular weight (kDa) |
Vapor pressure (kPa at 20°C) |
Flash point (°C) |
Boiling point (°C at 100 kPa) |
| SBO | 256 | 0.0020 | 196 | 250 |
| Xylene | 106 | 0.8 | 25 | 137–143 |
Tissue samples
For pilot study, we collected a total of 80 specimens from the omentum, spleen, and kidney of 10 rats (provided by Key Laboratory of Pharmacology for Natural Products, Kunming Medical University, China). The samples were fixed for 6–8 h in 4% phosphate-buffered formaldehyde (pH 7.0), and subjected to tissue processing and paraffin embedding as described below. For large-scale tests, a total of 328 surgically resected human specimens from 8 different tissues, such as the liver, spleen, lung, kidney, and skin, were obtained from the First Affiliated Hospital of Kunming Medical University (Kunming, China). The specimens were fixed in 4% neutral buffered formaldehyde and processed as below for histological study. For each test, no less than 200 tissue blocks were used. The study protocol was approved by the Ethics Committee of Kunming Medical University, and written informed consent was obtained from each patient.
Histological procedure
Each of the specimens described above was equally divided into 2 parts, and each part was randomly assigned to either xylene or SBO groups. The specimens in the two groups were routinely processed with a similar protocol (Table 2), except for using different clearing and dewaxing agents (i.e., the xylene group with xylene and the SBO group with SBO). The obtained paraffin sections (3–4 µm in thickness) were then subjected to histological examination. The histological procedures included hematoxylin and eosin (HE) staining, van Gieson staining for collagen fibers (Noorlander et al., 2002), aldehyde-fuchsin staining for elastic fibers (Osanai et al., 2002), Gordon Sweet's staining for reticulin fibers (Bralet et al., 2000), and alcian blue staining for acid mucopolysaccharides (Singh and Gorton, 1989), Periodic acid-Schiff (PAS) staining for glycogen (Stankler and Walker, 1976), periodic acid-silver methenamine (PASM) staining for the glomerular basement membrane (Doyle and Campbell, 1976), Victoria blue staining for hepatitis B surface antigen (Dunsford et al., 1990), and immunostaining. Immunohistochemistry was carried out as previously (Masumori et al., 2001). The primary antibodies included anti-carcinoembryonic antigen (CEA) and anti- high-molecular-weight cytokeratins (CKH), which were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Following sequential incubations with the primary antibody and appropriate secondary antibodies conjugated with horseradish peroxidase (HRP), slides were developed with 3,3′-diaminobenzidine tetrahydrochloride (Sigma, St. Louis, MO, USA), and were counterstained with hematoxylin. After staining, slides were dehydrated in ethanol, cleared with xylene (in the xylene group) or SBO (in the SBO group), and mounted in neutral gum. The histological procedures for the two groups were carried out in parallel by the same pathologist from Kunming Medical University.
Table 2.
The protocol for tissue processing, embedding, and sectioning
| Procedure | Reagent | Time (min) | Temperature (°C) |
| Fixation | Formaldehyde 4% | 300 | 25 |
| Dehydration | Ethanol 75% | 30 | 25 |
| Ethanol 80% | 30 | 25 | |
| Ethanol 95% | 30 | 25 | |
| Ethanol 100% | 30 | 25 | |
| Ethanol 100% | 30 | 25 | |
| Clearing | Xylene or SBO | 30 | 25 |
| Xylene or SBO | 60 | 25 | |
| Xylene or SBO | 60 | 25 | |
| Infiltration | Paraffin wax | 30 | 58 |
| Paraffin wax | 60 | 58 | |
| Paraffin wax | 60 | 58 | |
| Embedding | Paraffin wax | Not determined | 58 |
| Sectioning | Not determined | −10 | |
| Dewaxing | Xylene or SBO | 10 | 25 |
| Xylene or SBO | 10 | 25 | |
| Xylene or SBO | 10 | 25 | |
| Rehydration | Ethanol 100% | 10 | 25 |
| Ethanol 100% | 10 | 25 | |
| Ethanol 95% | 2 | 25 | |
| Ethanol 95% | 2 | 25 | |
| Ethanol 85% | 2 | 25 | |
| Ethanol 75% | 2 | 25 | |
Assessment of stained sections
All sections were coded and evaluated by experimental pathologists who were blinded to the experiments. The criteria used for assessing section quality were the presence of folds, cracks, and artifacts. For assessing the quality of staining, we examined tissue and cell morphology and checked for the integrity and definition of cell structure.
Results
Pilot study
The paraffin blocks prepared by using SBO as a clearing agent were generally ready to section without any unpleasant odor produced. After being stored for no more than a few month, the paraffin blocks were well preserved. Assessment of the H&E-stained slides revealed that the staining results were comparable in both the xylene and SBO groups, and the cell architecture remained intact and clear, without evidence of distortion and shrinkage. SBO appeared to be more applicable than xylene in dealing with the tissues either rich in fat or vascular components.
Large-scale study
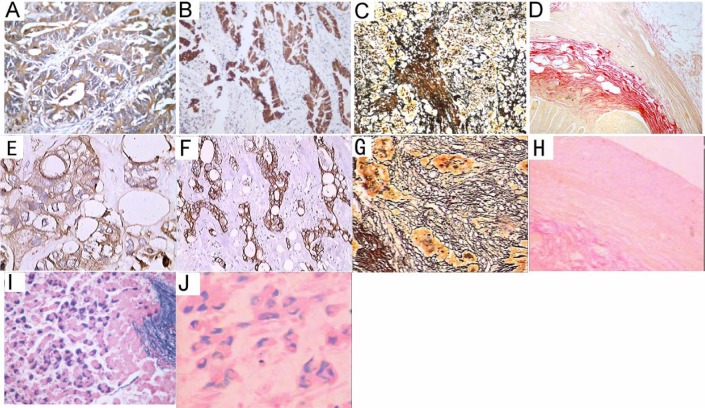
To further evaluate the efficiency of SBO in histology, a large-scale study was conducted using numerous human tissue blocks. The paraffin blocks made from SBO as a clearing agent presented with proper hardness, and were easy to be cut into 4-µm-thick consecutive sections. A thorough removal of wax was obtained in the SBO-treated slides. Representative staining results revealed similar good results achieved in both the xylene and SBO groups (Figures 1 and 2). There was no sign of cell shrinkage and deformation for the SBO-treated slides. A clear definition of the cytoplasm and the nucleus was observed.
Figure 1.
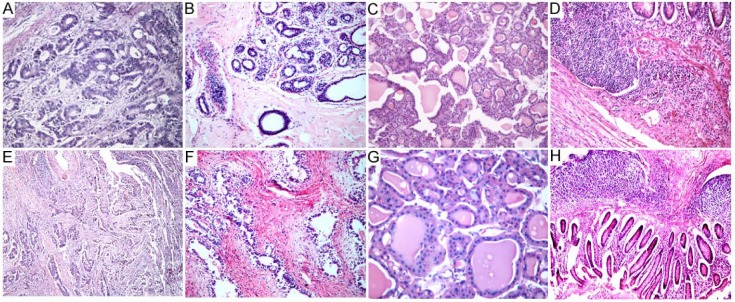
Routine HE staining analysis.
Human tissue samples of colorectal cancer (A, E), breast adenofibroma (B, F), thyroid gland (C, G), and vermiform appendix (D, H) were processed with xylene (upper panel) or SBO (lower panel) and subjected to HE staining. Representative staining results are shown. A–C, F, 200 ×; D, E, H, 100 ×; G, 400 ×.
Figure 2.
Examples of histochemical and immunohistochemical stainings of SBO- or xylene-treated sections.
Human tissue samples of colorectal cancer (A, B, E, F), lung cancer (C, G), vermiform appendix (D, H), and hepatitis B virus-infected liver (I, J) were processed with SBO (A-D, I) or xylene (E-H, J). Immunostaining for carcinoembryonic antigen (A, B) and low-molecular-weight cytokeratins (E, F), Gordon Sweet's staining for reticulin fibers (C, G), van Gieson staining for collagen fibers, and Victoria blue staining for hepatitis B surface antigen (I, J) were then performed. Representative staining results are shown. A, B, I, 400 ×; C, 200 ×; D, 100 ×.E,F,J,400 ×;G,200 ×;H,100 ×.
Clinical practice
SBO has been applied as a clearing agent for 6 months at the Pathological Department, the First Affiliated Hospital of Kunming Medical University. This agent was equally effective or superior to xylene when it comes to clearing, dewaxing, and sectioning. SBO was very stable, without any unpleasant odor produced and without any decomposition observed during 10 days of routine histological operations. Most importantly, the SBO-treated slides were successful used in various routine histological examinations and good staining results were obtained. With all these obvious advantages, SBO had completely replaced xylene in histology at this department for more than 2 months.
Discussion
Xylene traditionally has been employed as a clearing, dewaxing, and mounting agent for histology (Buesa and Peshkov, 2009; Chen et al., 2010). It is well documented, however, that xylene is an environmental hazard and highly toxic to humans. Accumulating evidences indicate that being repeatedly or excessively exposed to xylene can do harm to the nervous system, skin, liver, kidney, and lung tissues (Gamberale et al., 1978; Hass et al., 1995; Kum et al., 2007a; Chatterjee et al., 2005; Sandikci et al., 2009; Kum et al., 2007b). In addition, the xylene is also reported that it has many shortcomings, such as being highly flammable and volatile. It has a much lower boiling point(137-143°C), a flash point(25°C) and an ignition point (25°C), etc. Therefore, xylene is toxic as a clearing agent in histology. Although many xylene substitutes have been commercially developed (Buesa, 1997), they fail to completely replace xylene, partially due to the variable effectiveness and high costs. SBO is made from a mixture of nontoxic white oil and N-heptane, which was generated by hydrogenation of white oils through the addition hydrogen atoms to alkenes. The changes in the degree of saturation of oils are coupled with the alteration of important physical properties, i.e., SBO has an higher boiling point (250°C) , a flash point (196°C) and a very low vapor pressure. But Hydrogenation results in a decrease in the volatility, thereby reducing the pollution to the environment. Though SBO has the improved safety properties, it retains the miscibility with alcohol and paraffin, making it suitable for tissue processing.
In this study, the paraffin blocks prepared by using SBO as a clearing agent had proper hardness and were easy to be cut into ∼4-µm serial sections. Assessment of the stained sections revealed a good maintenance of cell morphology and structure. The cytoplasm and the nucleus were well stained in HE. These findings are consistent with a previous report (Piotrowska et al., 2002), Most importantly, the SBO-treated slides were applicable to various histological procedures including HE, van Gieson staining, aldehyde-fuchsin staining, Gordon Sweet's staining, alcian blue staining, PAS staining, PASM staining, Victoria blue staining, and immunostaining. The staining results were comparable or superior to those achieved by using xylene. Six-month clinical applications at one department of pathology support the potentials of SBO as a xylene substitute. SBO seems to have a broader range of applications than natural vegetable oils in histology, since some of the oil-processed tissues are somehow inappropriate for histochemical and immunohistochemical stainings (Piotrowska et al., 2002). Moreover, SBO is applicable to the tissues rich in fat or vascular components, from which a good result was not obtained by using olive oil as a clearing agent (Lyon et al., 1995).
Although our data suggested that a comparable staining result could be achieved in the SBO- and xylene-processed specimens, the criteria for assessing it were somewhat subjective. In this study, we mainly focused on whether there was cell shrinkage, whether the tissue and cell morphology was intact, and whether the definition of the cell structure was distinct. However, it is difficult to give a clear definition of the ideal appearance of a tissue section by using microscopes. Nevertheless, our data revealed that SBO is appropriate in the clearing and deparaffinization process, and the SBO-processed tissue is applicable to various histological examinations. A long-term clinical practice suggests that SBO may be a promising substitute of xylene in histology without losing valuable diagnostic information.
Acknowledgments
This work has been supported by the natural science foundation of Yunnan Province of China (No. 2008CD046).
References
- 1.Bralet MP, Régimbeau JM, Pineau P, Dubois S, Loas G, Degos F, Valla D, Belghiti J, Degott C, Terris B. Hepatocellular carcinoma occurring in nonfibrotic liver: epidemiologic and histopathologic analysis of 80 French cases. Hepatology. 2000;32:200–204. doi: 10.1053/jhep.2000.9033. [DOI] [PubMed] [Google Scholar]
- 2.Bruun Rasmussen B, Norring Hjort K, Mellerup I, Sether G, Christensen N. Vegetable oils instead of xylene in tissue processing. APMIS. 1992;100:827–831. doi: 10.1111/j.1699-0463.1992.tb04006.x. not cited in the text?? [DOI] [PubMed] [Google Scholar]
- 3.Buesa RJ. Mineral oil: the best xylene substitute for tissue processing yet? J Histotechnol. 2002;23:143–149. [Google Scholar]
- 4.Buesa RJ, Peshkov MV. Histology without xylene. Ann Diagn Pathol. 2009;13:246–256. doi: 10.1016/j.anndiagpath.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee A, Babu RJ, Ahaghotu E, Singh M. The effect of occlusive and unocclusive exposure to xylene and benzene on skin irritation and molecular responses in hairless rats. Arch Toxicol. 2005;79:294–301. doi: 10.1007/s00204-004-0629-1. [DOI] [PubMed] [Google Scholar]
- 6.Chen CY, He T, Mao XL, Friis TE, Qin RH, Jian YT. A novel xylene substitute for histotechnology and histochemistry. Biotech Histochem. 2010;85:231–240. doi: 10.3109/10520290903235445. [DOI] [PubMed] [Google Scholar]
- 7.Doyle GD, Campbell E. The periodic Schiff-methenamine (PASM) staining of renal biopsies—a light electron microscopic study. Ir J Med Sci. 1976;145:127–134. [PubMed] [Google Scholar]
- 8.Dunsford HA, Sell S, Chisari FV. Hepatocarcinogenesis due to chronic liver cell injury in hepatitis B virus transgenic mice. Cancer Res. 1990;50:3400–3407. [PubMed] [Google Scholar]
- 9.Gamberale F, Annwall G, Hultengren M. Exposure to xylene and ethylbenzene. III. Effects on central nervous functions. Scand J Work Environ Health. 1978;4:204–211. doi: 10.5271/sjweh.2705. [DOI] [PubMed] [Google Scholar]
- 10.Hass U, Lund SP, Simonsen K, Fries AS. Effects of prenatal exposure to xylene on postnatal development and behavior in rats. Neurotoxicol Teratol. 1995;17:341–349. doi: 10.1016/0892-0362(94)00093-s. [DOI] [PubMed] [Google Scholar]
- 11.Kum C, Kiral F, Sekkin S, Seyrek K, Boyacioglu M. Effects of xylene and formaldehyde inhalations on oxidative stress in adult and developing rats livers. Exp Anim. 2007a;56:35–42. doi: 10.1538/expanim.56.35. [DOI] [PubMed] [Google Scholar]
- 12.Kum C, Sekkin S, Kiral F, Akar F. Effects of xylene and formaldehyde inhalations on renal oxidative stress and some serum biochemical parameters in rats. Toxicol Ind Health. 2007b;23:115–120. doi: 10.1177/0748233707078218. [DOI] [PubMed] [Google Scholar]
- 13.Lyon H, Holm I, Prentø P, Balslev E. Non-hazardous organic solvents in the paraffin-embedding technique: a rational approach. Aliphatic monoesters for clearing and dewaxing: butyldecanoate. Histochem Cell Biol. 1995;103:263–269. doi: 10.1007/BF01457410. [DOI] [PubMed] [Google Scholar]
- 14.Masumori N, Thomas TZ, Chaurand P, Case T, Paul M, Kasper S, Caprioli RM, Tsukamoto T, Shappell SB, Matusik RJ. A probasin-large T antigen transgenic mouse line develops prostate adenocarcinoma and neuroendocrine carcinoma with metastatic potential. Cancer Res. 2001;61:2239–2249. [PubMed] [Google Scholar]
- 15.Matthews JB. Influence of clearing agent on immunohistochemical staining of paraffin-embedded tissue. J Clin Pathol. 1981;34:103–105. doi: 10.1136/jcp.34.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noorlander ML, Melis P, Jonker A, Van Noorden CJ. A quantitative method to determine the orientation of collagen fibers in the dermis. J Histochem Cytochem. 2002;50:1469–1474. doi: 10.1177/002215540205001106. [DOI] [PubMed] [Google Scholar]
- 17.Osanai H, Murakami G, Ohtsuka A, Suzuki D, Nakagawa T, Tatsumi H. Histotopographical study of human periocular elastic fibers using aldehyde-fuchsin staining with special reference to the sleeve and pulley system for extraocular rectus muscles. Anat Sci Int. 2002;84:129–140. doi: 10.1007/s12565-009-0017-2. [DOI] [PubMed] [Google Scholar]
- 18.Piotrowska D, Długosz A, Pajak J. Antioxidative properties of coenzyme Q10 and vitamin E in exposure to xylene and gasoline and their mixture with methanol. Acta Pol Pharm. 2002;59:427–432. [PubMed] [Google Scholar]
- 19.Pollard K, Lunny D, Holgate CS, Jackson P, Bird CC. Fixation, processing, and immunochemical reagent effects on preservation of T-lymphocyte surface membrane antigens in paraffin-embedded tissue. J Histochem Cytochem. 1987;35:1329–1338. doi: 10.1177/35.11.3309048. [DOI] [PubMed] [Google Scholar]
- 20.Revilla AS, Pestana CR, Pardo-Andreu GL, Santos AC, Uyemura SA, Gonzales ME, Curti C. Potential toxicity of toluene and xylene evoked by mitochondrial uncoupling. Toxicol In Vitro. 2007;21:782–788. doi: 10.1016/j.tiv.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Sandikci M, Seyrek K, Aksit H, Kose H. Inhalation of formaldehyde and xylene induces apoptotic cell death in the lung tissue. Toxicol Ind Health. 2009;24:455–461. doi: 10.1177/0748233709106824. [DOI] [PubMed] [Google Scholar]
- 22.Singh R, Gorton AW. Orcein-alcian blue staining: a new technique for demonstrating acid mucins in gastrointestinal epithelium. J Clin Pathol. 1989;42:881–884. doi: 10.1136/jcp.42.8.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stankler L, Walker F. Periodic acid-Schiff (PAS) staining for glycogen in clinically normal psoriatic and non-psoriatic skin. Br J Dermatol. 1976;95:599–601. doi: 10.1111/j.1365-2133.1976.tb07030.x. [DOI] [PubMed] [Google Scholar]