Abstract
Drymaria cordata (Linn.) Willd (Caryophyllaceae) is an herbaceous plant widely used in traditional African medicine (TAM) for the treatment of diverse ailments including painful and febrile conditions. This study was conducted to investigate the analgesic and antipyretic properties of the whole plant extract of D. cordata. The acetic acid-induced writhing, formalin, and tail clip tests were used to evaluate analgesic activity while the 2,4-dinitrophenol (DNP)-, d-amphetamine-, and yeast-induced hyperthermia tests were used to investigate antipyretic activity in rodents. D. cordata (100, 200, and 400 mg kg−1, p.o) produced significant (p<0.05) analgesic activity in the mouse writhing, formalin (second phase), and tail clip tests. The effects of D. cordata were generally comparable to those of acetylsalicylic acid (ASA, 100 mg kg−1, p.o) and morphine (2 mg kg−1, s.c). Also, D. cordata produced significant (p<0.05) dose-dependent inhibition of temperature elevation in the 2,4-DNP and yeast-induced hyperthermia models with peak effects produced at the dose of 400 mg kg−1. The effect at this dose was comparable to that of ASA in the two models. In the d-amphetamine method, D. cordata produced significant (p<0.05) dose- and time-dependent reduction of temperature elevation with peak effect produced at the dose of 200 mg kg−1. The effect of the extract at this dose was greater than that of ASA. The results obtained in this study demonstrate that the aqueous whole plant extract of Drymaria cordata possesses analgesic and antipyretic properties mediated through peripheral and central mechanisms.
Keywords: Drymaria cordata, Caryophyllaceae, analgesic activity, antipyretic activity, traditional African medicine (TAM)
Introduction
Over the years, plants have generally proven to be veritable sources of drugs used in orthodox medicine. This has in recent times encouraged the search for newer more efficacious and better tolerated drugs from plants. A criterion that has been used over the years for the selection of plants for pharmacological investigations is reported use in traditional medicine. Drymaria cordata (Linn.) Willd (Caryophyllaceae) is a weak spreading herb found widely dispersed in damp places all over the tropics of Africa, Asia and the Americas (Burkill, 1985). The plant appears spontaneously as a weed of cultivation, with stems growing to nearly one meter long and leaves being rounded, heart-shaped and hairless. It is usually found occupying grassland, forest margins, roadsides and cultivated areas, often under shade at mid to higher elevations. The use of the plant for agriculture and traditional medicine has been reported in Africa, Asia and the Americas (Burkill, 1985). In tropical Africa, D. cordata preparations are used for the treatment of diverse ailments including cold, headache, coryza, bronchitis, as poultice on sore (to treat aching, inflamed or painful parts), leprosy, tumors, as fumigant for eye troubles, as cerebral stimulant and antifebrile agent (Burkill, 1985). Extracts of D. cordata have previously been reported to possess antitussive (Mukherjee et al., 1997), anti-inflammatory (Adeyemi et al., 2008), anxiolytic (Barua et al., 2009), and cytotoxic (Sowemimo et al., 2009) activities.
In Nigeria, D. cordata (chick weed; “Calabar woman's eye”) is used in folk medicine to treat sleeping disorders, convulsions, and febrile conditions in children (Adeyemi et al., 2008). The aim of this study is to investigate the analgesic and antipyretic activities of the aqueous whole plant extract of D. cordata in rodents using different pharmacological models.
Materials and methods
Plant material and preparation of plant extract
Drymaria cordata plant (Figure 1) was obtained from a local farmland in Ibadan, Oyo State, Nigeria, in the month of June 2008. Botanical identification and authentication was done by Prof. J.D. Olowokudejo of the Department of Botany and Microbiology, Faculty of Science, University of Lagos, Lagos, Nigeria and Mr. T. K. Odewo, Senior Superintendent of the Forestry Research Institute of Nigeria (FRIN), Ibadan, Nigeria. The voucher specimen of the plant was deposited at the herbarium of the Institute and it was assigned reference number FHI 105715. Fresh D. cordata plant was separated from extraneous materials and washed. Sixty five grams of the whole plant was pounded using mortar and pestle, and macerated in distilled water (10 g in 1 L). This was kept at 4 °C in the refrigerator for 48 h. The filtrate was decanted and evaporated to dryness in an oven (Griffin 2/250 FC) set at 40 °C, giving a percentage yield of 14.68 %. The dried extract was weighed using a Mettler-Toledo GmbH digital weighing balance (Type BD202, SNR 06653) and dissolved in distilled water to give a concentration of 100 mg/ml before administration to experimental animals. The plant extract was prepared in this way based on the form in which it is used traditionally.
Figure 1.

Drymaria cordata
Yield (%) = (Weight of dried extract ÷ Weight of plant starting material) × 100.
Laboratory animals
Adult albino mice (20 — 25 g) and rats (100 — 200 g) of either sex used in this study were in-bred and obtained from the Laboratory Animal Centre of the College of Medicine, University of Lagos, Lagos, Nigeria. The animals were maintained under standard environmental conditions and fed with standard diet (Livestock Feeds PLC, Lagos, Nigeria) and water ad libitum. The experimental procedures used in this study were in strict compliance with the United States National Institutes of Health Guidelines for Care and Use of Laboratory Animals in Biomedical Research (1985).
Drugs and chemicals
Acetic acid (BDH Chemicals Ltd., Poole, England); formalin (UNIC Pharmaceuticals, Lagos, Nigeria); acetylsalicylic acid, ASA (Dispirin®; Reckitt & Coleman Ltd., Pakistan); morphine (Evans Medical Ltd., Leatherhead, England); amphetamine (BDH, Poole England); Yeast (Kunimed Pharmachem Ltd., Lagos, Nigeria); methylcellulose (Koch-Light Laboratories Ltd., Suffolk, England); 2,4-Dinitrophenol (DNP); and sodium chloride (May and Baker, Nigeria PLC).
Phytochemical screening
Qualitative and quantitative analysis of the aqueous whole plant extract of D. cordata was carried out using standard procedures (Sofowora, 1993; Edeoga et al., 2005).
Pharmacological studies
Analgesic activity: Mouse writhing test
Adult mice fasted overnight were divided into six groups of five animals each. Treatment was then carried out accordingly: saline (10 ml kg−1, p.o); D. cordata (100, 200, and 400 mg kg−1, p.o); ASA (100 mg kg−1, p.o); and morphine (2 mg kg−1, s.c). sixty minutes post-treatment for oral route or 30 min. post-treatment for subcutaneous route, mice were given acetic acid (0.6% v/v in saline, 10 ml kg−1, i.p). The number of writhes (characterized by contraction of the abdominal musculature and extension of the hind limbs) was then counted at 5 min. interval for 30 min. (Singh and Majumdar, 1995; Mbagwu et al., 2007).
Formalin test
Adult mice fasted overnight were divided into 6 groups of 6 animals each. Treatment was then carried out as scheduled: saline (10 ml kg−1, p.o); D. cordata (100, 200 and 400 mg kg−1, p.o); ASA (100 mg kg−1, p.o); and morphine (2 mg kg−1, s.c). Sixty minutes post-treatment for oral route or 30 min. post-treatment for subcutaneous route, formalin (20 µL of 1 % solution) was injected s.c into the right hind paw. The time (s) spent in licking and biting responses of the injected paw was taken as an indicator of pain response. Responses were measured for 5 min. after formalin injection (first phase) and 15–30 min. after formalin injection (second phase) (Shibata et al., 1989; Mbagwu et al., 2007).
Tail clip test
Mice used in this experiment were initially screened by applying a metal artery clip to the root of the mouse's tail to induce pain. Animals which failed to attempt to dislodge the clip in 10 s were discarded. After selecting mice that qualify for the experiment, animals were divided into groups as outlined in the formalin test. The initial reaction time of all mice to the tail clip was determined after which treatment was carried out as stated in the formalin test. The reaction time of each mouse was then determined at 30 min. interval for 3 h (Adeyemi et al., 2004). A post-treatment cut-off time of 30 s was observed.
Antipyretic activity
2,4-DNP-induced hyperthermia
The basal oral temperature of rats fasted for 12 h was recorded (To °C). Pyrexia was then induced by intraperitoneal injection of 2,4-DNP (prepared at a concentration of 1 mg/ml in 0.9 % sodium chloride solution) at a dose of 20 mg kg−1 (Berkan et al., 1991). After the confirmation of hyperthermia 30 min. after 2,4-DNP administration, treatment was then carried out orally in five groups of six animals each as outlined: distilled water (10 ml kg−1), D. cordata extract (100, 200 and 400 mg kg−1) and aspirin (100 mg kg−1). The oral temperature of rats was then recorded at 1, 2, 3 and 4 h post-treatment (Tx °C).
Amphetamine-induced hyperthermia
The basal oral temperatures of rats fasted for 12 h were recorded (To °C) prior to the induction of pyrexia by intraperitoneal injection of d-amphetamine 10 mg/kg (Berkan et al., 1991). After confirmation of hyperthermia in the experimental animals 30 min. after d-amphetamine administration, treatment was carried out in five groups of six animals each via the oral route; distilled water (10 ml kg−1), D. cordata extract (100, 200 and 400 mg kg−1) and aspirin (100 mg kg−1). The oral temperatures of the animals were then recorded 1, 2, 3, 4 h post-treatment (Tx °C).
Yeast-induced hyperthermia
This was carried out based on the method described by Mukherjee et al. (2002). Rats were randomly divided into five groups of six rats each. The basal oral temperatures of the animals were recorded (To °C) over a period of one hour and the average basal oral temperature of each animal was recorded. Ten ml kg−1 of yeast suspension (15 % in 0.5 % w/v methylcellulose) was injected subcutaneously into the rats to induce pyrexia. Nineteen hours after yeast injection, the oral temperatures of animals were taken and animals showing rises in temperature of less than 0.6 °C were discarded (Murkherjee et al., 2002). After the establishment of pyrexia, distilled water (10 ml kg−1), D. cordata extract (100, 200, and 400 mg kg−1) and aspirin (100 mg kg−1) were orally administered to qualified rats. The oral temperatures of animals were then recorded at 1, 2, 3, and 4 h post-treatment (Tx °C).
Effect of D. cordata on normal body temperature
The basal oral temperature of rats fasted for 12 h was recorded (To °C). Treatment was then carried out in five groups of four animals each as detailed below: distilled water (10 ml kg−1, p.o), D. cordata extract (100, 200 and 400 mg kg−1, p.o) and aspirin (100 mg kg−1, p.o). The oral temperature of rats was then recorded at 1, 2, 3 and 4 h after treatment.
Statistical analysis
Results obtained were expressed as mean ± SEM. The data were analyzed using one way ANOVA followed by Tukey's multiple comparison test using GraphPad Prism 5 (GraphPad Software Inc., CA, USA). Results were considered significant when p<0.05.
Results
Qualitative and quantitative assessment of the aqueous whole plant extract of D. cordata revealed the presence of glycosides, saponins (0.85 %), alkaloids (1.42 %), flavonoids (1.49 %), Phenols (1.96 %), and tannins (20.61 %).
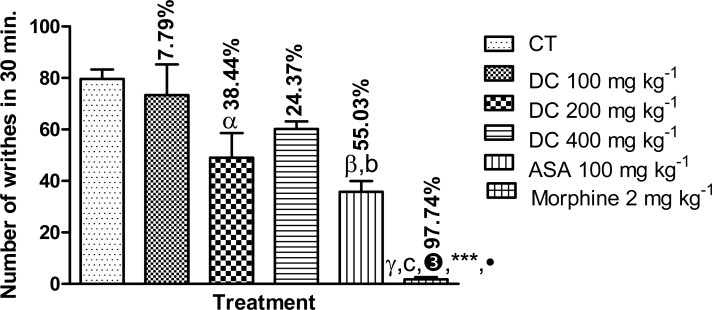
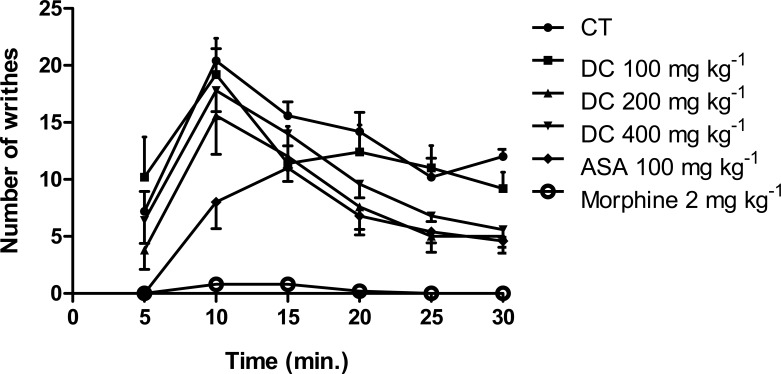
Intraperitoneal injection of acetic acid elicited the writhing reflex in control animals with the total number of writhes in 30 min. being 79.60 ± 3.67. D. cordata at the most effective dose, 200 mg kg−1, produced significant (p<0.05) reduction in the number of writhes (49.00 ± 9.63; 38.44 % inhibition) relative to control. Both ASA (100 mg kg−1) and morphine (2 mg kg−1) also significantly (p<0.01, 0.001) reduced the number of writhes relative to control giving values of 35.80 ± 4.16 (55.03 % inhibition) and 1.80 ± 0.80 (97.74 % inhibition), respectively. The effect of D. cordata at the dose of 200 mg kg−1 was not significantly different (p>0.05) from that of ASA but was significantly less (p<0.001) than that of morphine (Figure 2). In respect of time course of elicitation of the writhing reflex, the greatest number of writhes was generally produced at the 5–10 min. interval (Figure 3).
Figure 2.
Effect of D. cordata in the acetic acid-induced mouse writhing test. Data are mean ± SEM (n = 5). αp<0.05, βp<0.01,γp<0.001 vs. control; bp<0.01, cp<0.001 vs. D. cordata 100 mg kg−1; 3p<0.001 vs. D. cordata 200 mg kg−1; ***p<0.001 vs. D. cordata 400 mg/kg; ●p<0.05 vs. ASA 100 mg kg−1 (one way ANOVA followed by Tukey's multiple comparison test).
Figure 3.
Time course of elicitation of writhing in acetic acid-pain test in mice; data are mean ± SEM (n = 5).
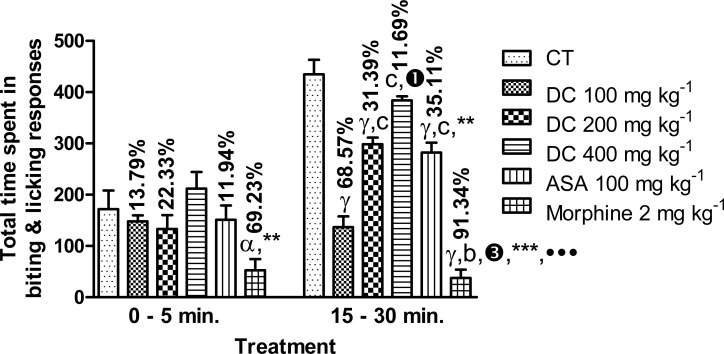
As shown in Figure 4, D. cordata extract (100–400 mg kg−1) and ASA (100 mg kg−1) did not produce any significant (p>0.05) effect on the duration of biting and licking responses induced by formalin injection into the hind paw of mice in the first phase (0–5 min.) of the formalin test. The effect of morphine (2 mg kg−1) was however significant (p<0.05), reducing the response duration from 171.67 ± 36.34 s in control animals to 52.83 ± 21.63 s (69.23 % inhibition). In the second phase of the formalin test, peak effect for D. cordata was produced at the dose of 100 mg kg−1 with the reduction of the response duration from 434.83 ± 28.38 s to 136.67 ± 20.89 s (68.57 % inhibition). Acetylsalicylic acid and morphine also produced significant (p<0.001) effects giving response duration values of 282.17 ± 19.49 s (35.11 % inhibition) and 37.67 ± 16.42 s (91.34 % inhibition) respectively, compared to control. The effect of morphine was significantly (p<0.001) greater than that of ASA. The effect of D. cordata at the most effective dose, 100 mg kg−1, was significantly greater than that of ASA (p<0.001) and less than that of morphine (p<0.01).
Figure 4.
Effect of D. cordata in the formalin test in mice. Data are mean ± SEM (n = 6). αp<0.05, γp<0.001 vs. control; bp<0.01, 6p<0.001 vs. D. cordata 100 mg kg−1; 1p<0.05, 3p<0.001 vs. D. cordata 200 mg kg−1; **p<0.01, ***p<0.001 vs. D. cordata 400 mg kg−1; •••p<0.001 vs. ASA 100 mg kg−1 (one way ANOVA followed by Tukey's multiple comparison test).
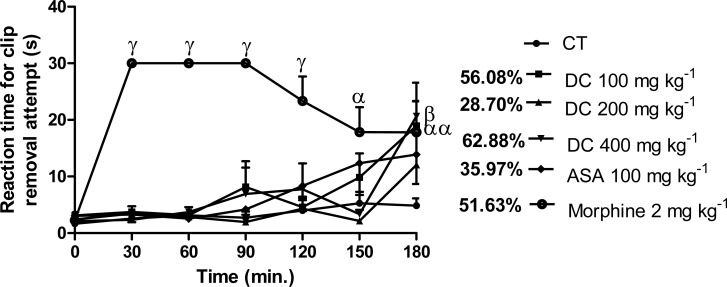
Comparing the reaction time of tail clip removal attempt pre-treatment and post-treatment, the effect of D. cordata was significant (p<0.05, 0.01) only at the post-treatment time of 180 min. at doses of 100 and 400 mg kg−1 with the reaction time increased from 4.84 ± 1.31 s in control animals to 18.95 ± 4.33 s and 20.66 ± 5.91 s, respectively. The effect of ASA (100 mg kg1; 35.97 %) was not significant (p>0.05) at this post-treatment time but the effect of morphine (2 mg kg−1), which increased the reaction time to 17.83 ± 5.52 s; 51.63 %, was significant (p<0.05). The effect of D. cordata at the most effective dose (400 mg kg−1; 62.88 % inhibition) was greater but not significantly different (p>0.05) from that of morphine (51.63 %). At post-treatment times of 30–90 min., morphine produced 100 % inhibition (maximum effect) which reduced to 51.63 % at 180 min (Figure 5).
Figure 5.
Effect of D. cordata in the tail clip test in mice. Data are mean ± SEM (n = 6). αp<0.05, βp<0.01, γp<0.001 vs. pretreatment time, 0 min. (one way ANOVA followed by Tukey's multiple comparison test).
Intraperitoneal injection of 2,4-DNP caused an increase in body temperature of control rats from 37.73 °C to 38.65 °C (Δ 0.92 °C) 0.5 h after administration. D. cordata (100, 200 and 400 mg kg−1) produced dose-dependent and significant (p<0.01, 0.001) inhibition of temperature elevation with peak effect produced at 4 h post-treatment with inhibition values of 90.60 %, 95.70 % and 104.30 %, respectively. At this time interval, the inhibition value obtained for the standard drug (107.20 %), aspirin (100 mg kg−1), was comparable and not significantly different from the value for the extract at the dose of 400 mg kg−1 (Table 1). Significant inhibition of temperature elevation by the extract was observed from 1 h post-treatment, 65.00 % being the highest at the dose of 400 mg kg−1.
Table 1.
Effect of D. cordata on 2,4-DNP-induced hyperthermia in rats.
| Treatment | Dose (mg kg−1) | TO | T0.5h | T1h | T2h | T3h | T4h |
| Distilled water | (10ml kg−1) | 37.73±0.10 | 38.65±0.14 | 38.93±0.14 | 39.12±0.13 | 39.10±0.15 | 39.08±0.15 |
| D. cordata | 100 | 37.80±0.14 | 38.77±0.15 | 38.48±0.15** (43.3%) | 38.23±0.14*** (69%) | 38.03±0.17*** (83%) | 37.93±0.23*** (90.6%) |
| D. cordata | 200 | 37.37±0.16 | 38.17±0.13 | 37.80±0.12*** (58.3%) | 37.68±0.14*** (79.9%) | 37.65±0.14*** (79.6%) | 37.47±0.18*** (95.7%) |
| D. cordata | 400 | 37.28±0.18 | 38.07±0.19 | 37.75±0.16*** (65%) | 37.50±0.14*** (84%) | 37.35±0.14*** (94.9%) | 37.22±0.17*** (104.3%) |
| ASA | 100 | 37.62±0.19 | 38.47±0.28 | 38.17±0.19*** (52.5%) | 37.93±0.19*** (77.7%) | 37.75±0.16*** (90.5%) | 37.52±0.19*** (107.2%) |
Values are mean ± SEM (n = 6).
p<0.01,
p<0.001 vs. control (one way ANOVA followed by Tukey's multiple comparison test) in respect of increase in temperature. Figures in parenthesis indicate inhibition (%) of temperature elevation.
The administration of amphetamine intraperitoneally caused an increase in the body temperature of control rats from the baseline value of 35.77 °C to 38.18 °C (Δ 2.41 °C) 0.5 h after administration. D. cordata (100, 200 and 400 mg kg−1) extract produced significant (p<0.05, 0.001) dose- and time-dependent reduction of temperature elevation. Peak effect (122.00 %) was observed at 3 h post-treatment with the dose of 200 mg kg−1. This effect was greater than that elicited by aspirin (86.90 %). As early as 1 h post-treatment, the extract produced significant inhibition of pyrexia with the highest value being 76.90 % at 200 mg kg−1 (Table 2). On administration of amphetamine, rats manifested increased alertness, aggressiveness and locomotor activity. They also showed stereotyped behaviors which consisted of repeated actions like licking, gnawing and rearing.
Table 2.
Effect of D. cordata on amphetamine-induced hyperthermia in rats.
| Treatment | Dose (mg/kg) | TO | T0.5h | T1h | T2h | T3h | T4h |
| Distilled water | (10ml/kg) | 35.77±0.28 | 38.18±0.35 | 38.15±0.15 | 38±0.33 | 38.68±0.14 | 38.78±0.15 |
| D. cordata | 100 | 36.67±0.34 | 37.75±0.36 | 38.78±0.40 (11.3%) | 38.35±0.36*** (24.7%) | 37±0.31 (88.7%) | 36.25±0.47*** (112.3%) |
| D. cordata | 200 | 36.62±0.35 | 38.07±0.39 | 37.17±0.51* (76.9%) |
36.62±0.50*** (100%) |
36.42±0.52* (122%) |
36.15±0.54*** (115.6%) |
| D. cordata | 400 | 36.50±0.31 | 38.43±0.75 | 37.78±0.31 (44.1%) |
37.45±0.29*** (42.6%) |
36.88±0.40 (86.9%) |
36.40±0.34*** (103.3%) |
| ASA | 100 | 36.35±0.32 | 37.58±0.39 | 38.67±0.53 (2.52%) |
37.30±0.34*** (57.4%) |
36.73±0.39 (86.9%) |
36.47±0.27*** (96%) |
Values are mean ± SEM (n = 6).
p<0.05,
p<0.001 vs. control (one way ANOVA followed by Tukey's multiple comparison test) in respect of increase in temperature. Figures in parenthesis indicate inhibition (%) of temperature elevation.
As shown in Table 3, subcutaneous injection of yeast caused elevation of the body temperature in control rats from 36.87 °C to 38.4 °C (Δ 1.53 °C) 19 h after administration. D. cordata (100, 200 and 400 mg kg−1) produced inhibition of temperature elevation with effect being most pronounced (83.60 %) and significant (p<0.05) at the dose of 400 mg kg−1 23 h posttreatment. This effect was comparable and not significantly different from that produced by aspirin (86.70 %).
Table 3.
Effect of D. cordata on yeast-induced hyperthermia in rats.
| Treatment | Dose (mg kg−1) | TO | T19h | T20h | T21h | T22h | T23h |
| Distilled water |
(10ml kg−1) | 36.87±0.31 | 38.40±0.18 | 38.73±0.13 | 39.03±0.10 | 38.88±0.11 | 39.12±0.15 |
| D. cordata | 100 | 36.65±0.16 | 38.47±0.19 | 38.15±0.37 (19.4%) |
38.07±0.26 (34.3%) |
37.92±0.37 (36.8%) |
37.72±0.49 (52.4%) |
| D. cordata | 200 | 36.42±0.41 | 38.53±0.19 | 38.63±0.27 (24.29%) |
38.48±0.26 (4.6%) |
38.20±0.39 (11.4%) |
37.87±0.30 (35.6%) |
| D. cordata | 400 | 36.97±0.22 | 38.45±0.18 | 38.38±0.21 (24.29%) |
37.98±0.33 (53.2%) |
37.65±0.22 (66.2%) |
37.33±0.14* (83.6%) |
| ASA | 100 | 36.58±0.07 | 37.83±0.37 | 37.45±0.34 (53.2%) |
37.45±0.34 (59.7%) |
36.83±0.16 (87.6%) |
36.88±0.23* (86.7%) |
Values are mean ± SEM (n=6).
p<0.05 vs. control (one way ANOVA followed by Tukey's multiple comparison test) in respect of increase in temperature. Figures in parenthesis indicate inhibition (%) of temperature elevation.
As shown in Table 4, the effect of D. cordata on normal body temperature of rats was only significant (p<0.05) at the dose of 400 mg kg−1 at 1 h post-treatment. At this dose and time interval, basal body temperature of rats was reduced from 38.28 °C to 36.85 °C (Δ 1.43 °C).
Table 4.
Effect of D. cordata on normal body temperature of rats.
| Treatment | Dose (mg kg−1) |
TO | T1h | T2h | T3h | T4h |
| Distilled water |
(10ml kg−1) | 38.23±0.10 | 37.68±0.09 | 37.68±0.16 | 37.53±0.18 | 37.48±0.19 |
| D. cordata | 100 | 38.25±0.10 | 37.35±0.09 | 37.40±0.18 | 37.45±0.06 | 37.40±0.14 |
| D. cordata | 200 | 37.95±0.19 | 36.85±0.13 | 36.75±0.31 | 36.80±0.28 | 36.85±0.31 |
| D. cordata | 400 | 38.28±0.29 | 36.85±0.09* | 36.75±0.18 | 36.73±0.17 | 36.68±0.11 |
| ASA | 100 | 38.10±0.27 | 37±0.18 | 37.25±0.23 | 37.05±0.30 | 36.95±0.24 |
Values are mean ± SEM (n = 6).
p<0.05 vs. control (one way ANOVA followed by Tukey's multiple comparison test) in respect of increase in temperature.
Discussion
In the evaluation of medicinal plants and herbal products for analgesic activity and in the search for newer more efficacious and better tolerated pure analgesic compounds, various pharmacological methods of pain evaluation have been used. Apart from helping to determine presence or absence of analgesic activity, the various models help to elucidate the mechanism of action of active medicinal plants, herbal products, and pure compounds. In this study, the mouse writhing, formalin, and tail clip tests have been used to screen for analgesic activity of D. cordata extract.
The mouse writhing test is useful for the evaluation of mild analgesic non-steroidal anti-inflammatory compounds (Berkenkopf and Weichman, 1988; Nunez Guillen et al., 1997) and peripherally acting drugs like ASA have been reported to exhibit analgesic activity in the writhing test only (Zakaria et al., 2008). Writhings induced by chemical substances like acetic acid involves stimulation of the peripheral receptor system and is partly attributed to the local peritoneal receptors found at the surface of the cells lining the peritoneal cavity (Zakaria et al., 2008). Prolonged irritation of the peritoneal cavity by acetic acid results in increase in the levels of peritoneal fluid prostaglandins, like PGE2 and PGF2α, which enhances inflammatory pain by increasing capillary permeability (Vogel and Vogel, 1997; Zakaria et al., 2008). The analgesic effect demonstrated by D. cordata extract in the mouse writhing test in this study suggest a peripheral mechanism of action involving direct action on nociceptors, direct inhibition of prostaglandin action or indirect inhibition of prostaglandin synthesis by inhibition of cyclo-oxygenase (COX) activity. The effectiveness of D. cordata extract in this test indicates its potential usefulness in the treatment of acute pain as the abdominal constriction test is said to be indicative of acute pain (Cowan, 1990).
However, effectiveness in the mouse writhing test is not specifically indicative of peripheral and/or central activity (Chan et al., 1995) as centrally acting drugs like morphine are also effective in the mouse writhing test as confirmed by the result obtained in this study. This formed the basis for the use of the formalin and tail clip tests. Further justification for the use of these methods is the fact that in addition to non-steroidal anti-inflammatory drugs, anti-histaminics and anti-cholinergics can inhibit the writhing response (Singh and Majumdar, 1995).
The formalin test produces distinct biphasic nociceptive response; an initial acute response (early phase) and a prolonged response (late phase; Malmberg and Yaksh, 1992), 0–5 min. and 15–30 min. respectively, as observed in this study. Injection of formalin causes immediate and intense increase in the spontaneous activity of afferent C fibers and evokes distinct quantifiable behavior indicative of pain (Zakaria et al., 2008); biting and licking of the injected paw as observed in this study. The early phase is due to direct effect of formalin on nociceptors while the late phase is a tonic response involving inflammatory processes and neurons in the dorsal horns of the spinal cord are activated (Tjolsen et al., 1992; Zakaria, 2008). Drugs which act mainly centrally, like narcotic analgesics e.g. morphine, inhibit both phases of the formalin test while peripherally acting drugs like ASA inhibit the late phase only (Santos et al., 1994; Chan et al., 1995). According to Cowan (1990), the formalin test can also be used to indicate the potential of analgesic agents to treat chronic pain due to inflammation. D. cordata extract in this study did not demonstrate effective analgesic activity in the first phase of the formalin test but was active in the second phase. In agreement with literature, morphine was effective in both phases of the formalin test while ASA was only effective in the second phase. Findings in the formalin test suggest that D. cordata extract acts through peripheral mechanism of action, as established in the mouse writhing test, demonstrating possible effectiveness in the treatment of chronic inflammatory pain by inhibition of associated inflammatory processes, basically release and/or action of inflammatory mediators. This assertion is supported by the report of Adeyemi et al. (2008) that D. cordata extract possess anti-inflammatory activity.
The tail clip test was used in this study to investigate the involvement of central mechanisms in the analgesic activity of D. cordata extract, as centrally acting analgesic drugs like morphine elevate the pain threshold of rodents towards heat and pressure (Singh and Majumdar, 1995). In the mouse writhing and formalin tests, D. cordata extract demonstrated analgesic activity at doses of 200 and 100 mg kg−1 body weight respectively at 90 min., considering the time of treatment with test and standard drugs and the observation period after pain induction. However, in the tail clip test D. cordata demonstrated analgesic activity at the highest dose of 400 mg kg−1 body weight at 180 min. post-treatment. This finding suggest that the mechanism of action of the analgesic effect of D. cordata extract shifts from peripheral to central mechanism with increasing dose and posttreatment time.
Over the years, various approaches in terms of duration and time interval of counting of number of writhes in the mouse writhing test have been adopted. This has ranged from 15-30 min. as used by various researchers (Singh and Majumdar, 1995; Nunez Guillen et al., 1997; Akindele and Adeyemi, 2006; Zakaria et al., 2008). In this study, a consideration of the time course of acetic acid-induced mouse writhing shows that the greatest number of writhes was generally produced at the 5–10 min. time interval. We therefore suggest the adoption of the 5–10 min. interval in the counting of writhes in the mouse writhing test as a way of strengthening the rapidity of the model in the screening of agents for analgesic activity.
Fever is one of the most common signs of illness and it is best defined as an increase in temperature over what is normal for a given individual at that particular time of day (Wolff et al., 1975). The normal body temperature is regulated by a centre in the hypothalamus, which ensures a balance between heat loss and heat production. Fever occurs when there is a disturbance of this hypothalamic thermostat that leads to the set point of body temperature being raised (Rang et al., 1999). According to Uzcategui et al. (2004), pyretic activity involves stimulation of the region in the hypothalamus that controls body temperature via prostaglandins synthesized within the central nervous system (CNS) and antipyretic agents must access the brain through the blood-brain barrier (BBB) which prevents drug molecules and chemicals from entering the CNS (Begley et al., 2000). In this study, D. cordata extract demonstrated effective antipyretic activity as evident in the results obtained in all the different experimental models of pyrexia. It inhibited temperature elevation in all the models employed. Its antipyretic effect may be due to the inhibition of prostaglandin synthesis in the hypothalamus because prostaglandins play an important role in the development of pain, inflammation and fever, especially since the extract had been shown to possess anti-inflammatory activity (Adeyemi et al., 2008). The antipyretic activity demonstrated by D. cordata extract in this study provides a further justification for a central mechanism of analgesic action of the extract at the highest dose used as ability to cross the BBB is a possible factor contributing to antipyretic activity (Zakaria, 2008). The plant did not generally affect the normal body temperature since its significance was at 1 h after administration but for d-amphetamine and 2,4-DNP models, its significance was at the 3rd and 4th hour which implies that it is not due to any hypothalamic effect. The behavioral observations made in rats in the amphetamine model such as increased alertness, aggressiveness and locomotor activity, stereotyped behavior (consisting of repeated actions like licking, gnawing and rearing) are due to the fact that amphetamine is a psychomotor (CNS) stimulant (Rang et al., 2003).
The aqueous whole plant extract of D. cordata was found in this study to contain glycosides, saponins, alkaloids, flavonoids, phenols, and tannins in increasing order of abundance. The extract was previously reported to contain these phytochemicals (Adeyemi et al., 2008). One or a combination of this phytoconstituents may be responsible for the analgesic and antipyretic activities observed with D. cordata in this study, especially as synergy is an important concept in the pharmacology of phytochemicals of botanical medicines (Larkins and Wynn, 2004). Saponins (glycosides with the sugar moiety attached to the hydrophilic end) and related phytosterols have been associated with anti-inflammatory, antipyretic, and immune-modulating properties (Larkins and Wynn, 2004). Alkaloids which generally have wide pharmacological actions are analgesics and narcotics (Raaman, 2006) and their ability to cross the blood-brain barrier and interact with various neurotransmitter receptors determine much of their pharmacology (Larkins and Wynn, 2004). Some alkaloids have also been reported to possess anti-inflammatory activity (Larkins and Wynn, 2004). Phenols including flavonoids have been shown to possess various pharmacological and biochemical actions including antipyretic and anti-inflammatory properties (Larkins and Wynn, 2004). Specific flavonoids and related compounds e.g. genistein, kaempferol, quercetin, resorcinol, and resveratrol have been reported to exhibit cyclooxygenase (COX)-2 inhibition activity and dose-dependent decreases in inflammatory-mediating cytokine TNFα (Mutoh et al., 2000). However, according to Maekawa et al. (1999) plant-based COX-2 inhibitors, unlike pharmacologic nonsteroidal anti-inflammatory drugs (NSAIDs), have a slow onset of pain relief. The abundance of tannins in the whole plant extract of D. cordata provides a justifaction for further pharmacological investigations as this class of phytochemicals have been associated with antisecretory, antibacterial, wound healing, antidiarrhea, and antioxidant activities (Larkins and Wynn, 2004).
In a previous study, Adeyemi et al. (2008) reported the safety of oral administration of the aqueous extract of D. cordata based on the fact that no mortality and visible signs of toxicity were recorded given up to 2 g kg−1. The LD50 administered intraperitoneally was estimated to be 133.35 mg kg−1.
Conclusion
The results obtained in this study demonstrate that the aqueous whole plant extract of Drymaria cordata possesses analgesic and antipyretic properties mediated through peripheral and central mechanisms. This finding justifies the use of the extract in traditional medicine for the treatment of painful and febrile conditions. A bioactivity guided fractionation of the extract will reveal the specific chemical agent(s) responsible for the observed effects of D. cordata and the exercise could also possibly throw up lead compound(s) for the development of newer, more efficacious and better tolerated drugs.
References
- 1.Adeyemi OO, Akindele AJ, Ndubuisi N. Anti-inflammatory activity of Drymaria cordata extract. J Nat Rem. 2008;8(1):93–100. [Google Scholar]
- 2.Adeyemi OO, Okpo SO, Okpaka O. The analgesic effect of the methanolic extract of Acanthus montanus. J Ethnopharmacol. 2004;90(1):45–48. doi: 10.1016/j.jep.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 3.Akindele AJ, Adeyemi OO. Analgesic activity of the aqueous leaf extract of Byrsocarpus coccineus. Niger J Health Biomed Sci. 2006;5(1):43–46. [Google Scholar]
- 4.Amanlou M, Dadkhah F, Salehnia A, Farsam H, Dehpour AR. Anti-inflammatory and anti-nociceptive effects of hydrochloric extract of Satureja khuzistanica Jamzad extract. J Pharm Pharmaceut Sci. 2005;8:102–106. [PubMed] [Google Scholar]
- 5.Barua CC, Roy JD, Buragohain B, Barua AG, Borah P, Lahkar M. Anxiolytic effect of hydroethanolic extract of Drymaria cordata L Willd. Indian J Exp Biol. 2009;47(12):969–973. [PubMed] [Google Scholar]
- 6.Begley DJ, Bradbury MWB, Kreuter J. The blood-brain barrier and drug delivery to the CNS. New York: Marcel Dekker; 2000. [Google Scholar]
- 7.Berkan T, Ustunes L, Lermioglu F, Ozer A. Anti-inflammatory, analgesic and antipyretic effects of an aqueous extract of Erythracea centarium. Planta Med. 1991;57:34–37. doi: 10.1055/s-2006-960011. [DOI] [PubMed] [Google Scholar]
- 8.Berkenkopf JW, Weichmann BM. Production of prostacylin in mice following intraperitoneal injection of acetic acid, phenylbenzoquinone and zymosan: its role in the writhing response. Prostaglandins. 1988;36:693–709. doi: 10.1016/0090-6980(88)90014-7. [DOI] [PubMed] [Google Scholar]
- 9.Burkill HM. The useful plants of West Tropical Africa. 2nd Edn. Vol. 1. Royal Botanic Gardens, Kew: 1985. p. 343. [Google Scholar]
- 10.Chan TF, Tsai HY, Tian-Shang W. Anti-inflammatory and analgesic activities from the roots of Angelica pubescens. Planta Med. 1995;61:2–8. doi: 10.1055/s-2006-957987. [DOI] [PubMed] [Google Scholar]
- 11.Cowan A. Modern methods in pharmacology: testing and evaluation of drugs abuse. Vol. 6. New York: Wiley Liss; 1990. pp. 33–42. [Google Scholar]
- 12.Edeoga HO, Okwu DE, Mbaebie BO. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol. 2005;4(7):685–688. [Google Scholar]
- 13.Larkins N, Wynn S. Pharmacognosy: phytomedicines and their mechanisms. Vet Clin Small Anim. 2004;34:291–327. doi: 10.1016/j.cvsm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Maekawa K, Yoshikawa N, Du J, Nishida S, Kitasato H, Okamoto K, Tanaka H, Mizushima Y, Kawai S. The molecular mechanism of inhibition of interleukin-1beta-induced cyclooxygenase-2 expression in human synovial cells by Tripterygium wilfordii Hook F extract. Inflamm Res. 1999;48(11):575–581. doi: 10.1007/s000110050506. [DOI] [PubMed] [Google Scholar]
- 15.Malmberg AB, Yaksh TL. Antinociceptive actions of spinal nonsteroidal anti-inflammatory agents on the formalin test in the rat. J Pharmacol Exp Ther. 1992;263:136–146. not cited in the text?? [PubMed] [Google Scholar]
- 16.Mbagwu HO, Anene RA, Adeyemi OO. Analgesic, antipyretic and anti-inflammatory properties of Mezoneuron benthamianum Baill (Caesalpiniaceae) Nig Q J Hosp Med. 2007;17(1):35–41. doi: 10.4314/nqjhm.v17i1.12540. [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee PK, Saha K, Bhattacharya S, Giri SN, Pal M, Saha BP. Studies on antitussive activity of Drymaria cordata Willd. (Caryophyllaceae) J Ethnopharmacol. 1997;56(1):77–80. doi: 10.1016/s0378-8741(97)01512-2. [DOI] [PubMed] [Google Scholar]
- 18.Mukherjee K, Saha BP, Mukherjee PK. Evaluation of antipyretic potential of Leucas lavandulaefolia (Labiatae) aerial part extract. Phytother Res. 2002;16:686–688. doi: 10.1002/ptr.1013. [DOI] [PubMed] [Google Scholar]
- 19.Mutoh M, Takahashi M, Fukuda K, Matsushima-Hibiya Y, Mutoh H, Sugimura T, Wakabayashi K. Suppression of cyclooxygenase-2 promoter-dependent transcriptional activity in colon cancer cells by chemopreventive agents with a resorcin-type structure. Carcinogenesis. 2000;21(5):959–963. doi: 10.1093/carcin/21.5.959. [DOI] [PubMed] [Google Scholar]
- 20.Nunez Guillen ME, Emim JA, Souccar C, Lapa AJ. Analgesic and anti-inflammatory activities of the aqueous extract of Plantago major L. Int J Pharmacog. 1997;35(2):99–104. [Google Scholar]
- 21.Raaman N. Phytochemicals techniques. Pitam Pura, New Delhi: New India Publishing Agency; 2006. Categories of phytochemicals; pp. 197–274. [Google Scholar]
- 22.Rang HP, Dale MM, Ritter JM. Pharmacology. 4 Edn. Churchill Livingstone, Edinburgh: 1999. Anti-inflammatory and immuno-suppressant drugs; pp. 229–247. [Google Scholar]
- 23.Rang HP, Dale MM, Ritter JM, Moore PK. Pharmacology. 5 Edn. Edinburgh: Churchill Livingstone; 2003. CNS Stimulants and Psychotomimmetic Drugs; pp. 585–593. [Google Scholar]
- 24.Santos ARS, Filho VC, Niero R, Viana AM, Morenof N, Campos MM, Yunes RA, Calixto JB. Analgesic effects of callus culture extracts from selected species of Phyllantus in mice. J Pharm Pharmacol. 1994;46:755–759. doi: 10.1111/j.2042-7158.1994.tb03897.x. not cited in the text?? [DOI] [PubMed] [Google Scholar]
- 25.Shibata M, Ohkubo T, Takahashi H, Inoki R. Modified formalin test; characteristic biphasic pain response. Pain. 1989;38:347–352. doi: 10.1016/0304-3959(89)90222-4. [DOI] [PubMed] [Google Scholar]
- 26.Singh S, Majumdar DK. Analgesic activity of Ocimum sanctum and its possible mechanism of action. Int J Pharmacogn. 1995;33:188–192. [Google Scholar]
- 27.Sofowara A. Medicinal plants and Traditional medicine in Africa. Ibadan, Nigeria: Spectrum Books Ltd.; 1993. p. 289. [Google Scholar]
- 28.Sowemimo A, van de Venter M, Baatjies L, Koekemoer T. Cytotoxic activity of selected Nigerian plants. Afr J Tradit Complement Altern Med. 2009;6(4):526–528. doi: 10.4314/ajtcam.v6i4.57186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.United States National Institutes for Health publication; 1985. No. 85-23. [Google Scholar]
- 30.Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. not cited in the text?? [DOI] [PubMed] [Google Scholar]
- 31.Uzcategui B, Avila D, Suarez-Roca H, Quintero L, Ortega J, Gonzalez B. Anti-inflammatory, antinocicepive, and antipyretic effects of Lantana trifolia Linnaeus in experimental animals. Invest Clin. 2004;45:317–322. [PubMed] [Google Scholar]
- 32.Vogel HG, Vogel WH. Drug discovery and evaluation: pharmacological assays. Lewisville: J. A. Majors Company; 1997. pp. 360–418. [Google Scholar]
- 33.Wolff SM, Fauci AS, Dale DC. Unusual etiologies of fever and evaluation. Annu Rev Med. 1975;26:277–281. doi: 10.1146/annurev.me.26.020175.001425. [DOI] [PubMed] [Google Scholar]
- 34.Zakaria ZA, Abdul Ghani ZDF, Raden Mohd Nor RNS, Gopalan HK, Sulaiman Mohd R, Mat Jais AM, Somchit MN, Kader AA, Ripin J. Antinociceptive, anti-inflammatory, and antipyretic properties of an aqueous extract of Dicranopteris linearis leaves in experimental animal models. J Nat Med. 2008;62(2):179–187. doi: 10.1007/s11418-0070224-x. [DOI] [PubMed] [Google Scholar]