Abstract
Purpose
To study a commonly used Astragalus-based herbal formula previously found effective in non-small cell lung cancer (NSCLC) on the pharmacokinetics of docetaxel in patients with NSCLC.
Methods
Patients with advanced NSCLC who progressed after prior platinum-containing chemotherapy were accrued and received docetaxel at 35 mg/m2 for 3 weeks followed by 1 week of rest. At 4 days prior to the second dosing, Jinfukang was given orally. Pharmacokinetic studies of initial-dose docetaxel (in the absence of Jinfukang) and the third dose (in the presence of Jinfukang) were compared.
Results
Of the 24 patients enrolled, 21 started Jinfukang and docetaxel. Jinfukang had no significant impact on the pharmacokinetics of docetaxel. Median time to progression or withdrawal from treatment was 7 weeks. Twelve patients were removed from study for progression of disease; nine patients withdrew.
Conclusions
Jinfukang did not alter the pharmacokinetics of docetaxel nor appear to affect survival in this study.
Keywords: Lung cancer, Chemotherapy, Docetaxel, Botanicals, Astragalus
Introduction
Lung cancer continues to be the leading cause of cancer deaths in the United States. An estimated 215,020 new cases will be diagnosed in the year 2008, and 161,840 deaths, 29% of all cancer deaths, are anticipated in that year. Non-small cell lung cancer (NSCLC) accounts for approximately 80% of lung cancer diagnoses. More than three-quarters of patients present at diagnosis with locally advanced or disseminated disease that is not amenable to surgery, and many resected patients relapse and present with disseminated disease (American Cancer Society. Cancer facts and Wgures, 2008, http://www.cancer.org).
The primary treatment for patients with locally advanced or metastatic NSCLC includes systemic chemotherapy, sometimes in combination with radiation therapy and targeted biological therapy such as bevacizumab (Avastin®) or erlotinib (Tarceva®). No single regimen has been demonstrated as superior Wrst-line therapy for advanced NSCLC. A cisplatin-based or carboplatin-based combination regimen that includes one of the new agents (i.e., paclitaxel, docetaxel, gemcitabine, vinorelbine or irinotecan) remains the standard of care for Wrst-line therapy in patients with stage IV NSCLC. Patients who fail Wrst-line therapy most commonly receive docetaxel, pemetrexed or erlotinib for second-line treatment. Despite advances, the median survival for patients who progress after Wrst-line therapy is approximately 3 months [1], and toxicity remains a common problem.
Astragalus-based herbal formulas, including Jinfukang, were previously reported to reduce side effects and improve the efficacy of chemotherapy in NSCLC patients [2, 3]. Such agents also were shown to improve response rates when combined with chemotherapy [4]. Jinfukang is an oral liquid formulation developed in China, where it is approved by the State Drug Administration (SDA), the Chinese equivalent of the US FDA, for use in the treatment of NSCLC.
Jinfukang contains extracts from 12 botanicals, as indicated in Table 1 and as detailed further below. Prior positive Wndings engaged our interest in this agent. Because botanical preparations are known to produce unpredictable toxicity or detrimental drug–herb interactions when combined with chemotherapy [5, 6], we initiated the safety and pharmacokinetic study of docetaxel with Jinfukang in patients with advanced NSCLC reported here.
Table 1.
Composition of Jinfukang
| Latin name | Chinese name | Plant part | Weighta (g) |
|---|---|---|---|
| Astragalus membranaceus | Huang Qi | Root | 146 |
| Glehnia littoralis | Bei Sha Shen | Root | 146 |
| Asparagus cochinchinensis | Tian Men Dong | Root | 49 |
| Ligustrum lucidum | Nu Zhen Zi | Fruit | 49 |
| Selaginella doederleinii | Shi Shang Bai | Leaf | 146 |
| Paris polyphylla | Chonglou | Root | 73 |
| Epimedium sagittatum | Yin Yang Huo | Leaf | 49 |
| Gynostemma pentaphyllum | Jiao Gu Lan | Leaf | 49 |
| Cornus officinalis | Shan Zhu Yu | Fruit | 49 |
| Salvia chinensis | Shi Jian Chuan | Leaf | 146 |
| Ophiopogon japonicus | Maidong | Root | 49 |
| Trigonella foenum graecum | Huluba | Seed | 49 |
Weights are those needed to produce one liter of finished product
Methods
Study design
This single arm, before/after study compared the pharmacokinetics of docetaxel with and without Jinfukang pretreatment.
Study agents and dosages
Docetaxel, an antimicrotubule taxane class agent, was supplied by Sanofi-Aventis US LLC as a standard formulary drug. Docetaxel was administered intravenously at 35 mg/m2 once weekly for 3 weeks followed by a week of rest (Days 1, 8, and 15 every 28 days). Treatment cycles were 4 weeks each.
Jinfukang was supplied by Jianxi Yicun Pharmaceutical Company (China) in 10-ml vials to be patient self-administered orally. Quality standards for Jinfukang are determined via thin layer chromatography (TLC) by the presence of four major constituent botanicals: Huang Qi (Astragalus membranaceus); Shan Zhu Yu (Cornus officinalis); Nu Zhen Zi (Ligustrum lucidum) and Yin Yang Huo (Epimedium sagittatum). Batch consistency is based on the standardized marker compound icariin, a major constituent of Yin Yang Huo (E. sagittatum), by HPLC. An independent lab, Adpen Laboratories of Jacksonville, Florida, was retained to conduct quality tests on the product used in this study before it was administered to patients. An Investigational New Drug application was Wled with the Food and Drug Administration.
The dose of Jinfukang was 75 ml/m2 per day rounded to the nearest 10 ml, taken in three doses at least 1 h after food. A typical patient takes four 10-ml vials three times each day. The study agent was given on a 4-week cycle, with the Wrst 3 days a rest period between cycles (given in days 4–28 during chemotherapy cycles).
Subjects
Per protocol, subjects included patients with advanced non-small cell lung cancer who had progressed following platinum-containing chemotherapy and who were to receive second-line treatment with docetaxel. We chose to include 20 patients in this trial as a Wrst experience in using the agent. Accrual was stopped when data for the primary endpoint was available for 20 patients, although patients still on the trial were allowed to continue. Inclusion criteria included pathologic conformation of stage III or IV NSCLC, docetaxel clinically indicated, and KPS ≥ 60%. Exclusion criteria included inadequate bone marrow, liver or kidney function; concurrent use of any botanical supplement; history of allergy to any constituent botanicals in Jinfukang; pregnant or lactating women; concurrent active cancer; concurrent use of immunomodulators.
Consent was obtained prior to subject enrollment according to a protocol approved by the Memorial Sloan-Kettering Cancer Center (MSKCC) Institutional Review Board. Patients were recruited from the MSKCC Thoracic Oncology Service from 2/2005 to 8/2006 and followed thereafter, with the final follow-up in August, 2008. Patients remained on study until progression of disease, intolerable toxicity or death.
Docetaxel pharmacokinetic studies
Blood samples were collected for the Wrst (without Jinfukang) and third (with Jinfukang) dose of docetaxel at 0, 15, 45 min, and 1, 3, 5, 6 and 24 h after starting docetaxel infusion as previously described [7]. Blood samples were collected only for the Wrst cycle. Samples were centrifuged at 4°C with plasma stored at −20 or at −80°C if held for more than 1 week. Plasma docetaxel concentrations were measured by high performance liquid chromatography following published methods [8].
Statistical methods
Differences in pharmacokinetic measures between docetaxel alone and docetaxel plus Jinfukang were tested with the Wilcoxon signed-rank test, a non-parametric test for paired data. Time-to-event analyses were conducted with two endpoints: time to progression or withdrawal from treatment (i.e. time on trial), or time to progression where patients who withdrew were censored at the time of withdrawal. All analyses were conducted using Stata 9.2 (Stata Corp., College Station, TX).
The primary outcome measurement was area-under-the-curve for docetaxel concentration in the pharmacokinetic studies. PK data were analyzed using the WinNonlin program (ver. 5.1, Pharsight), a 2-compartmental model method of analysis. Parameters analyzed included the maximal plasma concentration (Cmax) and time (Tmax), area under the plasma concentration curve (AUC 0–24), area under the plasma concentration curve to infinity from time zero (AUC0-inf), half life (T½) and. systemic clearance (CL_F). The AUC was calculated using the 2-compartmental analysis with linear interpolation to infinity. Toxicity was evaluated every 2 weeks or more frequently if needed per National Cancer Institute Common Terminology Criteria for Adverse Events, Version 3.0 (NCI-CTC 3.0). Tumor response was evaluated according to response evaluation criteria in solid tumors (RECIST) revised version, June 1999.
Results
Twenty-four patients were enrolled initially, 21 started Jinfukang and docetaxel. They represent the study sample reported here as indicated in the flow chart (Figure 1).
Fig. 1.
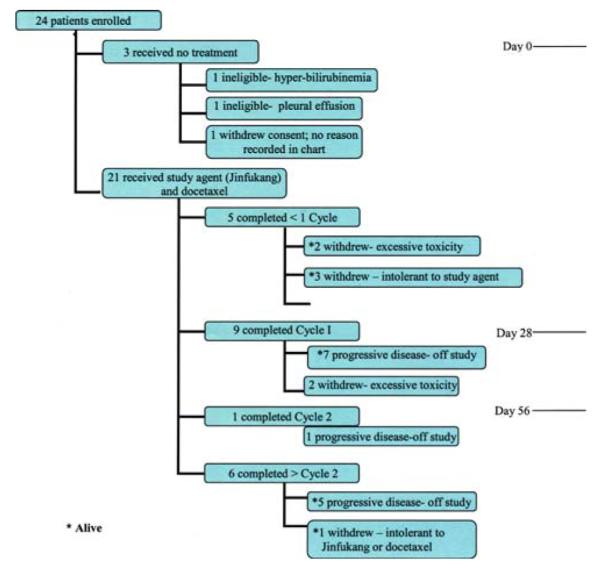
Patient flow diagram
Baseline characteristics of the sample are given in Table 2. All patients had failed platinum-based chemotherapy for NSCLC. At study entry, all but one patient had performance status (KPS) of 70 or higher (Table 2).
Table 2.
Characteristics of study participants at enrollment
| Gender | 11 male, 10 female |
| Age | Range, 39–83; mean, 65 years; median, 67 years |
| Ethnicity | 15 (71%) White non-Hispanic |
| 3 (14%) White Hispanic | |
| 3 (14%) Asian | |
| Stage | 19 (91%) Stage IV; 2 (9%) Stage IIIB |
| Karnofsky performance status (KPS) |
Number of patients |
| 60 | 1 |
| 70 | 4 |
| 80 | 13 |
| 90 | 1 |
| Not recorded | 2 |
Pharmacokinetics
Pharmacokinetic measures of docetaxel with and without Jinfukang pretreatment are given in Table 3. Wide variation across patients is evident in the large standard deviations, but there were no obvious differences in docetaxel pharmacokinetics between the Wrst dose (no Jinfukang) and third dose (with Jinfukang).
Table 3.
Measures of docetaxel pharmacokinetics
| Variable | First dose mean (SD) |
After Jinfukang pretreatment mean (SD) |
|---|---|---|
| Concentration at 15 min | 4,463 (5,281) | 3,616 (1813) |
| Concentration at 45 min | 253 (193) | 348 (490) |
| Area-under-the-curve at infinity | 2,263 (2,059) | 2,159 (868) |
| Area-under-the-curve at final time point |
2,180 (2,057) | 2,000 (825) |
| Clearance rate (L/h) | 36.5 (14) | 32.7 (12) |
| Volumes of distribution (Vd) by the steady-state method |
122 (71) | 161 (103) |
Table 4 shows mean change, with 95% CI, associated with Jinfukang. In no case was the difference between Wrst and third dose clinically or statistically significant. To illustrate changes on an individual basis, Table 5 displays the number of patients for whom a pharmacokinetic measure either increased or decreased by at least 33%. Although most patients experienced increased levels of docetaxel, no clear trend was evident.
Table 4.
Change in measures of docetaxel pharmacokinetics
| Variable | Difference Pre- to Post-JFK |
P value |
|---|---|---|
| Concentration at 15 min | 847 (−2,165, 3,859) | 0.7 |
| Concentration at 45 min | −95 (−369, 179) | 0.9 |
| Area-under-the-curve at infinity | 104 (−1,127, 1,335) | 0.3 |
| Area-under-the-curve at final time point |
180 (−1,037, 1,397) | 0.4 |
| Clearance rate (L/h) | 3.8 (−5.3, 13.0) | 0.3 |
| Volumes of distribution (Vd) by the steady-state method |
−39 (−102, 24) | 0.15 |
Data are given as difference between means and 95% CI
Table 5.
Individual patient responses to Jinfukang administration (N = 21)
| Variable | Decrease (<67% of value for initial dose) |
No change (67–133% of value for initial dose) |
Increase (>133% of value for initial dose) |
|---|---|---|---|
| Concentration at 15 min | 3 (14) | 9 (43) | 9 (43) |
| Concentration at 45 min | 4 (19) | 6 (29) | 11 (52) |
| Area-under-the-curve at infinity |
2 (10) | 7 (33) | 12 (57) |
| Area-under-the-curve at final time point |
3 (14) | 7 (33) | 11 (52) |
| Clearance rate (L/h) | 5 (24) | 8 (38) | 8 (38) |
| Volumes of distribution (Vd) by the steady- state method |
3 (14) | 4 (19) | 14 (67) |
Toxicity and tolerability
Six patients, all Stage IV, four females and two males, experienced no Grade 3 or higher toxicity. These patients received Jinfukang for a range of 4–109 days (mean 46 days). With the exception of one patient recorded to have had a single Grade 4 hyperglycemia, remaining toxicities deemed possibly or likely related to the study agent were Grade 3 or lower: eight patients had Grade 3 lymphopenia; three had Grade 3 hyperglycemia; and one patient each experienced a range of additional Grade 3 toxicities, myelosuppression not among them.
Nine patients withdrew because they could not tolerate the taste of Jinfukang or because they found treatment-related nausea and vomiting, fatigue or general “toxicity,” regardless of Grade level recorded, intolerable. Considering the possibility that cultural background influenced tolerance or response to the study agent, data on the three Asian study subjects were reviewed. Two went off study for progression of disease after 26 and 60 days, respectively, and one experienced excessive toxicity and withdrew from study after 19 days.
Disease progression and survival
Disease progression occurred in 13 patients while on Jinfukang. Figure 2 shows time on treatment. Median time until progression or withdrawal was 7.0 weeks (95% CI 4.1, 9.0). Figure 3 shows time to progression where patients who withdrew were censored at time of withdrawal. In this analysis, median time to progression was 8.3 weeks (95% CI 6.7, 11.4). In both analyses, the longest time to progression was 24 weeks. There were no partial responses.
Fig. 2.
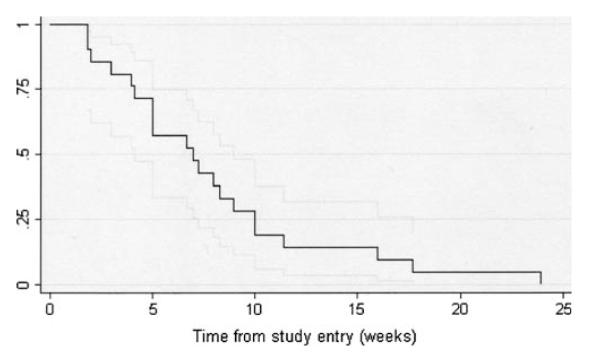
Time on study (progression or withdrawal from treatment), with 95% CI N = 21
Fig. 3.
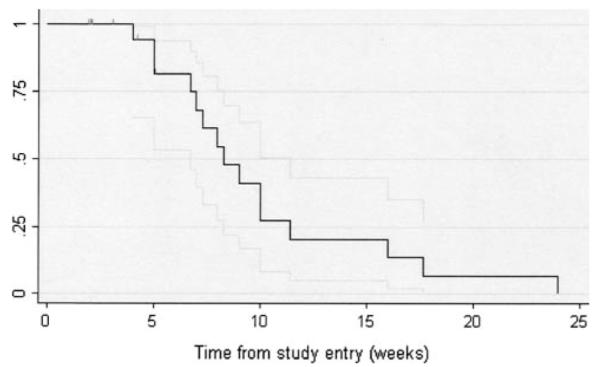
Time to progression, with 95% CI Patients who withdrew were censored at time of withdrawal (vertical marks)
Four patients, two women and two men, remain alive at final follow-up in August, 2008, 2 years or more post-diagnosis. All were Stage IV at diagnosis. The living patients remained on Jinfukang for 4, 18, 48 and 122 days, respectively. Of three patients who used Jinfukang for 90 days or more, one remains alive.
Discussion
Many anti-cancer agents are derived from botanical sources. Careful investigative pursuit of botanical agents previously found helpful is worthwhile. Because botanical agents may alter the metabolism of chemotherapeutic agents and increase their toxicity or reduce their effectiveness, such potential herb–drug interactions Wrst must be ruled out. This Phase II trial tested a popular botanical compound previously reported to reduce side effects and improve the efficacy of chemotherapy.
We did not find evidence that the concurrent use of Jinfukang altered the pharmacokinetics of docetaxel. Although wide variation occurred in pharmacokinetics across individual patients, there was no significant change in docetaxel pharmacokinetics with the addition of Jinfukang.
We also explored the toxicity and efficacy of docetaxel in combination with Jinfukang, and found, contrary to expectation, little reduction in serious side effects with the study agent. Four of the 21 study participants (19%) remain alive more than 2 years after diagnosis of Stage IV NSCLC. However, only one remained on Jinfukang for the 3 months or more believed to produce optimal results, and the other three living patients maintained use of the study agent for 4, 18 and 48 days each. Thus, no clear relationship between use of the study agent and survival is evident.
Contrary to expectations, this study failed to support the belief that Jinfukang improves survival benefit and reduces chemotherapeutic toxicity in the treatment of non-small cell lung cancer. Our conclusions about the effects of Jinfukang on survival are not related to a direct analysis of overall survival times. However, we found that disease in most patients on Jinfukang progressed in a short period of time. We think it unlikely that an agent could benefit lung cancer patients if their tumors continue to grow rapidly while receiving that agent.
Any future research with this agent should aim for longer-term administration, as most patients remained on Jinfukang for relatively short periods of time. Longer-term administration would be facilitated with a more tolerable form of the agent. Encapsulation of dehydrated Jinfukang would eliminate the need for patients to consume large quantities of a liquid that many found unpleasant, to the point that its taste and odor generally were perceived to be intolerable. Following Phase I testing to ensure a tolerable dose, further studies would be more successful if Jinfukang were used in conjunction with a chemotherapeutic agent associated with less gastrointestinal toxicity than docetaxel. It appears that combining two agents, each with GI toxicity, exacerbated diarrhea and related symptoms, and further compromised patients’ ability to remain on study.
The common public belief that long-term use per se conveys effectiveness is incorrect. This study demonstrates that historic acceptance of a therapeutic modality is not sufficient to warrant its use. Time-honored practice does not provide evidence of safety or efficacy; proper research is the only route to that goal.
Acknowledgments
This study was supported by the Memorial Sloan-Kettering Cancer Center under the Integrative Medicine/Translational Research Grant, and the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements (ODS) under grant 1-P50-AT002779-01. We thank Drs. Azoli, Fiori, Krug and other oncologists of the MSKCC Thoracic Oncology Service who accrued patients to this study, James Lozada, and other research assistants for their important efforts, and our patients for their willingness to participate in this clinical trial.
Footnotes
Conflict of interest statement Dr. Mark G. Kris received lecture fees from SanoW Aventis, the maker of Docetaxel. The remaining authors have no conflict of interest regarding this study.
Contributor Information
Barrie R. Cassileth, Integrative Medicine Service, Memorial Sloan-Kettering Cancer Center, 1429 First Avenue at 74th Street, New York, NY 10021, USA
Naiyer Rizvi, Thoracic Oncology Service, Memorial Sloan-Kettering Cancer Center, New York, USA.
Gary Deng, Integrative Medicine Service, Memorial Sloan-Kettering Cancer Center, 1429 First Avenue at 74th Street, New York, NY 10021, USA.
K. Simon Yeung, Integrative Medicine Service, Memorial Sloan-Kettering Cancer Center, 1429 First Avenue at 74th Street, New York, NY 10021, USA.
Andrew Vickers, Integrative Medicine Service, Memorial Sloan-Kettering Cancer Center, 1429 First Avenue at 74th Street, New York, NY 10021, USA; Biostatistics, Memorial Sloan-Kettering Cancer Center, New York, USA.
Stacey Guillen, Integrative Medicine Service, Memorial Sloan-Kettering Cancer Center, 1429 First Avenue at 74th Street, New York, NY 10021, USA.
Derek Woo, Integrative Medicine Service, Memorial Sloan-Kettering Cancer Center, 1429 First Avenue at 74th Street, New York, NY 10021, USA.
Marci Coleton, Integrative Medicine Service, Memorial Sloan-Kettering Cancer Center, 1429 First Avenue at 74th Street, New York, NY 10021, USA.
Mark G. Kris, Thoracic Oncology Service, Memorial Sloan-Kettering Cancer Center, New York, USA
References
- 1.Socinski MA, Morris DE, Masters GA, Lilenbaum R. Chemotherapeutic management of stage IV non-small cell lung cancer. Chest. 2003;123:226S–243S. doi: 10.1378/chest.123.1_suppl.226s. [DOI] [PubMed] [Google Scholar]
- 2.Liu J, Shi Z, Li H, Xu Z, Zhu Y, Zhao L, et al. Clinical observation on 271 cases of non-small lung cancer treated with Yifei Kangliu Yin (Jin Fu Kang) Chin J Integr Tradit West Med. 2001;7:247–250. [Google Scholar]
- 3.McCulloch M, See C, Shu XJ, Broffman M, Kramer A, Fan WY, Gao J, Lieb W, Shieh K, Colford JM., Jr Astragalus-based Chinese herbs and platinum-based chemotherapy for advanced non-small cell lung cancer: meta-analysis of randomized trials. J Clin Oncol. 2006;24:419–430. doi: 10.1200/JCO.2005.03.6392. [DOI] [PubMed] [Google Scholar]
- 4.Liu JPM, Li Y, Ye D, Guo Y, Li Y. Clinical study of oral liquid Jin Fu Kang for the treatment of primary non-small cell lung cancer. Tumor (Shanghai) 2001;21:463–465. [Google Scholar]
- 5.Madabushi R, Frank B, Drewelow B, Derendorf H, Butterweck V. Hyperforin in St. John’s wort drug interactions. Eur J Clin Pharmacol. 2006;62:225–233. doi: 10.1007/s00228-006-0096-0. [DOI] [PubMed] [Google Scholar]
- 6.Meijerman I, Beijnen JH, Schellens JH. Herb–drug interactions in oncology: focus on mechanisms of induction. Oncologist. 2006;11:742–752. doi: 10.1634/theoncologist.11-7-742. [DOI] [PubMed] [Google Scholar]
- 7.Bhargava P, Marshall JL, Fried K, Williams M, Lefebvre P, Dahut W, Hanfelt J, Gehan E, Figuera M, Hawkins MJ, Rizvi NA. Phase I and pharmacokinetic study of two sequences of gemcitabine and docetaxel administered weekly to patients with advanced cancer. Cancer Chemother Pharmacol. 2001;48:95–103. doi: 10.1007/s002800100317. [DOI] [PubMed] [Google Scholar]
- 8.Vergniol JC, Bruno R, Montay G, Frydman A. Determination of Taxotere in human plasma by a semi-automated high-performance liquid chromatographic method. J Chromatogr. 1992;582:273–278. doi: 10.1016/0378-4347(92)80333-l. [DOI] [PubMed] [Google Scholar]


