Abstract
Elephant endotheliotropic herpesviruses (EEHVs) can cause acute hemorrhagic disease with high mortality rates in Asian elephants (Elephas maximus). Recently, a new EEHV type known as EEHV5 has been described, but its prevalence and clinical significance remain unknown. In this report, an outbreak of EEHV5 infection in a herd of captive Asian elephants in a zoo was characterized. In February 2011, a 42-yr-old wild-born female Asian elephant presented with bilaterally swollen temporal glands, oral mucosal hyperemia, vesicles on the tongue, and generalized lethargy. The elephant had a leukopenia and thrombocytopenia. She was treated with flunixin meglumine, famciclovir, and fluids. Clinical signs of illness resolved gradually over 2 wk, and the white blood cell count and platelets rebounded to higher-than-normal values. EEHV5 viremia was detectable starting 1 wk before presentation and peaked at the onset of clinical illness. EEHV5 shedding in trunk secretions peaked after viremia resolved and continued for more than 2 mo. EEHV5 trunk shedding from a female herd mate without any detectable viremia was detected prior to onset of clinical disease in the 42-yr-old elephant, indicating reactivation rather than primary infection in this elephant. Subsequent EEHV5 viremia and trunk shedding was documented in the other five elephants in the herd, who remained asymptomatic, except for 1 day of temporal gland swelling in an otherwise-healthy 1-yr-old calf. Unexpectedly, the two elephants most recently introduced into the herd 40 mo previously shed a distinctive EEHV5 strain from that seen in the other five elephants. This is the first report to document the kinetics of EEHV5 infection in captive Asian elephants and to provide evidence that this virus can cause illness in some animals.
Keywords: Asian elephant, Elephas maximus, elephant endotheliotropic herpesviruses, Proboscivirus, real-time PCR, viremia
INTRODUCTION
Elephant endotheliotropic herpesviruses (EEHVs) can cause lethal hemorrhagic disease in Asian elephants (Elephas maximus), especially in juveniles.6 Several types of EEHVs have been described, with EEHV1A and EEHV1B types being associated with the vast majority of lethal cases.2–4,7 Recently, a new EEHV type was discovered during a routine blood screen from a 69-yr-old asymptomatic female Asian elephant that displayed 30% nucleotide divergence from EEHV1 and 22% from EEHV2 over a 463–base pair (bp) segment of the DNA polymerase gene locus.4 A similar level of divergence has been found subsequently at many other characteristic genomic loci as well, thus confirming its designation as a distinctive species within the Probosci-virus genus (Richman et al. unpubl. data). Designated EEHV5, the prevalence of this virus within captive or range country elephants is unknown, and it is unclear whether the virus can cause hemorrhagic disease or is associated with any other clinical syndrome. In February 2011, nonquantitative polymerase chain reaction (PCR) indicated that a 42-yr-old wild-born female Asian elephant that presented with bilaterally swollen temporal glands, oral mucosal hyperemia, vesicles on the tongue, and generalized lethargy had detectable EEHV5 DNA in blood samples but was negative for all other known EEHVs. In this report, a quantitative real-time PCR test for EEHV5 was used to track EEHV5 viremia and shedding in this zoo elephant and her herd mates from January through May of 2011.
CASE REPORT
On 7 February 2011 a 42-yr-old wild-born female Asian elephant (E. maximus) presented with bilaterally swollen temporal glands, oral mucosal hyperemia, vesicles on the tongue (Fig. 1), and generalized lethargy (elephant 1) (Table 1). The elephant had a leukopenia and thrombocytopenia (Table 2). She was treated with flunixin meglumine (Banamine®, Schering-Plough Animal Health Corp., Union, New Jersey 07083, USA; 3 g i.m., single dose), Ceftiofur (Excede®, Pharmacia & Upjohn Company, Division of Pfizer, Inc., New York, New York 10017, USA; 18 g s.q., single dose), famciclovir (Novopharm Limited, Toronto, M1b2K9, Canada; 60 g p.o. initial single dose, then rectally t.i.d. for 12 days), and fluids. Fluids consisted of rectal administration of warm tap water to capacity (i.e., until it flowed back out of the elephant) q.i.d. for 7 days (7–14 February) and then b.i.d. for an additional 6 days (14–13 February). Aside from generalized lethargy, none of the symptoms observed in elephant 1 are hallmarks of EEHV-associated fatal illness.11 Signs resolved gradually over the course of 2 wk (until 21 February 2011), and the white blood cell count and platelets rebounded to levels above normal (Table 2). Upon the first signs of illness, blood samples were sent to Baylor College of Medicine and the National Elephant Herpesvirus Laboratory (Washington, D.C.) for EEHV testing.4,10 Real-time and conventional PCR tests were negative for EEHVs 1–4 and 6 (data not shown). However, conventional PCR testing and DNA sequence analysis indicated the presence of EEHV5 DNA.4
Figure 1.
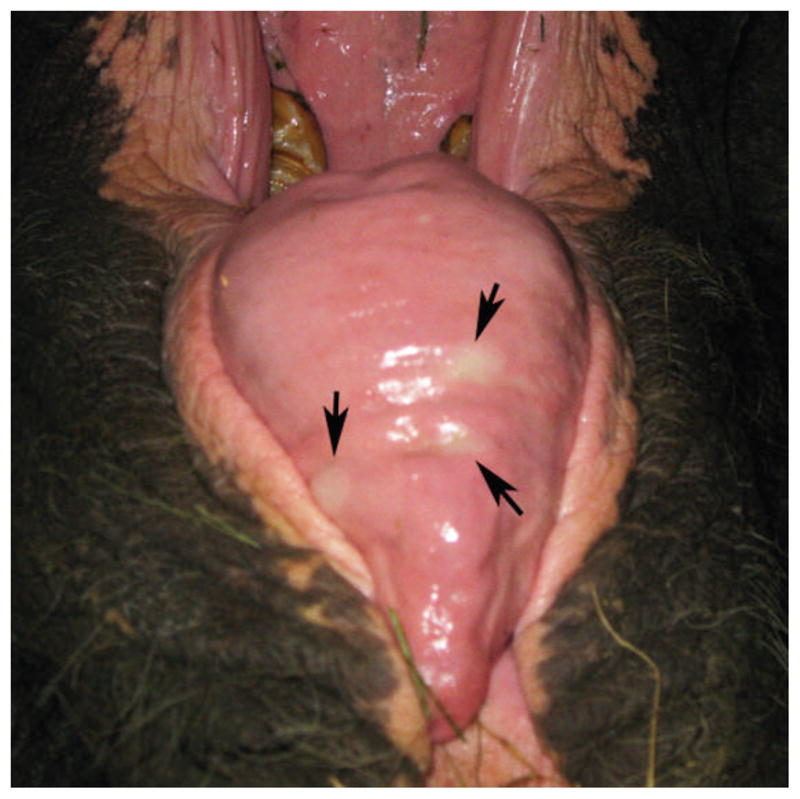
Oral hyperemia and tongue vesicles during peak period of clinical illness with apparent association with elephant endotheliotropic herpesvirus 5 (EEHV5) infection. Arrows indicate tongue vesicles in elephant 1 on 7 February 2011.
Table 1.
Herd demographics and summary of elephant endotheliotropic herpesvirus 5 (EEHV5) detection data.
| Elephant | Age (yr) | Sex | Weighta (kg) | Presenting clinical signs (date) | Dates of detectable viremiab | Peak whole blood viral loadc (date) | Peak trunk wash leveld (date) | Antiviral chemotherapy (route) | EEHV5 straine | WBg Samples tested | TWh no. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 42 | Female | 3,664 | Temporal gland swelling (2/7/2011) | 1/31/2011–3/21/2011 | 18,561 (2/10/2011) | 2,081,452 (3/14/2011) | Famciclovir (PO/PR)f2/9/11-2/21/11 | A | 36 | 33 |
| 2 | 28 | Female | 3,071 | None | None | NA | 46,000 (1/23/2011) | None | B | 27 | 23 |
| 3 | 21 | Female | 3,505 | None | 1/31/2011–3/31/2011 | 2,680 (3/7/2011) | 27,312 (3/14/2011) | None | A | 24 | 21 |
| 4 | 0.75 | Male | 518 | Temporal gland swelling (2/14/2011) | 2/14/2011–2/28/2011 | 18,518 (2/16/2011) | 33,934 (2/21/2011) | None | A | 6 | 21 |
| 5 | 0.3 | Female | 273 | None | 2/14/2011–2/28/2011 | 1,232 (2/28/2011) | 15,068 (3/14/2011) | None | A | 6 | 22 |
| 6 | 47 | Male | 5,709 | None | 3/28/2011–4/25/2011 | 1,320 (4/4/2011) | 39,176 (4/25/2011) | None | A | 33 | 21 |
| 7 | 6 | Male | 1,880 | None | 4/1/2011–5/12/2011 | 34,538 (4/21/2011) | 649,735 (5/23/2011) | None | B | 23 | 22 |
Weight in February 2011 during the beginning of EEHV5 outbreak.
Detectable EEHV5 DNA in whole blood DNA by real-time quantitative polymerase chain reaction (qPCR).
Viral genome equivalents per milliliter of whole blood as determined by EEHV1 qPCR assay.
Viral genomes detected per test reaction from trunk wash DNA by real-time qPCR.
Two different EEHV5 strains were found in the herd and are provisionally designated as A and B (Fig. 2).
PO, per os; PR, per rectum. The first dose was given PO and the rest PR.
Number of whole blood samples (WB) tested for EEHV5 during the study period.
Number of trunk wash (TW) samples tested for EEHV5 during the study period.
Table 2.
Complete blood counts (CBCs) for elephant 1 before, during, and after elephant endotheliotropic herpesvirus 5 (EEHV5)–associated illness.
| Elephant 1 meana | 31 Jan 2011 | 7 Feb 2011 | 8 Feb 2011 | 10 Feb 2011 | 16 Feb 2011 | 28 Feb 2011 | 4 Apr 2011 | |
|---|---|---|---|---|---|---|---|---|
| WBCb | 9.77 ± 1.63 | 9.72 | 5.64 | 7.90 | 10.98 | 19.26 | 13.22 | 9.62 |
| RBCc | 2.57 ± 0.25 | 2.64 | 2.74 | 2.64 | 3.13 | 3.31 | 2.98 | 2.86 |
| Hgbd | 10.43 ± 1.06 | 10.4 | 10.7 | 10.8 | 11.7 | 13.5 | 12.6 | 10.8 |
| Spun PCVe | 32.69 ± 3.6 | 29.0 | 34.0 | 31.0 | 34.0 | 35.0 | 40.0 | 33.0 |
| Neutrophilsb | 2.62 ± 2.53 | 2.43 | 4.40 | 4.11 | 3.18 | 2.89 | 1.32 | 1.64 |
| Lymphocytesb | 1.17 ± 0.41 | 0.68 | 0.06 | 0.16 | NDf | 2.31 | 0.93 | 0.58 |
| Monocytesb | 6.11 ± 1.45 | 6.51 | 0.06 | 3.63 | 7.58 | 14.06 | 10.97 | 7.41 |
| Eosinophilsb | 0.19 ± 0.10 | 0.10 | 1.13 | ND | 0.22 | ND | ND | ND |
| Plateletsb | 304.76 ± 40.51 | 364 | 142 | 152 | 212 | 441 | 556 | 336 |
Mean and standard deviation values for elephant 1, taken from 99 samples collected once a week from July 2008 through 26 July 2010.
WBC, white blood cell; value ×103/mm3.
RBC, red blood cell; value ×106/mm3.
Hgb, hemoglobin; value as g/dl.
PCV, packed cell volume; % red blood cells from packed cell volume.
ND, not detected.
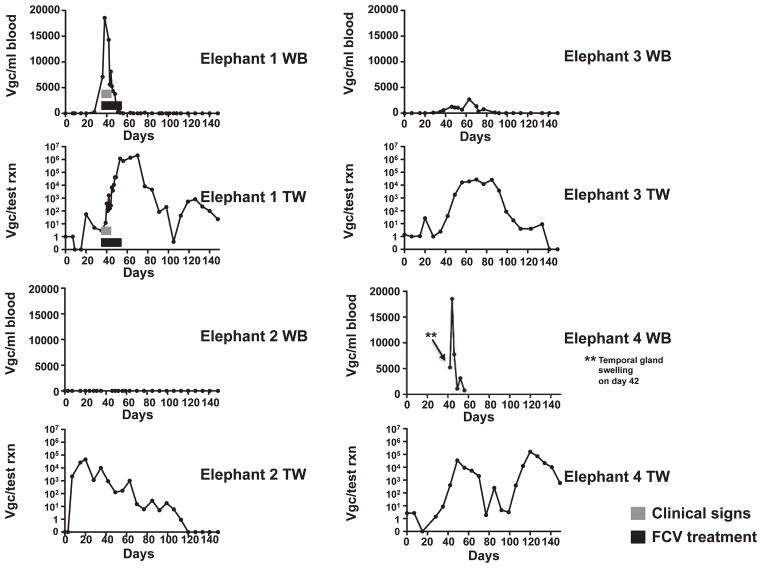
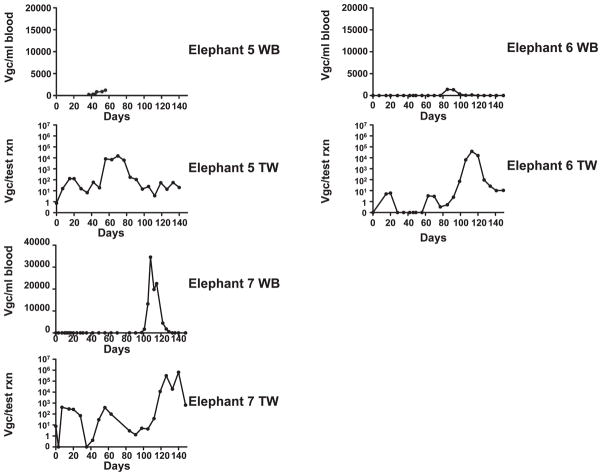
A real-time PCR EEHV5 test9 was used retrospectively to determine levels of EEHV5 viremia and shedding in the entire herd (Table 1) starting 3 January 2011 through the end of May 2011 (Fig. 1). The number of whole blood (WB) and trunk wash (TW) samples analyzed for each elephant during the study period is summarized in Table 1. Interestingly, no EEHV5 could be detected in the same WB or TW samples analyzed 1 yr earlier from this herd for EEHV110 (data not shown). EEHV5 viremia was first detected in elephant 1 on 7 February, peaked on 14 February at close to 20,000 viral genomes/ml of blood, and returned to undetectable levels by 15 March. Clinical signs of illness, which were present from 7 through 21 February, correlated with peak viral blood levels (Fig. 2; Table 1). As viremia began to resolve, a substantial increase of EEHV5 of more than four logs was observed in the elephant’s nasal secretions, peaking at over 1 million viral genomes per test reaction (Fig. 2; Table 1). EEHV5 shedding continued to be detectable for more than 2 mo.
Figure 2.
Kinetics of elephant endotheliotropic herpesvirus 5 (EEHV5) viral loads detected in whole blood (WB) and trunk wash (TW) preparations from seven elephants. VGC: viral genome equivalents. DNA prepared from WB and TW from seven elephants was screened for the presence of EEHV5 nucleic acid using a real-time quantitative polymerase chain reaction (qPCR) assay. Data for TW is expressed as VGC per test reaction and for WB as VGC per milliliter of blood. The y-axis on the graphs shows the quantity of EEHV5 genome copies, and the x-axis shows the number of days over which the study was conducted. Day 0 represents the first day that samples were collected (3 January 2011). Graphs for each animal’s WB and TW EEHV5 profiles are indicated. The time frame during which clinical signs were observed for elephant 1 is designated by the gray bar, and the duration of famciclovir treatment is indicated by a black bar.
While tracking EEHV5 WB and TW samples from elephant 1, available WB and TW samples from other animals in the herd that were archived before and after elephant 1’s illness were analyzed for EEHV5. Another female in the herd, elephant 2, had no detectable EEHV5 in her blood during the entire time period of this study (Fig. 2; Table 1), including in blood samples going back through November 2010 (data not shown). No clinical signs were evident for this elephant during the study period. Interestingly, elephant 2 had detectable EEHV5 in her nasal secretions to almost 50,000 viral genomes per test reaction, which peaked approximately 2 wk before the onset of illness in elephant 1 and continued for nearly 2 mo (Fig. 2; Table 1). Elephant 2 and her calf, elephant 7, joined the herd in August 2008. The herd has been closed since then. Of all the elephants in the herd, elephant 2 was the only one that never had any detectable EEHV5 viremia during the winter–spring of 2011, yet she had the earliest substantial levels of viral shedding in nasal secretions within the herd.
A third female elephant in the herd, elephant 3, also experienced detectable blood viremia during a similar time frame as elephant 1, although her peak EEHV5 blood levels were significantly lower (Table 1; Fig. 2). Like elephant 1, elephant 3’s EEHV5 levels in TWs rose by more than 3 logs following emergence of viremia and continued for several weeks. In contrast to elephant 1, no clinical signs were evident for elephant 3 during the study period. One week following elephant 1’s presentation with temporal gland swelling, one of the juvenile elephants, elephant 4, presented with bilateral temporal gland swelling (14 February 2011), which spontaneously resolved within 1 day. Aside from temporal gland swelling, this elephant had no other observable clinical signs of illness, and so he was not treated. Following this observation, blood samples were collected from elephant 4 and elephant 5 over a limited period of time during which both experienced detectable EEHV5 viremia, which was followed by significant EEHV5 shedding in nasal secretions (Fig. 2; Table 1). Limited blood samples were available for these two young calves (elephants 4 and 5) because of the fact that they had to be mildly sedated to obtain them. The calves were lightly sedated for sample collection using butorphanol (butorphanol tartrate, ZooPharm, Laramie, Wyoming 82070, USA; 30 mg/ml) at 0.02 to 0.05 mg/kg i.m. and detomidine (Dormosedan, Pfizer Animal Health, Exton, Pennsylvania 19341, USA; 10 mg/ml) at 0.01 mg/kg i.m. Sedation was antagonized with atipamezole (Antisedan, Pfizer Animal Health; 5 mg/ml) at 0.05 to 0.07 mg/kg i.m. For several elephants, variable low levels of EEHV5 DNA below 500 viral genome equivalents per test reaction in TWs were detectable prior to much higher sustained levels of shedding that typically exceeded 104 viral genomes per test reaction following viremia.
Two other bull elephants in the herd, elephant 6 and elephant 7, had variable contact with the rest of the herd (as opposed to daily contact between the females and very young calves) or each other from 1 February through 28 March 2011. Nevertheless, although neither animal showed any signs of clinical illness, both had detectable EEHV5 viremia at the end of March to the beginning of April (Table 1; Fig. 2) followed by an increase in EEHV5 shedding in nasal secretions that extended into May 2011 (Fig. 2).
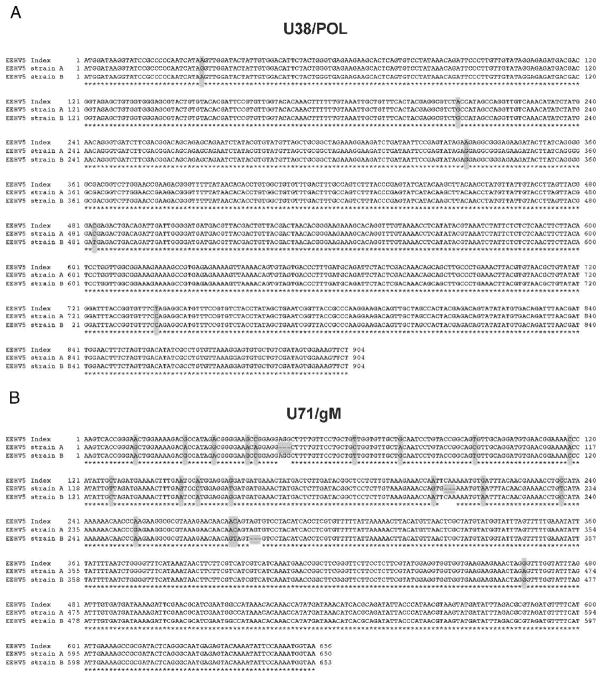
Preliminary genetic analysis of the EEHV5 viruses circulating within this herd was carried out on WB and TW samples from this cohort of elephants. Two genetic loci were chosen to work with, including a 904-bp region within the well-conserved DNA polymerase gene (U38/POL) and a more variable 650-bp region overlapping with the glycoprotein M gene (U71/gM). DNA from at least two independent TW and WB samples was PCR amplified and sequenced for each elephant, as described previously.10 Analysis of the virus DNA present in the WB and TW samples from elephant 1 gave identical DNA sequences across both genomic loci (Fig. 3A, B). Interestingly, there were several differences between these sequences and those from the original EEHV5 index case (also known as North American Proboscivirus Case 28, or NAP28) (Fig. 3A, B).4 Elephant 1 differed from the index case by four of 904 bp for the U38/POL locus and by 24 of 650 bp for the U71/gM locus. For purposes of this study, elephant 1’s strain will be referred to as strain A. Surprisingly, genetic evaluation of elephant 2’s TW virus revealed that it was a novel and significantly different EEHV5 strain (referred to as strain B) compared with EEHV5 strain A found in both the WB and TW samples from elephant 1 (Fig. 3A, B). The A and B strains differed by two of 904 bp for the U38/POL locus and by 28 of 656 bp for the U71/gM locus. Strain B differed from the index strain by four of 904 bp for the U38/POL loci and by six of 653 bp for the U71/gM locus. Genetic analysis of the virus DNA present in the TW of elephant 3 and in both the WB and TW samples from elephants 4 and 5 revealed that they all had identical sequences to those found in elephant 1 (i.e., strain A). The TW virus found at the peak of elephant 6’s shedding proved to be strain A, whereas at the peak of elephant 7’s shedding strain B was found (same as elephant 2).
Figure 3.
Sequence comparison between the elephant endotheliotropic herpesvirus 5 (EEHV5) index case and two new strains. A. Sequence comparison of 904 base pairs (bp) from the index case DNA polymerase gene (U38/ POL) (see GenBank accession number JN983100), to the same locus from EEHV5 strain A found in elephants 1 and 3–6 (GenBank accession number JN983108.1) and EEHV5 strain B found in elephants 2 and 7 (GenBank accession number JX011012). B. Sequence comparison of 656 bp from the index case glycoprotein M gene (U71/ gM) (see GenBank accession number JN983105), to the same locus from EEHV5 strain A found in elephants 1 and 3–6 (GenBank accession number JN983113.1) and EEHV5 strain B found in elephants 2 and 7 (GenBank accession number JX011021). Sequences were aligned using ClustalW. Asterisks below the sequences indicate sequence identity. Bases highlighted in gray indicate sequence differences or deletions. The polymerase chain reaction (PCR) sequencing primers used for the U71/gM locus were the same as those used previously for EEHV1 strains,10 whereas those for the U38/POL locus were specific for EEHV5.4
DISCUSSION
To the authors’ knowledge, this is the first report describing EEHV5 infection kinetics in Asian elephants and its apparent association with illness in at least one animal. A simple explanation for the apparent successive infection of the entire herd is that it began with a reactivation and shedding event initiated by a single elephant (elephant 2), which then spread to susceptible naive herd mates. However, the fact that two different virus strains were involved does not exclude the alternative possibility that some of the elephants (especially the adults) in the herd had already been previously infected with EEHV5 (probably strain B in elephants 2 and 7, which came from a different facility 40 mo previously, and strain A in the rest) and that some precipitating environmental factor (seasonal allergens, nearby zoo exhibit construction, etc.) caused multiple reactivations within this herd of elephants. Another scenario might be that elephant 2 was latently infected with both the A and B strains and reactivated them both (despite the detection of only one predominant strain in all samples evaluated). One assumption is that viremia followed by a burst of shedding in nasal secretions indicates primary infection. However, it cannot be fully ruled out that in some cases viremia followed by increased shedding could be due to reactivation. At this time it is unclear whether the initial low levels of virus shedding in some of the elephants preceding their viremia represent spurious cross contamination of trunk secretions from other elephants in the herd that were shedding high levels of EEHV5 DNA or if it was virus that was derived from active replication within that animal prior to detectable viremia. Development of an EEHV5-specific serologic assay could help to resolve the issue of which animals were undergoing reactivation versus primary infections but obviously would be unlikely to distinguish between prior latent infections with the A strain versus the B strain.
The more limited exposure of the two bull elephants to the rest of the herd, especially during February 2011, is consistent with their delayed infection with EEHV5. The fact that the bull elephant 6 had the same virus as his long-time female companions elephants 1 and 3 (strain A), whereas the 6-yr-old juvenile bull (elephant 7) had the same virus (strain B) as his mother (elephant 2), might be equally consistent with all of them having already been infected previously many years before and reactivating in response to some common but somewhat staggered triggering event. On the other hand, surprisingly, elephant 2’s much younger female calf, born recently at this facility (elephant 5), was instead shedding the same virus (strain A) as the other young male calf (elephant 4) and the three long-term adult occupants of the facility, rather than her mother’s strain. It seems particularly important to note that both of these young sub–1-yr-old calves survived the EEHV5 infection without major symptoms or drug treatments, in apparent contrast to the observations of severe life-threatening acute disease commonly caused by apparent primary infections by EEHV1A and EEHV1B in young Asian elephant calves.
At this time, it is unclear why only elephant 1 developed signs of clinical illness from EEHV5, although it is notable that the two animals that displayed temporal gland swelling were those with the highest levels of viremia. Given the age of elephant 1 (42 yr), it might be predicted that she would have been exposed or infected with this virus already. In humans, primary herpesvirus infections during childhood are often benign or subclinical, while primary infection later in life can result in severe and even life-threatening disease.1,8 Thus, in the event that she had not been infected previously, it is possible that she suffered illness due to acute primary infection. Alternatively, the nature of EEHV5 reactivations in older elephants remains unknown, and there is a precedent for other herpesviruses to cause disease following reactivation, especially in older or immunocompromised individuals.1,5,8 At this time, it remains unclear whether anti-herpesvirus drugs and supportive care had an impact on elephant 1’s ability to recover from clinical illness. Since there is no control animal with which to compare and no internal control for elephant 1 (e.g., with and without famciclovir over a period of high viremia), it cannot be determined if the EEHV5 viremia might have been even greater without treatment or if it had no effect. An interesting question is whether prior infection with EEHV5 might provide some cross-protective immunity to EEHV1 or if EEHV1 might confer some cross protection to EEHV5. Sequence identification of additional EEHV5 genes will be required to help with a detailed molecular epidemiologic characterization of EEHV5 strains and development of EEHV5-specific serologic assays. Answers to major questions about the frequency of EEHV5 infection in Asian elephants or the frequency with which it can cause disease remain unknown, and will require additional surveillance of other captive herds and of Asian elephants in range countries.
Acknowledgments
Support for this study was provided by the Houston Zoo and a grant from the Dan L. Duncan Foundation, as well as by grants from the International Elephant Foundation to the National Zoological Park Elephant Herpesvirus Laboratory and by National Institutes of Health grant R01 AI24576 to G.S. Hayward at Johns Hopkins. Sally Nofs was supported by National Institutes of Health training grant T32-AI-07471.
LITERATURE CITED
- 1.Cohen JI, Straus SE, Arvin AM. Varicellazoster virus replication, pathogenesis, and management. In: Knipe DM, Howley PM, editors. Fields’ Virology. 5. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia, Pennsylvania: 2007. pp. 2773–2818. [Google Scholar]
- 2.Fickel J, Richman LK, Montali R, Schaftenaar W, Goritz F, Hildebrandt TB, Pitra C. A variant of the endotheliotropic herpesvirus in Asian elephants (Elephas maximus) in European zoos. Vet Microbiol. 2001;82:103–109. doi: 10.1016/s0378-1135(01)00363-7. [DOI] [PubMed] [Google Scholar]
- 3.Garner MM, Helmick K, Ochsenreiter J, Richman LK, Latimer E, Wise AG, Maes RK, Kiupel M, Nordhausen RW, Zong JC, Hayward GS. Clinicopathologic features of fatal disease attributed to new variants of endotheliotropic herpesviruses in two Asian elephants (Elephas maximus) Vet Pathol. 2009;46:97–104. doi: 10.1354/vp.46-1-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Latimer E, Zong JC, Heaggans SY, Richman LK, Hayward GS. Detection and evaluation of novel herpesviruses in routine and pathological samples from Asian and African elephants: identification of two new probosciviruses (EEHV5 and EEHV6) and two new gammaherpesviruses (EGHV3B and EGHV5) Vet Microbiol. 2011;147:28–41. doi: 10.1016/j.vetmic.2010.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mocarski ES, Shenk T, Pass RF. Cytomegalovirus. In: Knipe DM, Howley PM, editors. Fields’ Virology. 5. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia, Pennsylvania: 2007. pp. 2701–2772. [Google Scholar]
- 6.Richman LK, Hayward GS. Elephant herpesviruses. In: Fowler M, editor. Fowler’s Zoo and Wild Animal Medicine: Current Therapy. Elsevier/Saunders Co; St. Louis, Missouri: 2011. pp. 496–502. [Google Scholar]
- 7.Richman LK, Montali RJ, Garber RL, Kennedy MA, Lehnhardt J, Hildebrandt T, Schmitt D, Hardy D, Alcendor DJ, Hayward GS. Novel endotheliotropic herpesviruses fatal for Asian and African elephants. Science. 1999;283:1171–1176. doi: 10.1126/science.283.5405.1171. [DOI] [PubMed] [Google Scholar]
- 8.Rickinson AB, Kieff E. Epstein-Barr virus. In: Knipe DM, Howley PM, editors. Fields’ Virology. 5. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia, Pennsylvania: 2007. pp. 2655–2700. [Google Scholar]
- 9.Stanton JJ, Nofs S, Peng RS, Hayward GS, Ling PD. Development and validation of quantitative real-time polymerase chain reaction assays to detect elephant endotheliotropic herpesviruses-2, 3, 4, 5, and 6. J Virol Methods. 186:73–77. doi: 10.1016/j.jviromet.2012.07.024. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanton JJ, Zong JC, Latimer E, Tan J, Herron A, Hayward GS, Ling PD. Detection of pathogenic elephant endotheliotropic herpesvirus in routine trunk washes from healthy adult Asian elephants (Elephas maximus) by use of a real-time quantitative polymerase chain reaction assay. Am J Vet Res. 2010;71:925–933. doi: 10.2460/ajvr.71.8.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiedner E, Howard LL, Isaza R. Treatment of elephant endotheliotropic herpesviruses. In: Fowler M, editor. Fowler’s Zoo and Wild Animal Medicine: Current Therapy. Elsevier/Saunders Co; St. Louis, Missouri: 2011. pp. 537–543. [Google Scholar]