Abstract
In this study, isoegomaketone(IK) was isolated from Perilla frutescens(L.), a Chinese herbal. The effects of IK were examined by cell viability assay, colony formation assay, xenograft tumor assay and western blotting in HCC cells. We found that IK inhibited cell viability, and its administration decreased tumor volume and weight profoundly. The presence of IK(10nmol/l) produced a dramatic decrease of pAkt, while total Akt level was not affected. The data suggested that IK from perilla suppressed HCC tumor growth via blocking PI3K/Akt signaling pathway.
Keywords: Perilla Frutescens, IK, Tumor Proliferation, HCC, PI3K/AKT
Introduction
Hepatocellular carcinoma(HCC) is the fifth most common cancer and the third leading cause of cancer-related death. It is estimated that every year more than 1 million people die of HCC worldwide (Hussain et al., 2009). Many signaling molecules involved in proliferation or metastasis are found activated in human HCC specimens. Thus people designed drugs targeting activated signaling pathways for the chemotherapy. But in HCC usual treatment, drugs such as first generation platinum compounds exhibit severe hepatotoxicity. For the increasing incidence of HCC, it is urgent to develop novel target agent and better chemotherapeutic regiment.
Perilla frutescens(L.) is a popular herbaceous plant for its pleasant aroma and taste. Its leaves are delicious vegetable and seeds are full of oil. It is also a traditional herb in China. Ingredients isolated from Perilla frutescens(L.) such as rosmarinic acid, caffeic acid and apigenin have been demonstrated to inhibit cell proliferation in various type of cancers. However, there are less reports about the effects of isoegomaketone(IK) on tumor. Cho and his colleagues have found that IK exhibited apoptosis-inducing effects on colon cancer cells (Cho et al., 2011), but the effects of IK on other cancer cells and the precise mechanism have not been reported.
Phosphoinositide 3-kinase(PI3K)-Akt signaling pathway plays a pivotal role in regulating the proliferation, apoptosis and metastasis of tumor cells. It is a key juncture in signaling communication network. It makes crosstalk between several important pathways, for example, Bcl/Bcl/caspases, AMPK/mTOR/survivin, GSK/ERK/c-fos, p21/p27/cyclin-D1 etc. Therefore, the PI3K-Akt signaling network is acknowledged as a potential target for novel agents of chemoprevention and chemotherapy. So we detected the change of PI3K/Akt in IK treated HCC cells. The demonstration of the target of IK is helpful to disclose the potential molecular mechanism of this novel chemoprevention agent.
In this study, we explored the anti-tumor effects of IK separated from Perilla frutescens(L.) on human HCC cells. This is the first report about IK inhibiting proliferation via PI3K/Akt signaling pathway in human HCC cells.
Methods
Extracts preparation
Perilla Frutescens (voucher No.08045) were purchased from local herb stores in Luoyang City. Voucher specimen of plant materials were preserved in the Herbarium at Henan Technology University. The leaves of Perilla Frutescens were dried, and extracted in MeOH over 3 days at room temperature. The extract was partitioned by ethyl acetate(EA), butanol and water. The EA fraction was separated by a silica gel column using a gradient of hexane-EA. The fraction was further segregated to yield IK. The final IK solution was prepared in DMSO.
Cell lines and animals
Two hepatoma cell carcinoma-derived cell line-Huh-7 and Hep3B were used as experimental materials. Cells were cultured in Dulbecco's modified Eagle's medium(Invitrogen) supplemented with 10%(V/V) fetal bovine serum(GIBCO) and incubated at 37° C in an atmosphere of 5% CO2. Male BALB/c nude mice(5–6 wk old) were purchased from Experimental Animal Center of Henan Technology University. These mice were housed in a windowless room at 22±1°C. The animal experiments were approved by the Ethics Committees of Henan Technology University.
Cell proliferation and colony formation
Cell viability was examined by Cell Counting Kit-8 (Dojindo) according to the instructions of the manufacturer. Huh-7 and Hep3B cells were cultured on 10-cm plate and G418(Invitrogen) (0.6–1mg/ml) screening was done for colony formation.
Western blot assay
Cells were lysed in Laemmli buffer and quantified using bradford assay (Bio-Rad). 20 µg protein was analyzed by standard procedures, with antibodies for pAkt (Cell Signaling, 1:1000) and β-tubulin (Sigma, 1:2000). The secondary antibodies were goat anti-mouse-HRP and goat anti-rabbit-HRP (IgG; Pierce, 1:3000). Finally, the PVDF membrane was washed and detected with ECL substrate solution (GE healthcare). Signals were detected by Syngene™ gel imaging analysis system and quantified by Genetools software. β-tubulin was considered as a loading control.
Tumorigenicity assays and drug administration
Nude mice were randomized by weight into 2 groups (5 animals/group), and each was injected 2×106 Huh-7 cells subcutaneously to establish xenograft animal model. IK(10µM/ml,0.2ml/d) or vehicle(0.1%DMSO) were administrated intragastrically to treated group or control group for 8 weeks. Tumor size was measured by calipers, and tumor volume was confirmed using following formula: volume=0.5×width2×length. Kinetics of tumor formation was evaluated by keeping the records of tumor size/volume change at every 3-day interval.
Xenograft immunohistochemistry staining
The pAkt immunostaining was carried out on 5 µm sections of formalin fixed paraffin embedded xenograft tumors. Antigen retrieval was performed by pretreating the dewaxed and rehydrated slides in 96° C water bath for 30 minutes in sodium citrate buffer(pH 6.0). Sections were cooled at room temperature and incubated in rabbit anti-pAkt Ser473 (1:500, Cell Signaling, Rome, Italy) overnight, followed by PBS washing and incubation with secondary antibody. Immunoreactivity was revealed by enhanced immunoperoxidase system (Novocastra, Florence, Italy) and a chromogenic substrate-3,3′-diaminobenzidine according to manufacturer's instructions.
Statistical analysis
All data were presented as Mean±SD. Tumor weight and volume was compared by Mann-Whitney U test. P values of <0.05 were considered to be significant. All Statistical analyses were performed using SPSS for windows 16.0.
Results and Discussion
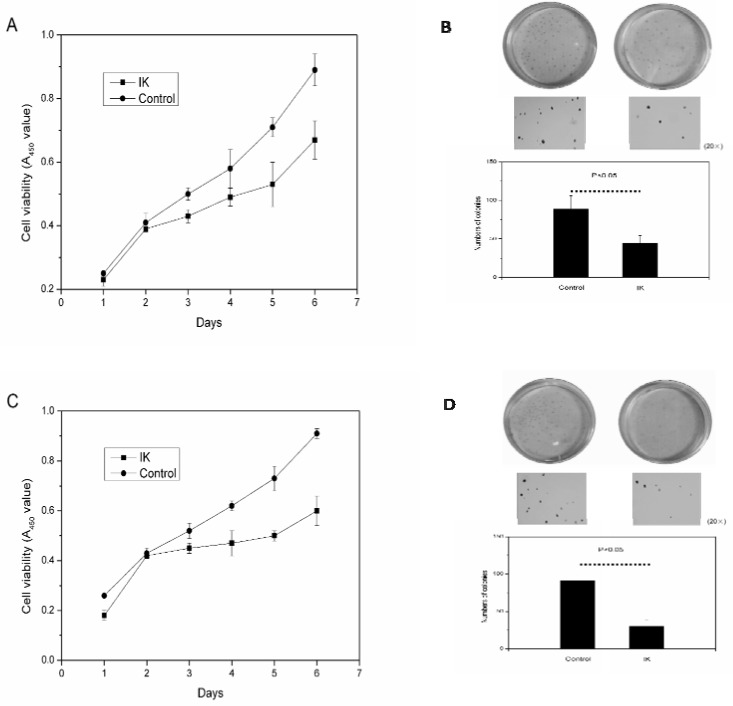
The results obtained in Figure 1 suggested that IK inhibited cellular proliferation and colony formation in Huh-7 and Hep3B cell lines. After IK addition, the A450 of viable HCC cells decreased and colonies number was significantly reduced.
Figure 1.
IK inhibits the cellular proliferation and colony formation. A, IK suppressed the proliferation of Huh-7 cells. B, IK inhibited the colony formation of Huh-7 cells, as shown by representative dishes treated with 10nM IK or 0.1%DMSO(control). Columns, mean(n=3); bars, SD. C, IK suppressed the proliferation of Hep3B cells. D, IK inhibit the colony formation of Hep3B cells.
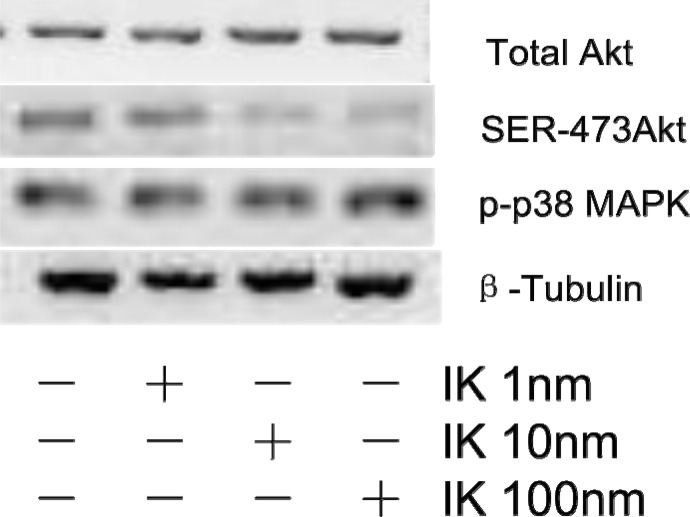
The expression level of Akt was examined by western blotting assay. Figure 2 showed that IK decreases pAkt level, not MAPK. Our data clearly showed that IK treatment influenced PI3K/Akt signal pathway, not MAPK pathway.
Figure 2.
IK inhibits Akt phosphorylation in Huh-7 cells. Western blotting assay for expression of total Akt, Ser 473 pAkt, Thr 180 p-p38 MAPK. Protein(20µg/lane) was separated by SDS-PAGE and blotted to PVDF membrane. Protein levels were normalized by a loading control-β-tubulin.
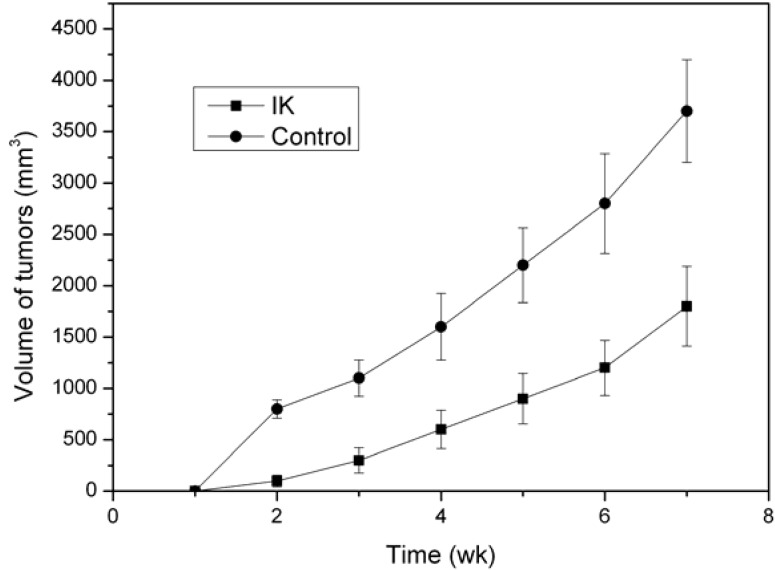
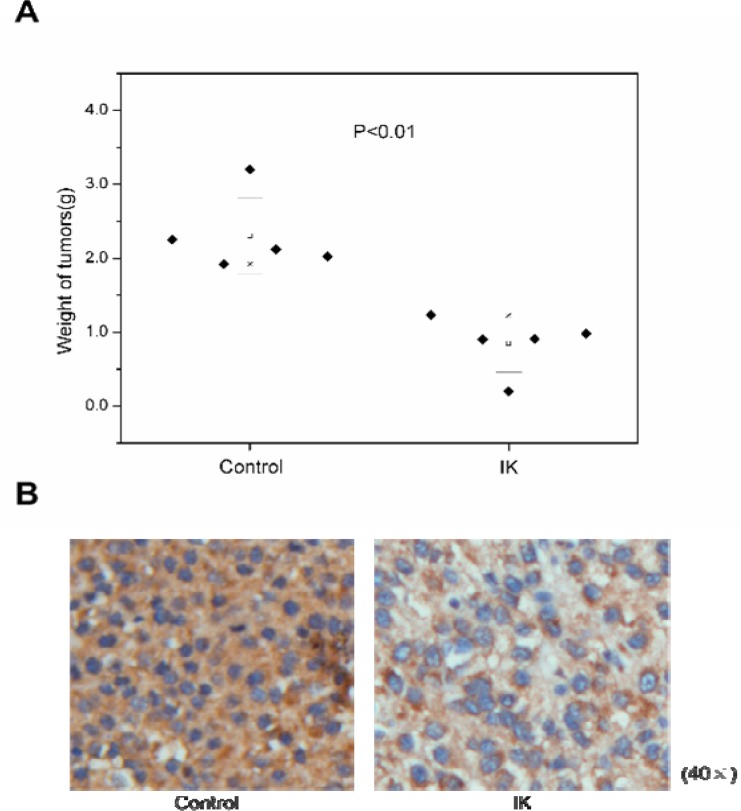
As shown in Figure 3 and Figure 4, a decrease of tumor volume and weight indicated that IK suppressed the tumorigenicity and reduced tumor burden.
Figure 3.
IK treatment decreases the xenograft tumor volume. IK suppressed tumorigenicity of Huh-7 cells in a flank of nude mice(n=5). Tumor size was estimated by serial calipation.
Figure 4.
The weight and immunohistochemistry analysis of xenograft tumors. A. IK treatment decreased the tumor weight. Tumor was estimated by analytical balance. The results showed the statistically significant difference(Mann-Whitney test; n=5, p<0.01) . B. IHC confirms that pAkt was down-regulated in xenograft tumors of IK group.
There is an old saying in china: Food and medicine have same action. Perilla, a plant genus of the family Lamiaceae and it is an ingredient of Banxia Houpu (DRUGS,CHINESE HERBAL). It is an aromatic vegetable which is always used as sapid substance in salad or noodles, but it is also commonly known for its anti-oxidant, anti-allergic, anti-tumor properties (Zhao et al., 2011; Kim et al., 2005; Makino et al., 2001; Ueda et al., 2002; Makino et al., 2003; Takano et al., 2004; Kwak et al., 2009; Banno et al., 2004).
In our research, we found one ingredient of perilla-IK inhibited the tumor growth and decreased pAkt level. It provided new evidence of the anti-tumor property of perilla and shed light on the molecular mechanism of its action. As we all know, Akt is the key protein in PI3K/Akt pathway. Its phosphorylation is the activation step of this pathway. PI3K/Akt is very important in the whole signaling network. It is the center of a lot of signal pathways involved in apoptosis, inflammation, angiogenesis, metastasis, infiltration and etc.(Yuan et al., 2012; Chen et al., 2012; Kirouac et al., 2012; Blunt et al., 2012; Okumura et al., 2012; Tenbaum et al., 2012; Wallin et al., 2012; Chiu et al., 2012; Kim et al., 2012; Cheng et al., 2011). From the data obtained in this study, we speculated that IK initiated signaling pathways which are correlated with anti-tumor growth via inhibiting Akt phosphorylation. Thus it decreased the volume and weight of xenograft tumor.
The anti-cancer effects of perilla provide a beautiful perspective of traditional medicine. It warrants further researches on drug development and medicated diet regiment.
Conclusion
The results in this study indicated that one ingredient of Perilla frutescens(L.)-IK was a potential antitumor agent against HCC cells at least partly via PI3K/Akt signaling pathway. IK significantly inhibited cell viability and xenograft tumor formation of HCC cells. Our results indicated that Akt phosphorylation was inhibited after IK administration but not Akt or p38 expression. This suggested that the mechanism of IK inhibiting HCC growth was related to PI3K/Akt signaling pathway. This is the first article about effects of IK on HCC cells, which disclosed the precise functional mechanism of the traditional herb-Perilla frutescens(L.). As the relationship between IK and signaling pathways becoming clearer, new therapeutic strategies would be further explored and developed.
References
- 1.Hussain K, El-Serag HB. Epidemiology, screening, diagnosis and treatment of hepatocellular carcinoma. Minerva Gastroenterol Dietol. 2009;55:123–138. [PubMed] [Google Scholar]
- 2.Cho BO, Jin CH, Park YD, Ryu HW, Byun MW, Seo KI, Jeong IY. Isoegomakeone induces apoptosis through caspase-dependent and caspase-independent pathways in human DLD1 cells. Biosci Biotechnol Biochem. 2011;75(7):1306–1311. doi: 10.1271/bbb.110088. [DOI] [PubMed] [Google Scholar]
- 3.Zhao G, Zang SY, Jiang ZH, Chen YY, Ji XH, Lu BF, Wu JH, Qin GW, Guo LH. Postischemic administration of liposome-encapsulated luteolin prevents against ischemia-reperfusion injury in a rat middle cerebral artery occlusion model. J Nutr Biochem. 2011;22(10):929–936. doi: 10.1016/j.jnutbio.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 4.Kim DS, Kim HR, Woo ER, Hong ST, Chae HJ, Chae SW. Inhibitory effects of rosmarinic acid on adriamycin-induced apoptosis in H9c2 cardiac muscle cells by inhibiting reactive oxygen species and the activations of c-Jun N-terminal kinase and extracellular signal-regulated kinase. Biochem Pharmacol. 2005;70(7):1066–1078. doi: 10.1016/j.bcp.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 5.Makino T, Furuta A, Fujii H, Nakagawa T, Wakushima H, Saito K, Kano Y. Effect of oral treatment of Perilla frutescens and its constituents on type-I allergy in mice. Biol Pharm Bull. 2001;24(10):1206–1209. doi: 10.1248/bpb.24.1206. [DOI] [PubMed] [Google Scholar]
- 6.Ueda H, Yamazaki C, Yamazaki M. Luteolin as an anti-inflammatory and anti-allergic constituent of Perilla frutescens. Biol Pharm Bull. 2002;25(9):1197–1202. doi: 10.1248/bpb.25.1197. [DOI] [PubMed] [Google Scholar]
- 7.Makino T, Furuta Y, Wakushima H, Fujii H, Saito K, Kano Y. Anti-allergic effect of Perilla frutescens and its active constituents. Phytother Res. 2003;17(3):240–243. doi: 10.1002/ptr.1115. [DOI] [PubMed] [Google Scholar]
- 8.Takano H, Osakabe N, Sanbongi C, Yanagisawa R, Inoue K, Yasuda A, Natsume M, Baba S, Ichiishi E, Yoshikawa T. Extract of Perilla frutescens enriched for rosmarinic acid, a polyphenolic phytochemical, inhibits seasonal allergic rhinoconjunctivitis in humans. Exp Biol Med (Maywood) 2004;229(3):247–254. doi: 10.1177/153537020422900305. [DOI] [PubMed] [Google Scholar]
- 9.Kwak CS, Yeo EJ, Moon SC, Kim YW, Ahn HJ, Park SC. Perilla leaf, Perilla frutescens, induces apoptosis and G1 phase arrest in human leukemia HL-60 cells through the combinations of death receptor-mediated, mitochondrial, and endoplasmic reticulum stress-induced pathways. J Med Food. 2009;12(3):508–517. doi: 10.1089/jmf.2008.1103. [DOI] [PubMed] [Google Scholar]
- 10.Banno N, Akihisa T, Tokuda H, Yasukawa K, Higashihara H, Ukiya M, Watanabe K, Kimura Y, Hasegawa J, Nishino H. Triterpene Acids from the Leaves of Perilla frutescens and their anti-inflammatory and antitumor-promoting effects. Biosci Biotechnol Biochem. 2004;68(1):85–90. doi: 10.1271/bbb.68.85. [DOI] [PubMed] [Google Scholar]
- 11.Yuan Q, Cai S, Zhang X, Liu Z, Li Z, Luo X, Xiong C, Wang J, Hu J, Ruan J. A new protoapigenone analog RY10-4 induces apoptosis and suppresses invasion through the PI3K/Akt pathway in human breast cancer. Cancer Lett. 2012 doi: 10.1016/j.canlet.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 12.Chen YT, Tan KA, Pang LY, Argyle DJ. The class I PI3K/Akt pathway is critical for cancer cell survival in dogs and offers an opportunity for therapeutic intervention. BMC Vet Res. 2012;8(1):73. doi: 10.1186/1746-6148-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirouac DC, Saez-Rodriguez J, Swantek J, Burke JM, Lauffenburger DA, Sorger PK. Creating and analyzing pathway and protein interaction compendia for modelling signal transduction networks. BMC Syst Biol. 2012;6(1):29. doi: 10.1186/1752-0509-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blunt MD, Ward SG. Targeting PI3K isoforms and SHIP in the immune system: new therapeutics for inflammation and leukemia. Curr Opin Pharmacol. 2012 doi: 10.1016/j.coph.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Okumura N, Yoshida H, Kitagishi Y, Murakami M, Nishimura Y, Matsuda S. PI3K/AKT/PTEN Signaling as a Molecular Target in Leukemia Angiogenesis. Adv Hematol. 2012 doi: 10.1155/2012/843085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tenbaum SP, Ordónez-Morán P, Puig I, Chicote I, Arqués O, Landolfi S, Fernández Y, Herance JR, Gispert JD, Mendizabal L, Aguilar S, Cajal SR, Schwartz S, Jr, Vivancos A, Espín E, Rojas S, Baselga J, Tabernero J, Munoz A, Palmer HG. β-catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer. Nat Med. 2012 doi: 10.1038/nm.2772. [DOI] [PubMed] [Google Scholar]
- 17.Wallin JJ, Guan J, Edgar KA, Zhou W, Francis R, Torres AC, Haverty PM, Eastham-Anderson J, Arena S, Bardelli A, Griffin S, Goodall JE, Grimshaw KM, Hoeflich KP, Torrance C, Belvin M, Friedman LS. Active PI3K Pathway Causes an Invasive Phenotype Which Can Be Reversed or Promoted by Blocking the Pathway at Divergent Nodes. PLoS One. 2012 doi: 10.1371/journal.pone.0036402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiu CF, Ho MY, Peng JM, Hung SW, Lee WH, Liang CM, Liang SM. Raf activation by Ras and promotion of cellular metastasis require phosphorylation of prohibitin in the raft domain of the plasma membrane. Oncogene. 2012 doi: 10.1038/onc.2012.86. [DOI] [PubMed] [Google Scholar]
- 19.Kim DI, Kim SR, Kim HJ, Lee SJ, Lee HB, Park SJ, Im MJ, Lee YC. PI3K-γ inhibition ameliorates acute lung injury through regulation of IκBα/NF-κB pathway and innate immune responses. J Clin Immunol. 2012;32(2):340–351. doi: 10.1007/s10875-011-9628-1. [DOI] [PubMed] [Google Scholar]
- 20.Cheng C, Ho WE, Goh FY, Guan SP, Kong LR, Lai WQ, Leung BP, Wong WS. Anti-malarial drug artesunate attenuates experimental allergic asthma via inhibition of the phosphoinositide 3-kinase/Akt pathway. PLoS One. 2011;6(6) doi: 10.1371/journal.pone.0020932. [DOI] [PMC free article] [PubMed] [Google Scholar]