Abstract
Juglans mandshurica Maxim is a traditional herbal medicines in China, and its anti-tumor bioactivities are of research interest. Bioassay-guided fractionation method was employed to isolate anti-tumor compounds from the stem barks of the Juglans mandshurica Maxim. The anti-tumor effect and biological activities of the extracted compound JMM6 were studied in BEL-7402 cells by MTT, Cell cycle analysis, Hoechst 33342 staining, Annexin V-FITC/PI assay and Detection of mitochondrial membrane potential (ΔΨm). After treatment with the JMM6, the growth of BEL-7402 cells was inhibited and cells displayed typical morphological apoptotic characteristics. Further investigations revealed that treatment with JMM6 mainly caused G2/M cell cycle arrest and induced apoptosis in BEL-7402 cells. To evaluate the alteration of mitochondria in JMM6 induced apoptosis. The data showed that JMM6 decreased significantly the ΔΨm, causing the depolarization of the mitochondrial membrane. Our results show that the JMM6 will have a potential advantage of anti-tumor, less harmful to normal cells. This paper not only summarized the JMM6 pick-up technology from Juglans mandshurica Maxim and biological characteristic, but also may provide further evidence to exploit the potential medicine compounds from the stem-barks of the Chinese Juglans mandshurica Maxim.
Keywords: Juglans mandshurica Maxim, stem-barks, anti-tumor, apoptosis, mitochondrial membrane potential, cell cycle
Introduction
Traditional Chinese medicine-based herbal medicines have gained more and more attention in the recent years and are being pursued by pharmaceutical companies as rich sources for drug discovery (Hsiao and Liu, 2010). Juglans mandshurica Maxim is a traditional herbal medicine in China. In fact, Juglans mandshurica Maxim is widely distributed throughout the northeast of China, Korean Peninsula, Japan and India, and it is also one of rare species of trees for pharmacy resources (Bai et al., 2010). Its leaves, roots, stem-barks and green husks have been used as a traditional herbal medicine to treat or prevent various diseases (Kim et al., 1998; Lee et al., 2002; Lee et al., 2005; Reutrakul et al., 2006). Many components such as quinones, flavones, polyphenols, glycosides, volatile oils have been paid attention in this plant (Machida et al., 2009; Li et al., 2009; Liu et al., 2004). Cytotoxic activities of the extracts of Juglans mandshurica Maxim have been demonstrated by certain reports (Inbaraj and Chignell, 2004; Xu et al., 2010). However, little attention has been paid to screen the main extracts by Bioassay-guided fractionation and search their bioactivities, especially, the anti-tumor biological mechanism of the medicinal compound from the Chinese Juglans mandshurica Maxim stem-barks.
Material and Methods
Plant material
The stem-barks of Juglans mandshurica Maxim were collected from Yiliga Mountain, Heilongjiang Province, China, dried at room temperature for 3 weeks. The plant was kindly identified and authenticated by pharmacist Liping Liu (Heilongjiang Province Corporation of Traditional and Herbal Medicine, China (Identified No. 2009-02-124).
Extraction and fractionation
The dry stem-barks of Juglans mandshurica Maxim (5kg) were grounded, and were exhaustively extracted with 70% EtOH (3 × 150) with reflux extract for 2h each time at 85°C. Then the rude extract was suspended in water and partitioned with the same volumes between petroleum ether (60–90°C), EtOAc, and n-BuOH. The EtOAc layer was subjected to column chromatography on silica gel eluted with a gradient of CHCl3-MeOH from (1:0, 30:1, 15:1, 7:1, 3:1, 1:1 ) to (0:1, flow rate 5ml/min) and 7 separated fractions, JMM(1–7), were obtained from differences in composition by Thin Layer Chromatography. The products JMM6 were recrystallized in CHCl3-ethanol (v/v = 3:1) and dried under vacuum, and afforded a yellow solid of 5.3g which represented 0.106% of the total dry stem-barks of the Juglans mandshurica Maxim.
Cell culture and Cell viability assay
The BEL-7402 cells were routinely cultured in RPMI-1640 medium, supplemented with 10% fetal calf serum. The culture was maintained at 37°C with a gas mixture of 5% CO2/95% air. Cell viability was assessed by measuring their ability to metabolize 3-(4,5-dimethydiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). The half-maximal inhibitory concentration (IC50) was obtained from the dose-response curve with original 6.0 software. The JMM6 showed the most potent anti-proliferative activity among JMM(1–7), so we made the follow-up experiments on the extracted JMM6 from Juglans mandshurica Maxim.
Cell cycle analysis
Analysis of the cell cycle of control and treated cancer cells was determined. Using standard methods, the DNA of cells were stained with PI, and the proportion of non-apoptotic cells in different phases of the cell cycle was recorded. The cancer cells were treated with the JMM6, harvested by centrifugation at 1000×g for 5 min, and then washed with ice-cold PBS. The collected cells were fixed overnight with cold 70% ethanol, and then stained with PI solution consisting of 50 µg/ml PI, 10 µg/ml RNase. After a 10 min incubation at room temperature in the dark, fluorescence-activated cells were sorted in a FACScan flow cytometer using CellQuest 3.0.1 software.
Fluorescence microscopy of Apoptosis assays
This method was modified from a previous report (Xu et al., 2010). Briefly, after exposure to the JMM6 for 48h, BEL-7402 cells were washed twice with PBS, then stained with 10 µg/ml Hoechst 33342 staining solution at 37°C for 30min according to the manufacturer's instructions. Finally, the cells were observed under the fluorescence microscope.
Annexin V-FITC/PI assay of apoptotic cells
BEL-7402 cells treated with the JMM6 for 24h or 48h were determined by flow cytometry using a commercially available Annexin V-FITC/PI apoptosis detection Kit. After treatment, cells were harvested and washed twice in ice-cold PBS, and resuspended in 500 µl of binding buffer at 1∼5×105 cells/ml. The samples were incubated with 5 µl of Annexin V-FITC and 5 µl propidium iodide in the dark for 15min at room temperature. Finally, samples were analyzed by flow cytometry and evaluated based on the percentage of cells for Annexin V positive.
Detection of mitochondrial membrane potential (ΔΨm)
In our study, ΔΨm was measured by using Rhodamine123. Treated with the JMM6 for 24h, 48h and 72h, BEL-7402 cells were incubated with Rhodamine123 (2µM/ml) at 37°C for 30min, and washed with PBS. The cell pellets were collected by centrifugation (1000×g, 5min), and resuspended in 500 µl PBS. Fluorescence intensities of Rhodamine 123 in cells were analyzed by flow cytometer.
Statistical analysis
Data were expressed as the Mean±SD from these independent experiments. Statistic analysis was performed using the SPSS 13.0 for Windows. Comparisons between two groups were performed by unpaired T-test. Multiple comparisons between more than two groups were performed by one-way analysis of variance (ANOVA). Significance was accepted at P value lower than 0.05.
Results
Anti-proliferative activity of the JMM6 on BEL-7402 and chang liver cell line
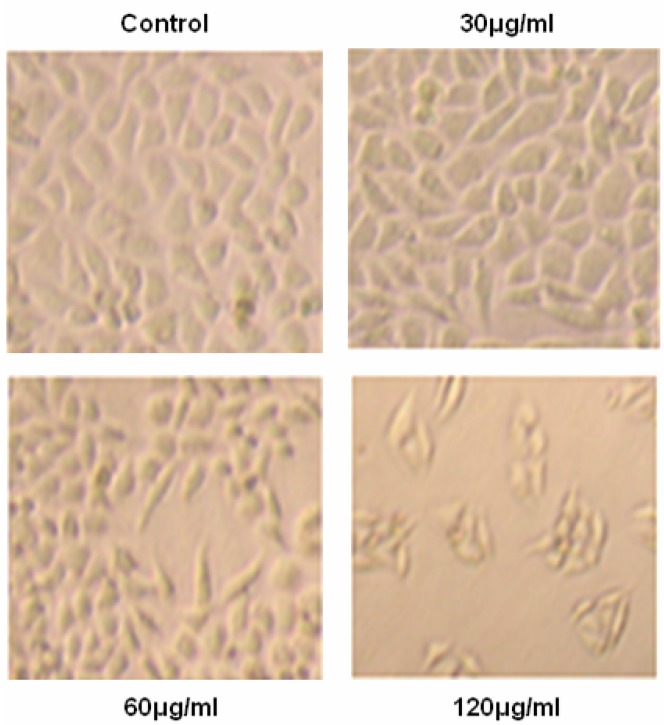
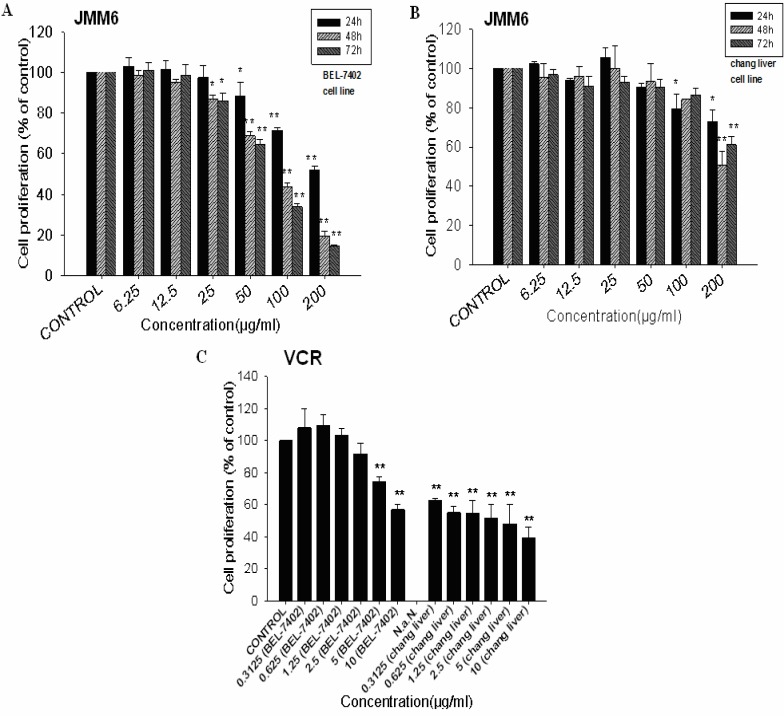
The extracted JMM6 was evaluated for its ability to inhibit the growth of human hepatocellular carcinoma cell line BEL-7402. After treating for 48h with the different concentrations of JMM6 (30, 60 and 120 µg/ml), the cultured situation was observed on BEL-7402 cells (Figure 1). The JMM6 showed the potent anti-proliferative activity with its IC50 value was 83.0µg/ml (Figure 2A). To determine the cytotoxicity of the JMM6 on normal human hepatocyte cell line, chang liver cell line was treated by the JMM6 at different concentrations and incubation time (Figure 2B). The results showed that when chang liver cells were treated for 48h, the IC50 value of the JMM6 was 155.4 µg/ml. It indicated that the BEL-7402 is more sensitive to JMM6-induced cytotoxicity than normal human hepatocyte cell line. After incubated with BEL-7402 and chang liver cells for 48h, the IC50 values of positive control Vincristine Sulfate (VCR) were 9.41µg/ml and 2.49µg/ml (Figure 2C).
Figure 1.
The cell culture photos without staining after 48h of treatment with the different JMM6 concentrations (30, 60 and 120µg/ml) on BEL-7402 cells.
Figure 2.
Anti-proliferative activity of the JMM6 detected by MTT assay after 24, 48 and 72h of treatment on BEL-7402 (A) and chang liver (B) cell. Cytotoxicity of the positive control vincristine sulfate was evaluated by MTT after 48h incubation on BEL-7402 an chang liver cell (C).
Cell cycle analysis
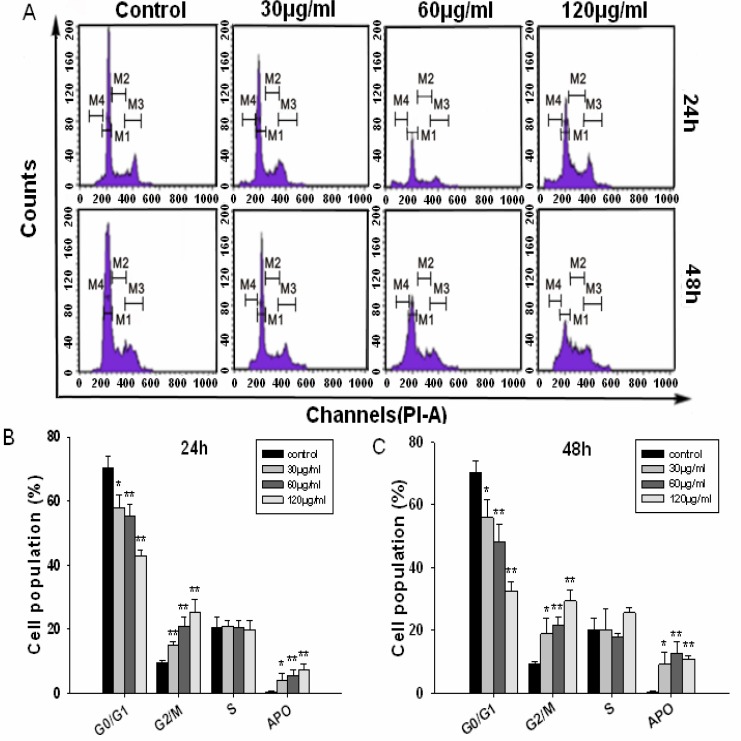
The BEL-7402 cells were treated with 30, 60 and 120µg/ml of JMM6 for 24 and 48h, respectively. We observed that the G2/M phase was arrested significantly after BEL-7402 cells were exposed to 120µg/ml JMM6 for 24 and 48h. Treatment with 30 or 60 µg/ml JMM6 resulted in modest G2/M phase arrest of BEL-7402 cells at 24 and 48h time point (Figure 3A, Table 1), respectively. The results showed that there were significant decreased G0/G1 phase distribution and increased G2/M phase distribution in a dose-dependent manner. Apoptotic cells and cell debris were significantly increased after BEL-7402 cells were exposed to the JMM6 (Figures 3B, C).
Figure 3.
BEL-7402 cells were cultured with either 0.1% DMSO (control), 30, 60 and 120µg/ml of JMM6 for 24h or 48h. The cell cycle was determined by flow cytometry. (A) Cell cycle distribution map. (B) Cell cycle bar graph for 24h. (C) Cell cycle bar graph for 48h.
Table 1.
The cell cycle analysis of the BEL-7402 cells induced by the JMM6
| concentration (µg/ml) |
the relative proportion of different phase in the cell cycle (%) |
||||
| G0/G1 | G2/M | S | APO | ||
| Control | 70.25±3.59 | 9.44±0.65 | 20.30±3.51 | 0.43±0.15 | |
| 30 | 58.00±3.95* | 15.08±0.95** | 20.92±1.77 | 4.03±2.14* | |
| 24h | 60 | 55.10±3.73** | 20.87±2.89** | 20.54±2.16 | 5.51±1.85** |
| 120 | 42.92±1.77** | 25.34±3.91** | 19.66±2.84 | 7.21±1.91** | |
| 30 | 55.86±5.67* | 18.90±5.01* | 20.31±6.45 | 9.18±3.84* | |
| 48h | 60 | 48.02±5.66** | 21.68±2.68** | 17.77±1.27 | 12.54±3.86** |
| 120 | 32.67±2.86** | 29.33±3.35** | 25.62±1.63 | 10.93±0.90** | |
Data are the mean ± SD of at least three independent experiments. *P < 0.05; **P < 0.01.* P<0.05 versus the control, the difference was significant. ** P<0.01 versus the control, the difference was markedly significant.
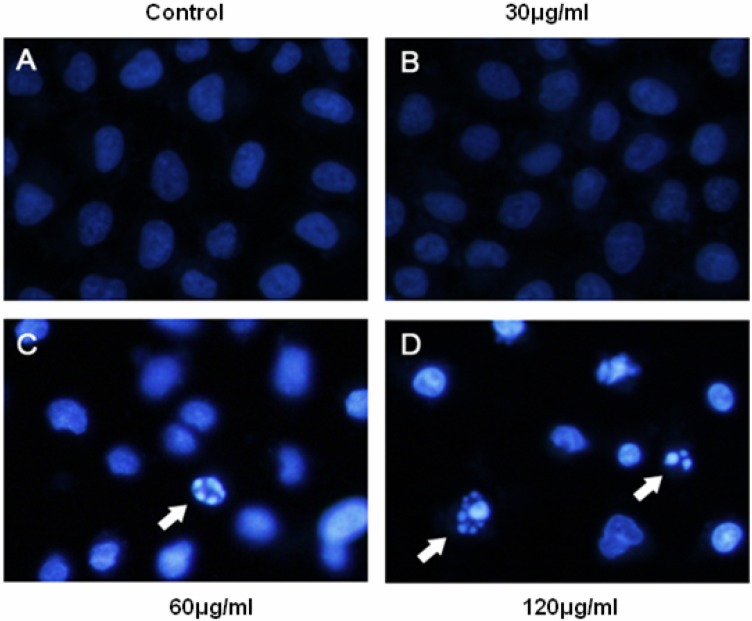
Induction of apoptosis as evidenced by Hoechst 33342 staining
The occurrence of apoptosis was identified with Hoechst 33342 staining.Figure 4 showed representative Hoechst 33258 fluorescence photomicrographs of cultured BEL-7402 cells treated with or without JMM6, respectively. In control cultures (Figure 4A), nuclei of BEL-7402 cells appeared with regular contours and were round, and large in size. By contrast, the condensation of nuclei characteristic of apoptotic cells were evident in BEL-7402 cells treated with 60 or 120 µg/ml JMM6 for 24h (Figure 4C, D). And most nuclei of JMM6 treated BEL-7402 cells appeared hypercondensed (brightly stained), and the typical apoptotic bodies were observed, which were different from that in the control cells (Figures 4C, D).
Figure 4.
BEL-7402 cells were incubated with the JMM6 for 24h and stained by Hoechst 33342. The arrows indicate apoptotic cells identified by the Hoechst 33342 staining. (A) the Blank control group. (B) the treated group with 30µg/ml. (C) the treated group with 60µg/ml. (D) the treated group with 120µg/ml.
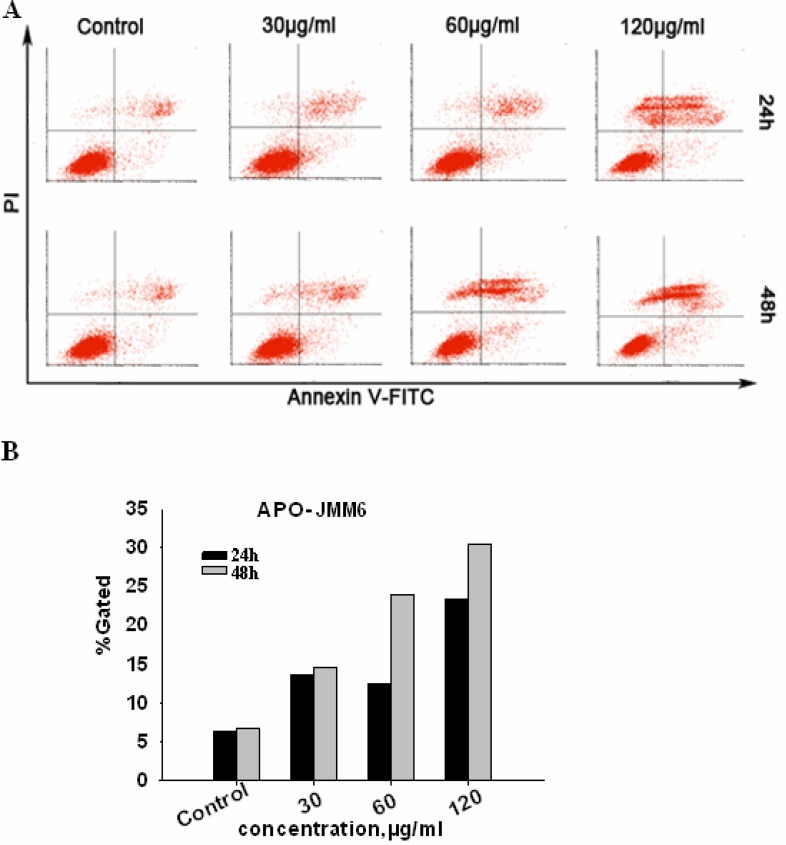
Apoptosis assessment by Annexin V-FITC/PI assay
As shown in Figure 5A, the percentage of Annexin V-FITC stained cells both the early and late apoptotic cells increased with the concentration of the JMM6 applied. It showed a remarked does-dependent manner in inducing apoptosis by the JMM6. Moreover, the JMM6 also showed a significant inducing effect in time-dependent manner (Figure 5B). In conclusion, the results suggested that JMM6-induced proliferative suppression of BEL-7402 cells was via the induction of apoptosis.
Figure 5.
Cells were incubated with JMM6 for 24h, 48h. and subjected to Annexin V-FITC/PI staining, and analyzed by flow cytometry. (A) Distribution map of cell apoptosis. (B) Bar graph of cell apoptosis. Abscissa denotes the different doses of JMM6, ordinate denotes the cell apoptosis positive rate.
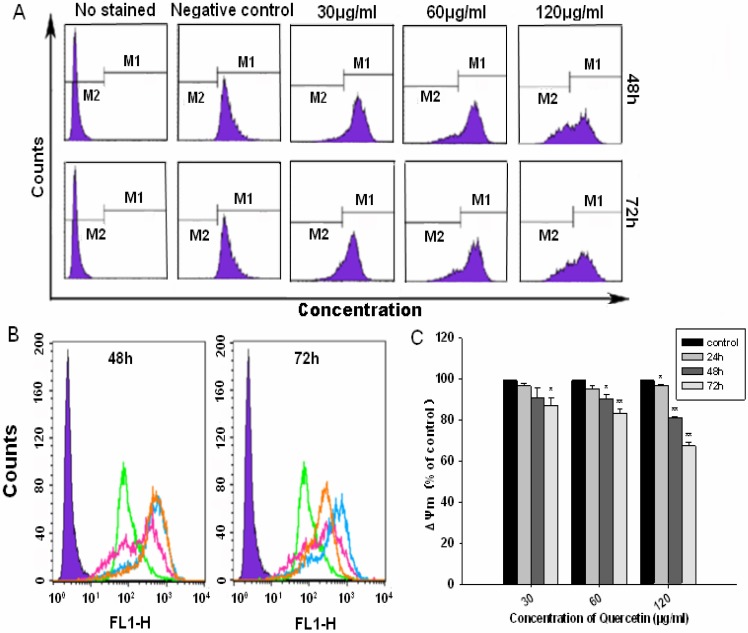
Loss mitochondrial membrane potential (ΔΨm)
To evaluate the function of mitochondria in the JMM6 induced apoptosis, we investigated its ability to induce alterations in mitochondrial potential. As shown in Figure 6A,B, the ΔΨm of the cells treated for 48h or 72h was gradually decreased with the JMM6 concentration increasing (30, 60 and 120µg/ml). Our results demonstrated that the ΔΨm was significantly decreased by the JMM6 in a dose and time dependent manner (Figure 6C). It is indicated that the JMM6 can affect the mitochondrial function, and lead to the ΔΨm value significantly decreasing, and causing the depolarization of the mitochondrial membrane, so the induction of apoptosis by the JMM6 may be associated with the mitochondrial pathway.
Figure 6.
(A) Distribution map of ΔΨm with JMM6 for 48h, 72h. (B) Superposition graph of ΔΨm with JMM6 for 48h, 72h. Note: negative control group (purple). blank control (green). low concentration(orange). medium concentration(blue). high concentration(pink). (C) Bar graph of ΔΨm with JMM6 for 24h, 48h, 72h.
Discussion
Hepatocellular Carcinoma (HCC) is the sixth most common cancer worldwide in terms of numbers of cases (626,000 or 5.7% of new cancer cases every year), but because of the very poor prognosis, the number of deaths every year is almost the same (598,000). Thus, HCC constitutes the sixth most frequent form of cancer worldwide, and it holds third place concerning malignancy-related mortality with survival rates of 3% to 5% in cancer registries for the United States and developing countries. Half of these cases and deaths were estimated to occur in China (Ferlay et al., 2010). In response to these threats, some anti-tumor chemicals or combined with radiation-therapy has been researched and developed to have prolong the life span, though, undesired or side effects have been occurring frequently. All of these may have troubles for treatment of tumor coming with problems of drug's genesis. However, the natural herbs tend to show very little toxicity or side effects in clinical treatment. It is reported that there are more than 11,000 species of herbal plants worldwide and about 500 species are commonly used in Asian and other countries. A large number of active ingredients from Chinese herbal medicines have been identified, and these naturally occurring compounds offer major opportunities for discovering novel lead structures against a wide range of therapeutic targets (Liu et al., 2011). Juglans mandshurica Maxim is sub-categoried species of Juglans regia. It has been used in treating some diseases since Chinese ancient time. Here, we screen the medicinal compound from the Chinese Juglans mandshurica Maxim stem-barks by Bioassay-guided fractionation and research its bioactivities, for the first time.
From the results of this MTT assay, we found that the JMM6 had anti-proliferative effect to Human hepatoma cells BEL-7402 in a time and does-dependent manner. VCR was used as a positive control since it has been used extensively as an efficient anticancer drug in clinical trials. Although the IC50 values of the JMM6 on BEL-7402 cells were higher than the positive control VCR, the JMM6 showed more minor cytotoxicity on human chang liver cell line than VCR. The cytotoxicity of JMM6 on human chang liver cells were 0.53 time more minor than in BEL-7402 cells, but the cytotoxicity of VCR on chang liver cells was 3.78 times more serious than in BEL-7402 cells. Therefore, it means that the JMM6 will have a potential advantage of minor side effects and less harmful to normal cells.
Apoptosis is an important continuous process of destruction of undesirable cells during development or homeostasis in multi-cellular organisms. This process is characterized by distinct morphological changes including membrane blebbing, cell shrinkage, dissipation ΔΨm, chromatin condensation and DNA fragmentation. Now, it's clear that cancer was caused by the disruption of cellular homeostasis between cell death and cell proliferation, so compounds which can induce apoptosis are considered to have potential as anticancer. According to theFigure 4, BEL-7402 cells with treated by JMM6 showed the significant characteristics of apoptosis cells. To further determine JMM6 induced apoptosis in cancer cells, the effect on the nuclear changes was investigated by flow cytometry. Compared with untreated cells, the G2/M phase was arrested significantly after BEL-7402 cells were exposed to JMM6 (Table 1,Figure 3). In addition, it is known that Phosphatidylserine (PS) externalization is an early feature of apoptosis and can be detected by the binding of Annexin V to PS on the cell surface, and the apoptosis assessment method by Annexin V-FITC/PI assay is well-recognized and accurate. According to the results of flow cytometric analysis on the Annexin V-FITC/PI, JMM6 efficiently induced apoptosis in BEL-7402 cells when applied at appropriate concentrations.
Disruption of the mitochondrial membrane potential is one of the earliest intracellular events that occur following the induction of apoptosis(Ly et al., 2003). Mitochondria play a central role in the life and death of cancer cells. They are not merely the centre for energy metabolism, but are also the headquarters for different catabolic and anabolic processes, calcium fluxes, and various signalling pathways(Scatena., 2012). At present, many studies have shown that mitochondria play a fundamental role in cell death by apoptosis, and modulation of mitochondrial respiration may induce an arrest of cancer cell proliferation and differentiation and apoptosis (Scatena., 2012). So, many drugs have been recently developed to target the mitochondria of cancer cells in order to trigger apoptosis or necrosis. There are many aspects about the role of mitochondria in the induced apoptosis of cancer cells: First once mitochondrial membrane permeabilization (MMP) occurs and the ΔΨm decrease, cells die either by apoptosis or necrosis. Key factors regulating MMP include calcium, the cellular redox status (including levels of reactive oxygen species) and the mobilization and targeting to mitochondria of Bcl-2 family members (Armstrong., 2006); Second, the release of proteins from the intermembrane space of mitochondria is one of the pivotal events in the apoptotic process, which can lead to the activation of caspases and the ultimate demise of the cell (Henry-Mowatt et al., 2004); Moreover, many studies suggest a role for mitochondria in maintaining genomic stability. Genomic stability appears to be dependent on mitochondrial functions involved in maintenance of proper intracellular redox status, ATP-dependent transcription, DNA replication, DNA repair and DNA recombination. If mitochondria are severely damaged, mitochondria damage checkpoint may not be able to repair the damage and protect cells. Such an event triggers apoptosis (Singh., 2004). To evaluate the function of mitochondria in JMM6 induced apoptosis, its ability to induce alterations in the mitochondrial potential was investigated. The data (Figure 6) showed that JMM6 decreased significantly the transmembrane potential of mitochondria, causing the depolarization of the mitochondrial membrane. Therefore, the loss of transmembrane potential was involved in JMM6 induced apoptosis. The two major apoptotic pathways ‘intrinsic or mitochondrial’ and ‘extrinsic or death receptor-related’ have been identified previously (Green, 1998).The intrinsic pathway involves the cell oxidative stress that triggers the mitochondria-dependent pathway, resulting in the induction of cytochrome c release from mitochondria into the cytosol and the activation of caspase cascade. The extrinsic pathway is triggered by the combination of ligands and receptors. To understand fully the mechanism involved in the induction of apoptosis, further investigation will be needed to be carried out.
In conclusion, the present study demonstrates that JMM6 treatment potentially inhibited the proliferation of BEL-7402 cells and induced apoptosis through a mitochondria dependent pathway. Our work intensively reveals the anti-tumor effect and biological activities of the extracted JMM6. These results also point out the direction for the following researching and exploiting natural herbal medicine Juglans mandshurica Maxim.
Acknowledgements
The work was supported by the National Natural Science Foundation of China (No. 81102753), the healthy industry base special project of Zhongshan City of China (No. 2009H021), and the Science and technology Research Project of Guangdong Province (No. 83046) and No.2012B031800431 from P. R. China.
References
- 1.Armstrong JS. Mitochondria: a target for cancer therapy. British Jornal of Pharmacology. 2006;147:239–248. doi: 10.1038/sj.bjp.0706556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai WN, Liao WJ, Zhang DY. Nuclear and chloroplast DNA phylogeography reveal two refuge areas with asymmetrical gene flow in a temperate walnut tree from East Asia. New Phytologist. 2010;188:892–901. doi: 10.1111/j.1469-8137.2010.03407.x. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 4.Green DR. Apoptotic pathways: the roads to ruin. Cell. 1998;94:695–698. doi: 10.1016/s0092-8674(00)81728-6. [DOI] [PubMed] [Google Scholar]
- 5.Henry-Mowatt J, Dive C, Martinou JC, James D. Role of mitochondrial membrane permeabilization in apoptosis and cancer. Oncogene. 2004;23:2850–2860. doi: 10.1038/sj.onc.1207534. [DOI] [PubMed] [Google Scholar]
- 6.Hsiao WL, Liu L. The role of traditional Chinese herbal medicines in cancer therapy from TCM theory to mechanistic insights. Planta Medica. 2010;76:1118–1131. doi: 10.1055/s-0030-1250186. [DOI] [PubMed] [Google Scholar]
- 7.Inbaraj JJ, Chignell CF. Cytotoxic action of juglone and plumbagin: a mechanistic study using HaCaT keratinocytes. Chemical Research in Toxicology. 2004;17:55–62. doi: 10.1021/tx034132s. [DOI] [PubMed] [Google Scholar]
- 8.Kim SH, Lee KS, Son JK, Je GH, Lee JS, Lee CH, Cheong CJ. Cytotoxic compounds from the roots of Juglans mandshurica. Journal of Natural Products. 1998;61:643–645. doi: 10.1021/np970413m. [DOI] [PubMed] [Google Scholar]
- 9.Lee KS, Li G, Kim SH, Lee CS, Woo MH, Lee SH, Jhang YD, Son JK. Cytotoxic diarylheptanoids from the roots of Juglans mandshurica. Journal of Natural Products. 2002;65:1707–1708. doi: 10.1021/np0201063. [DOI] [PubMed] [Google Scholar]
- 10.Lee SY, Min BS, Kim JH, Lee J, Kim TJ, Kim CS, Kim YH, Lee HK. Flavonoids from the leaves of Litsea japonica and their anti-complement activity. Phytotherapy Research. 2005;19:273–276. doi: 10.1002/ptr.1453. [DOI] [PubMed] [Google Scholar]
- 11.Li M, Wu Y, Jiang F, Yu X, Tang K, Miao Z. Isolation, identification and anticancer activity of an endophytic fungi from Juglans mandshurica. China Journal of Chinese Materia Medica. 2009;34:1623–1627. [PubMed] [Google Scholar]
- 12.Liu L, Li W, Koike K, Zhang S, Nikaido T. New alpha-tetralonyl glucosides from the fruit of Juglans mandshurica. Chemical & Pharmaceutical Bulletin (Tokyo) 2004;52:566–569. doi: 10.1248/cpb.52.566. [DOI] [PubMed] [Google Scholar]
- 13.Liu YH, Mo SL, Bi HC, Hu BF, Li CG, Wang YT, Huang L, Huang M, Duan W, Liu JP, Wei MQ, Zhou SF. Regulation of human pregnane X receptor and its target gene cytochrome P450 3A4 by Chinese herbal compounds and a molecular docking study. Xenobiotica. 2011;41:259–280. doi: 10.3109/00498254.2010.537395. [DOI] [PubMed] [Google Scholar]
- 14.Ly JD, Grubb DR, Lawen A. The mitochondrial membrane potential (deltapsi(m)) in apoptosis; an update. Apoptosis. 2003;8:115–128. doi: 10.1023/a:1022945107762. [DOI] [PubMed] [Google Scholar]
- 15.Machida K, Yogiashi Y, Matsuda S, Suzuki A, Kikuchi M. A new phenolic glycoside syringate from the bark of Juglans mandshurica MAXIM. var. sieboldiana MAKINO. Journal of Natural Medicines. 2009;63:220–222. doi: 10.1007/s11418-009-0312-1. [DOI] [PubMed] [Google Scholar]
- 16.Reutrakul V, Chanakul W, Pohmakotr M, Jaipetch T, Yoosook C, Kasisit J, Napaswat C, Santisuk T, Prabpai S, Kongsaeree P, Tuchinda P. Anti-HIV-1 constituents from leaves and twigs of Cratoxylum arborescens. Planta Medica. 2006;72:1433–1435. doi: 10.1055/s-2006-951725. [DOI] [PubMed] [Google Scholar]
- 17.Scatena R. Mitochondria and cancer: a growing role in apoptosis, cancer cell metabolism and dedifferentiation. Advances in Experimental Medicine and Biology. 2012;942:287–308. doi: 10.1007/978-94-007-2869-1_13. [DOI] [PubMed] [Google Scholar]
- 18.Scatena R. Mitochondria and drugs. Advances in Experimental Medicine and Biology. 2012;942:329–346. doi: 10.1007/978-94-007-2869-1_15. [DOI] [PubMed] [Google Scholar]
- 19.Singh K K. Mitochondria damage checkpoint in apoptosis and genome stability. FEMS Yeast Research. 2004;5:127–132. doi: 10.1016/j.femsyr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Xu HL, Yu XF, Qu SC, Zhang R, Qu XR, Chen YP, Ma XY, Sui DY. Anti-proliferative effect of Juglone from Juglans mandshurica Maxim on human leukemia cell HL-60 by inducing apoptosis through the mitochondria-dependent pathway. European Journal of Pharmacology. 2010;645:14–22. doi: 10.1016/j.ejphar.2010.06.072. [DOI] [PubMed] [Google Scholar]