Abstract
Current medical literature lacks any evidence of the protective effects of quince leaf on testes. Therefore, the aim of the present study was to assess the effect of quince (Cydonia oblonga Miller) leaf decoction on testicular injury and impaired spermatogenesis induced by hypercholesterolemia in rabbits. Eleven mature New Zealand white male rabbits were randomly divided into three groups: group 1 (hypercholesterolemia, n=3), group 2 (hypercholesterolemia plus quince treatment, n=6), and group 3 (control, n=2). Groups 1 and 2 received a cholesterol-enriched diet for six weeks. Group 2 received C. oblonga leaf decoction as drinking supplement as well. After six weeks, a normal diet was substituted in groups 1 and 2 for another six weeks. Group 3 (control group) was maintained throughout the study on a regular diet. At the end of the 12th week, the left testes of the animals were resected for light microscopic study with particular attention to the maturity of germ cells in seminiferous tubules using Johnsen's score. Increase in intertubular connective tissue and diameter of vessels, abundant spermatogonia and primary spermatocytes along the reduced germinal epithelium were noted in all rabbits of the group 1. The remaining animals in groups 2 and 3 had no significant changes in their testicular sections. The mean Johnsen's score of group 1 (4.20±1.92) was significantly lower than that of group 2 (7.33±0.52) and group 3 (7.05±0.07). (P=0.01). In conclusion, quince leaf decoction (C. oblonga Miller) protected rabbit testes and spermatogenesis from damage induced by hypercholesterolemia.
Keywords: Hypercholesterolemia, quince, testes, diet
Introduction
Hyperlipidemia and hypercholesterolemia have been found to be linked to male reproductive dysfunction. A number of human studies have indicated an association between hypercholesterolemia and poor semen quality and subsequently male infertility (Jones et al., 1979; Padron et al., 1989; Ramirez et al., 2000). On the other hand, investigations on high cholesterol-fed animals have found more details on such connection. Detrimental effects of hypercholesterolemia on testicular functions including spermatogenesis were noted following diet-induced hypercholesterolemia in animals (Gupta and Dixit, 1988; Shimamoto and Sofikitis, 1998; Yamamoto et al., 1999; Tanaka et al., 2001; Shalaby et al., 2004; Saez Lancellotti et al., 2010). Among numerous mechanisms proposed for the hypercholesterolemia-induced testicular injury, enhanced oxidative stress is of great research interest (Samir Bashandy, 2007).
Quince (Cydonia oblonga Miller) is a tree from Rosaceae family cultivated in gardens under warm temperature in the Caucasus and Northern Iran (Golgolab, 1961). Traditionally, preparations from different parts of quince have been used as remedies for cough, bronchitis, nausea, fever, diarrhea, cystitis, constipation, hemorrhoids, diabetes, and hypertension (Khoubnasabjafari and Jouyban, 2011). Thanks to its antioxidant properties, quince leaf possesses anti-hemolytic, anti-diabetic, anti-lipidperoxidative, anti-cancer, lipid-lowering, and renoprotective effects (Costa et al., 2009; Aslan et al., 2010; Carvalho et al., 2010; Osman et al., 2010; Jouyban et al., 2011). Nevertheless, the current medical literature lacks any evidence of the protective effects of quince leaf on testes. Therefore, the aim of the present study was to assess the effect of quince (C. oblonga Miller) leaf decoction on testicular injury and impaired spermatogenesis induced by hypercholesterolemia in rabbits.
Materials and Methods
Eleven mature New Zealand white male rabbits were maintained under controlled laboratory conditions with respect to humidity, illumination and temperature for two weeks prior to the study. Throughout the study, the international and local institutional guidelines for use of animals in research were applied. The animals were fed with drinking water and compact granules as standard food containing all essential nutrients (Hashemzadeh et al., 2012). The rabbits were randomly divided into three groups: group 1 (hypercholesterolemia, n=3), group 2 (hypercholesterolemia plus quince treatment, n=6), and group 3 (control, n=2). Groups 1 and 2 received a cholesterol-enriched diet. The cholesterol enriched diet elevated the serum cholesterol levels in both groups 1 and 2 by at least ten folds when compared to those in the control group fed with normal diet. Additionally, group 2 received C. oblonga leaf decoction as drinking supplement. After six weeks of experimental dietary changes, a normal diet was substituted for the hypercholesterolemic food of groups 1 and 2 for another six weeks. Group 3 (control group) was maintained throughout the study on a regular diet with neither cholesterol enhancement nor quince leaf decoction (Jouyban et al., 2011).
The cholesterol-enriched diet was prepared as following: cholesterol (Merck, Germany) was dissolved in ethanol and the solution was sprayed over the ground food in a 2% weight/weight ratio. The yielded food was then prepared, dried and granulated and the dried granules were later used in the present study. To prepare the quince leaf decoction, dried C. oblonga leaves (4.95 g) were added to boiling water (600 mL) and the mixture was preserved in a water bath (100°C) for two hours. The decoction was then cooled down to the room temperature and filtered through a membrane (Jouyban et al., 2011). The rabbits in group 2 (hypercholesterolemia plus quince treatment) drank and tolerated the decoction (100 ml/each). At the end of the experimental study (12th week), the animals were sacrificed with an anesthetic overdose of ketamine plus acepromazine (Somi et al., 2011). Thereafter, the left testis was immediately resected, then washed with 0.9% NaCl, cut into 5 × 5 mm pieces and fixed in 10% neutral buffered formaldehyde. Following graded dehydration through alcohol, the testicular tissues were embedded in paraffin and the blocks were retained until they were cut into sections for histochemical staining with hematoxylin and eosin (HE). The sections were evaluated by light microscopy with particular attention to the maturity of germ cells in seminiferous tubules using Johnsen's score (Johnsen, 1970; Manabe et al., 1997). The Johnsen's score is a subjective histopathology score of the testicular tissue revealing the maturation of the seminiferous tubules ranged from 0 to 10 (Table 1). We calculated the mean Johnsen's score in ten seminiferous tubules in each of the HE-stained testicular sections.
Table 1.
Johnsen's Score
| Score | Histopathology |
| 10 | Fully mature spermatogenesis is observed (mature hooked spermatozoa with dense nuclear chromatin lying within tubular lumen). Pyknotic bodies are present |
| 9 | Same criteria as for 10, but the germinal epithelium shows marked sloughing or obliteration of the lumen |
| 8 | More than 10 immature spermatozoa, with dense nuclear staining and hooked heads, are observed; the majority are peripherally placed within the tubule. Pyknotic bodies are sometimes present |
| 7 | Less than 10 spermatozoa are observed. The majority of cells are mature spermatids and are peripherally placed with less dense nuclear staining. No pyknotic bodies are present |
| 6 | Mid-phase spermatids, with pale chromatin and narrow oval heads, are observed; they are radial arranged. No pyknotic bodies are present |
| 5 | Immature spermatids, randomly arranged throughout the tubule, are observed; each has a rounded nucleus with pale chromatin. No pyknotic bodies are present |
| 4 | Only a few spermatocytes (less than 5) are observed and no spermatids or spermatozoa |
| 3 | Spermatogonia are the only germ cells present |
| 2 | No germ cells are present, but Sertoli cells are |
| 1 | No cells are present in the tubular section |
Data were presented as mean ± standard deviation (SD). Statistical analysis was performed with SPSS for windows version 19.0. The one-way analysis of variance (one-way ANOVA) with a post-hoc Tukey analysis was used to compare the mean Johnsen's scores among the three studied groups. A P value <0.05 was considered statistically significant.
Results
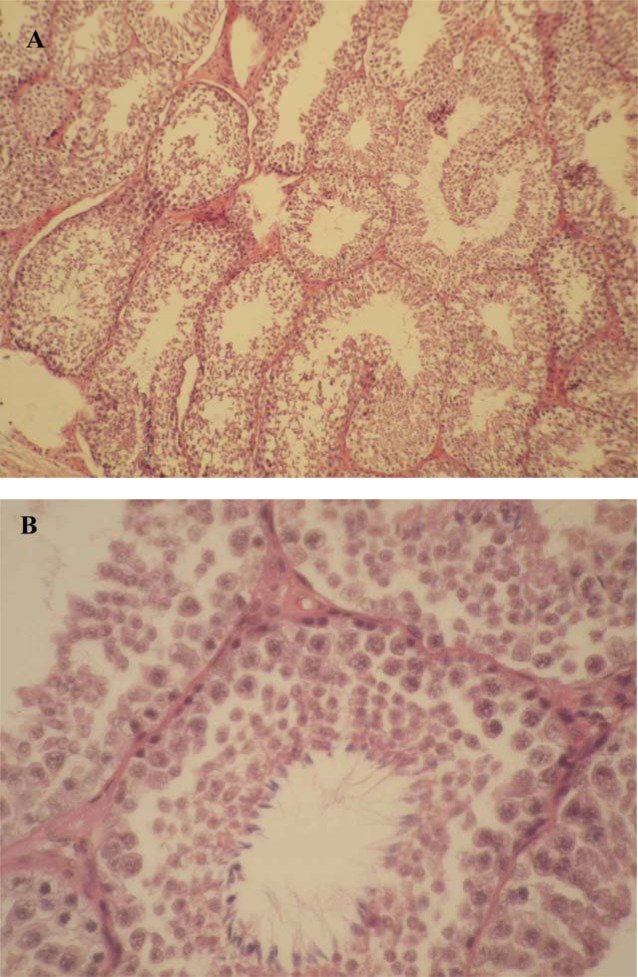
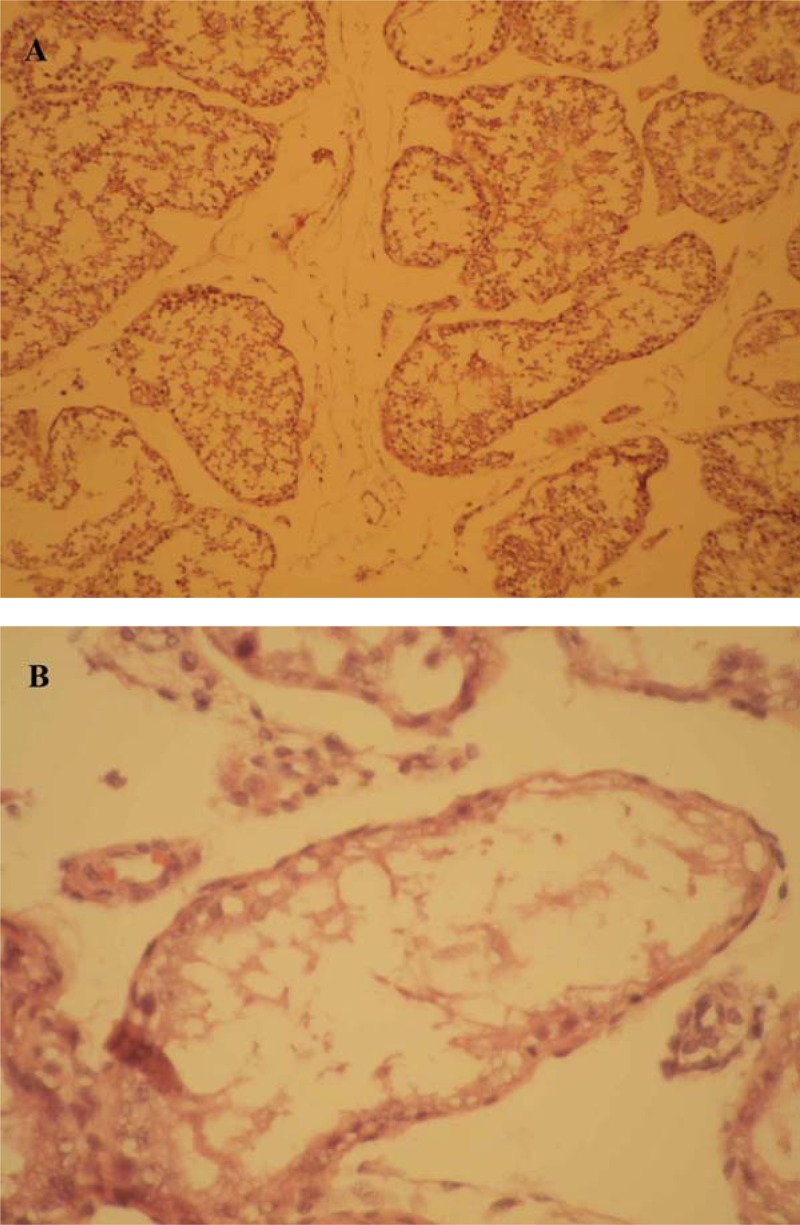
Light microscopy observations revealed thickening of tunica albuginea and increase in intertubular connective tissue in all rabbits of the group 1 (Figure 1A). The remaining animals in groups 2 (Figure 2A and B) and 3 had no significant changes in their HE-stained testicular sections. Moreover, significant structural alterations were noted in testicular sections of the group 1. These changes included disorganization of germinal epithelium, abundant spermatogonia and primary spermatocytes along the germinal epithelium, reduced thickness of the germinal epithelium, and vasodilatation of vessels in interstitial tissue (Figure 1B). The mean Johnsen's score of group 1 (4.20±1.92) was significantly lower than that of group 2 (7.33±0.52) and group 3 (7.05±0.07). (P=0.01).
Figure 1.
Histopathological findings in the testes of rabbits in group 1 (hypercholesterolemia) showed increase in intertubular connective tissue and vasodilatation of vessels in interstitial tissue (A), and abundant spermatogonia and primary spermatocytes along the reduced germinal epithelium (B).
Figure 2.
Microscopic examination of testicular specimens from rabbits in group 2 (hypercholesterolemia plus quince treatment) revealed normal histology of the tissue (A) and normal spermatogenesis (B).
Discussion
The present study revealed that cholesterol-fed rabbits had significant degenerative changes in the testes and atrophied seminiferous tubules associated with arrest of spermatogenesis. Nevertheless, cholesterol-fed animals treated with quince (C. oblonga Miller) leaf decoction supplement exhibited testicular structure and functioning seminiferous tubules similar to those of the control group. Altogether, it seems that quince leaf has a protective effect on the testes. To the best of our knowledge, this feature of the quince leaf has not been hitherto reported in the literature.
Hyperlipidemia in particular hypercholesterolemia is known to have detrimental effects on male reproductive function. In a few studies, hypercholesterolemia has been connected with testicular dysfunction in male patients (Jones et al., 1979; Padron et al., 1989; Ramirez et al., 2000). Further conclusions have been derived from investigations on hypercholesterolemic animals. Researchers found that sperm functionality and maturation, Leydig and Sertoli cells functions, and spermatogenesis were impaired following diet-induced hypercholesterolemia in animals (Gupta and Dixit, 1988; Shimamoto and Sofikitis, 1998; Yamamoto et al., 1999; Tanaka et al., 2001; Saez Lancellotti et al., 2010). In the present study, seminiferous tubules degeneration and subsequently impaired spermatogenesis was noted in hypercholesterolemic rabbits. This finding is consistent with that of the investigation by Shalaby and colleagues (2004). Although the core mechanism by which hypercholesterolemia induces reproductive and testicular damage is still debated, there has been particular focus on the role of reactive oxygen species (ROS) and increased oxidative stress which are extremely cytotoxic to the spermatozoma (Shalaby et al., 2004; Samir Bashandy, 2007; Ghabili et al., 2008). Similarly, administration of antioxidants and lipid-lowering agents has been shown to protect the testes and reproductive function during hypercholesterolemia (Shalaby et al., 2004; Samir Bashandy, 2007).
Theoretically, lipid-lowering pharmaceuticals belonging to a class of hydroxymethyl glutaryl coenzyme A (HMG-CoA) reductase inhibitors could adversely affect the male gonadal function. This hypothesis stems from the fact that these agents inhibit cholesterol biosynthesis, a process known as a precursor of steroid hormones such as androgens (Dobs et al., 2000). However, male hypercholesterolemic patients under six-month statin treatment, at doses effective in improving lipid profile, did not have impaired semen values and the gonadal endocrine dysfunction (Bernini et al., 1998; Dobs et al., 2000). On the other hand, in a recent study by Velasco-Santamaría and colleagues (2011), bezafibrate (a class of amphipathic carboxylic acids) was found to disrupt gonadal steroidogenesis and spermatogenesis in adult male zebrafish. These controversies over the probable detrimental effects of lipid-lowering pharmaceuticals on the male reproductive function might constitute the rationale for orchestrating investigations similar to the present study.
Different parts of quince are used as effective traditional remedies due to their antioxidant properties such as phenolic acids and flavonoids (Silva et al., 2004; Osman et al., 2010). Meanwhile, total concentration of phenolic compounds in quince leaves is higher than that in other parts of the plant (Oliveira et al., 2008). Therefore, quince leaf has been found to have diuretic, anti-hemolytic, anti-diabetic and anti-lipidperoxidative effects (Kültür, 2007; Costa et al., 2009; Aslan et al., 2010; Khoubnasabjafari and Jouyban, 2011). Furthermore, anti-cancer and lipid-lowering characteristics of the quince leaf have been reported in recent investigations (Carvalho et al., 2010; Osman et al., 2010). In addition, we recently found that quince leaf decoction protected rabbit kidneys from glomerular and tubular injuries during hypercholesterolemia (Jouyban et al., 2011). In the present study, the protective effects of quince leaf on the hypercholesterolemia-induced testicular damage might be attributed to both its antioxidants and its lipid-lowering characteristics.
This study has certain limitations; the studied groups (i.e. hypercholesterolemia, hypercholesterolemia plus quince treatment, and control groups) did not include an equal number of rabbits. Moreover, only testicular histopathological assessment was performed in the present study, while no measurement of the serum gonadal hormones (e.g. testosterone) was executed. Furthermore, the present investigation lacked semen retrieval and analysis study of the studied animals. However, to the best of our knowledge, the present study is the first investigation to study the protective effects of quince leaf on testes.
In conclusion, our results showed that quince leaf decoction (C. oblonga Miller) protected rabbit testes and spermatogenesis from damage induced by hypercholesterolemia. To the best of our knowledge, this is the first report concerning this effect of quince leaf.
Acknowledgement
The present investigation was financially supported by a grant from the Tuberculosis and Lung Disease Research Center, Tabriz University of Medical Sciences, Iran. Hamideh Ashrafi and Kamyar Ghabili had equal contribution to this work and should be regarded as joint First Authors
References
- 1.Aslan M, Orhan N, Orhan D D, Ergun F. Hypoglycemic activity and antioxidant potential of some medicinal plants traditionally used in Turkey for diabetes. J Ethnopharmacol. 2010;128:384–389. doi: 10.1016/j.jep.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 2.Bernini G P, Brogi G, Argenio G F, Moretti A, Salvetti A. Effects of long-term pravastatin treatment on spermatogenesis and on adrenal and testicular steroidogenesis in male hypercholesterolemic patients. J Endocrinol Invest. 1998;21:310–317. doi: 10.1007/BF03350334. [DOI] [PubMed] [Google Scholar]
- 3.Carvalho M, Silva B M, Silva R, Valentão P, Andrade P B, Bastos M L. First report on Cydonia oblonga Miller anticancer potential: differential antiproliferative effect against human kidney and colon cancer cells. J Agric Food Chem. 2010;58:3366–3370. doi: 10.1021/jf903836k. [DOI] [PubMed] [Google Scholar]
- 4.Costa R M, Magalhães A S, Pereira J A, Andrade P B, Valentão P, Carvalho M, Silva B M. Evaluation of free radical-scavenging and antihemolytic activities of quince (Cydonia oblonga) leaf: a comparative study with green tea (Camellia sinensis) Food Chem Toxicol. 2009;47:860–865. doi: 10.1016/j.fct.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 5.Dobs A S, Miller S, Neri G, Weiss S, Tate A C, Shapiro D R, Musliner T A. Effects of simvastatin and pravastatin on gonadal function in male hypercholesterolemic patients. Metabolism. 2000;49:115–121. doi: 10.1016/s0026-0495(00)90938-7. [DOI] [PubMed] [Google Scholar]
- 6.Ghabili K, Shoja M M, Agutter P S. Piezoelectricity and prostate cancer: proposed interaction between electromagnetic field and prostatic crystalloids. Cell Biol Int. 2008;32:688–691. doi: 10.1016/j.cellbi.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Golgolab H. Gia rahnema-ye giahi. Tehran: Tehran University Press; 1961. p. 107. [Google Scholar]
- 8.Gupta R S, Dixit V P. Effect of dietary cholesterol on spermatogenesis. Z Ernahrungswiss. 1988;27:236–243. doi: 10.1007/BF02019512. [DOI] [PubMed] [Google Scholar]
- 9.Hashemzadeh S, Hashemzadeh K, Ranjbari A, Halimi M, Estakhri R, Aligholipour R, Ghabili K. Erythropoietin does not ameliorate limb ischemia/reperfusion injury in rabbits. Afr J Pharm Pharmacol. 2012;6:1457–1461. [Google Scholar]
- 10.Johnsen S G. Testicular biopsy score count--a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones. 1970;1:2–25. doi: 10.1159/000178170. [DOI] [PubMed] [Google Scholar]
- 11.Jones R, Mann T, Sherins R. Peroxidative breakdown of phospholipids in human spermatozoa, spermicidal properties of fatty acid peroxides, and protective action of seminal plasma. Fertil Steril. 1979;31:531–537. doi: 10.1016/s0015-0282(16)43999-3. [DOI] [PubMed] [Google Scholar]
- 12.Jouyban A, Shoja M M, Ardalan M R, Khoubnasabjafari M, Sadighi A, Tubbs R S, Agutter P S, Ghabili K. The effect of quince leaf decoction on renal injury induced by hypercholesterolemia in rabbits: a pilot study. J Med Plant Res. 2011;5:5291–5295. [Google Scholar]
- 13.Khoubnasabjafari M, Jouyban A. A review of phytochemistry and bioactivity of quince (Cydonia oblonga Mill.) J Med Plant Res. 2011;5:3577–3594. [Google Scholar]
- 14.Kültür S. Medicinal plants used in Kirklareli Province (Turkey) J Ethnopharmacol. 2007;111:341–364. doi: 10.1016/j.jep.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 15.Manabe F, Takeshima H, Akaza H. Protecting spermatogenesis from damage induced by doxorubicin using the luteinizing hormone-releasing hormone agonist leuprorelin: an image analysis study of a rat experimental model. Cancer. 1997;79:1014–1021. doi: 10.1002/(sici)1097-0142(19970301)79:5<1014::aid-cncr19>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Oliveira A P, Pereira J A, Andrade P B, Valentao P, Seabra R M, Silva B M. Organic acids composition of Cydonia oblonga Miller leaf. Food Chem. 2008;111:393–399. doi: 10.1016/j.foodchem.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Osman A G, Koutb M, Sayed A E. Use of hematological parameters to assess the efficiency of quince (Cydonia oblonga Miller) leaf extract in alleviation of the effect of ultraviolet—A radiation on African catfish Clarias gariepinus (Burchell, 1822) J Photochem Photobiol B. 2010;99:1–8. doi: 10.1016/j.jphotobiol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Padrón R S, Más J, Zamora R, Riverol F, Licea M, Mallea L, Rodríguez J. Lipids and testicular function. Int Urol Nephrol. 1989;21:515–519. doi: 10.1007/BF02549590. [DOI] [PubMed] [Google Scholar]
- 19.Ramírez-Torres M A, Carrera A, Zambrana M. High incidence of hyperestrogenemia and dyslipidemia in a group of infertile men. Ginecol Obstet Mex. 2000;68:224–229. [PubMed] [Google Scholar]
- 20.Saez Lancellotti T E, Boarelli P V, Monclus M A, Cabrillana M E, Clementi M A, Espínola L S, Cid Barría J L, Vincenti A E, Santi A G, Fornés M W. Hypercholesterolemia impaired sperm functionality in rabbits. PLoS One. 2010;5:e13457. doi: 10.1371/journal.pone.0013457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samir Bashandy A E. Effect of fixed oil of Nigella sativa on male fertility in normal and hyperlipidemic rats. Int J Pharmacol. 2007;3:27–33. [Google Scholar]
- 22.Shalaby M A, el-Zorba H Y, Kamel G M. Effect of alpha-tocopherol and simvastatin on male fertility in hypercholesterolemic rats. Pharmacol Res. 2004;50:137–142. doi: 10.1016/j.phrs.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Shimamoto K, Sofikitis N. Effect of hypercholesterolaemia on testicular function and sperm physiology. Yonago Acta Med. 1998;41:23–29. [Google Scholar]
- 24.Silva B M, Andrade P B, Valentão P, Ferreres F, Seabra R M, Ferreira M A. Quince (Cydonia oblonga Miller) fruit (pulp, peel, and seed) and Jam: antioxidant activity. J Agric Food Chem. 2004;52:4705–4712. doi: 10.1021/jf040057v. [DOI] [PubMed] [Google Scholar]
- 25.Somi M H, Hajipour B, Abad G D, Hemmati M R, Ghabili K, Khodadadi A, Vatankhah A M. Protective role of lipoic acid on methotrexate induced intestinal damage in rabbit model. Indian J Gastroenterol. 2011;30:38–40. doi: 10.1007/s12664-011-0090-z. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka M, Nakaya S, Kumai T, Watanabe M, Matsumoto N, Kobayashi S. Impaired testicular function in rats with diet-induced hypercholesterolemia and/or streptozotocin-induced diabetes mellitus. Endocr Res. 2001;27:109–117. doi: 10.1081/erc-100107174. [DOI] [PubMed] [Google Scholar]
- 27.Velasco-Santamaría Y M, Korsgaard B, Madsen S S, Bjerregaard P. Bezafibrate, a lipid-lowering pharmaceutical, as a potential endocrine disruptor in male zebrafish (Danio rerio) Aquat Toxicol. 2011;105:107–118. doi: 10.1016/j.aquatox.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto Y, Shimamoto K, Sofikitis N, Miyagawa I. Effects of hypercholesterolaemia on Leydig and Sertoli cell secretory function and the overall sperm fertilizing capacity in the rabbit. Hum Reprod. 1999;14:1516–1521. doi: 10.1093/humrep/14.6.1516. [DOI] [PubMed] [Google Scholar]