Abstract
This present work describes an effective new method for study traditional Chinese medicine (TCM) on meridian tropism (MT) theory, which plays an essential role in clinical selection of TCM according to syndromes and strengthens the therapeutic effects. The new thread included material basis foundation and its tissue distribution study. Xiheliu, the most popular TCM on heart tropism, was investigated by simple and accurate high performance liquid chromatography (HPLC) method. The analysis of plasma after oral administration the total flavonoid of Xiheliu (TFX) exhibited that tamarixetin and kaempferide had the highest concentration and approximately the highest level within 25 min. The mixture of them could last accelerating the urine excretion more than 7 h after a single dose and could not cause the disorder of ion in rats, which was observed in diuretic activity experiment. In view of the reported biological activities was consistent with the effects of Xiheliu, tamarixetin and kaempferide were likely to be the material basis of it. Tissue distribution study showed that the highest level of analytes was in heart, lung, kidney and liver, and most tissues reached maximum level at 30 min post-dose. Since liver was the most important blood-supply tissue, the result of this experiment was in accordance with the MT record of Xiheliu and confirmed that tamarixetin and kaempferide was the material bases of it on MT. This is the first report for the illumination of material basis and the mechanism of Xiheliu on MT by analysis the record of Xiheliu in Compendium of Materia Medica and experimental study.
Keywords: Meridian Tropism, Material Basis, Tissue Distribution, Xiheliu
Introduction
MT theory, the core principle of TCM theory and the bridge between TCM theory and clinical medication (Huang and Tang, 2009), was found initially in “Huang Di Nei Jing”, which was the origin of TCM theory. After development of MT theory in Ming dynasty (1368–1644), especially included in Compendium of Materia Medica (Chinese name: Ben Cao Gang Mu), MT theory played an essential role in clinical selection of TCM according to syndromes and the therapeutic effects improvement (Zhao et al., 2008), and became the most important part of herbal description and clinical principles in China. The study of MT theory contributed to the efficient utilization and modernization of TCM.
Xiheliu, the twig of Tamarix chinensis, was the most popular TCM on heart tropism. Described in Compendium of Materia Medica, it had diuretic effect, could treat alcoholic damage and abdominal distension caused by parasite or dyspepsia. It was also used for the treatment of cough with dyspnea, wind chill cold, rheumatic bone pain and pruritus in China (Chinese pharmacopeia committee, 2010). The pharmacological study revealed Xiheliu extract owning good antihepatotoxic activity (Yang et al., 1987). It also could kill Pneumococcus, alpha-Streptococcus, Monilia albican, Haemophilus influenzae in vitro (Jiang and Zuo, 1988), and inhibit the growth of kinds of pest's larvae (Klocke et al., 1986). Clinical tests indicated the extract not only was good for treating rheumatoid arthritis (Wu, 1996), but also had significant analgesia and antipyretic effects (Zhao, 1995).
The variation of the cyclic adenosine monophosphate and the cyclic guanosine monophosphate, receptor theory and carrier theory had been used for MT theory explaination in the past (Zhao and Wei, 2003). At present, observation of the active compound and microelements distribution in vivo was the most popular experimental method for studying MT theory (Zhao et al., 2002; Xu, 2004). Every TCM had more than one active compound and variety of biological activities, how to find some certain active compound which owns the effects of TCM and do researches in further is the critical and complex problem.
Material basis was some constituents that possess the main effects of whole herbal. Therefore, finding material basis of TCM on MT was regarded as the most important thing to clarify the mechanism and the selectivity of drug actions in essence (Zhou, 2009). This paper was designed to determine the material basis of Xiheliu on MT theory, the plasma analysis and diuretic activity after oral administration were studied. After a single dose, the distribution of material basis in vivo was investigated and found tissue distribution result was consistent with the description of Xiheliu on MT in Compendium of Materia Medica. This result indicated that distribution of material basis was useful to clarify the mechanism of Xiheliu on MT. As we know, no literature was found on the study of TCM on MT through the material basis and its tissue distribution, and this is the first investigation on experimental study of Xiheliu on MT after oral administration the total flavonoid of Xiheliu (TFX) in rats.
Materials and Methods
Plant Source and Extract
Xiheliu was purchased from Tongrentang Drugstore (Lianyungang, Jiangsu Provincem, P. R. China). A voucher specimen (No. HS100305) was deposited in the Jiangsu Key Laboratory of Marine Biotechnology, Huaihai Institute of Technology. Xiheliu (3 kg) was extracted with 75% ethanol aqueous (3 × 1.5 h) under reflux. After evaporation of ethanol in vacuo, the concentrated extract was suspended in water and got supernatant by centrifugation, then the supernatant subjected to polyamide column and eluted by ethanol aqueous gradient solution. The total flavonoid of Xiheliu (TFX) was obtained from the 60% ethanol aqueous eluted fraction.
Chemicals and Drugs
Tamarixetin and kaempferide were isolated from TFX, their structures were identified on the basis of spectral data (Galeotti et al., 2008; Blasa, 2011) and the purity was more than 98.5%. Quercetin used as internal standard (IS) and its purity was more than 99.0%. HPLC-grade methanol was purchased from Honeywell International (Burdick and Jackson, Muskegon, MI, USA). Pure tamarixetin and kaempferide solutions were prepared in methanol to furnish working solutions at concentrations 800, 400, 160, 80, 16.0, 8.00, 3.20, 1.60 µg/mL and 1400, 700, 280, 140, 28.0, 14.0, 5.60, 2.80 µg/mL, respectively. Quercetin solution of concentration 275 µg/mL was prepared in methanol. For tissue distribution study, above working solutions were diluted by methanol and obtained the standard calibration sample in the concentration range of 0.32–160 µg/mL, 0.28–280 µg/mL and 27.5 µg/mL, respectively. All the solutions were stored at −20 °C and were brought to room temperature before use.
The TFX was distributed in 0.5% sodium carboxymethyl cellulose solution and the finial concentration was 30 mg/mL. Each rat was dosed 600 mg/kg of TFX solution orally, which contained 127 mg/kg tamarixetin and 103 mg/kg kaempferide, respectively.
Experimental Animals
Wistar rats (male, 150–170 g) were purchased from Lianyungang Institute of Drug Control (Lianyungang, P. R. China), and kept in environmentally controlled breeding room (relative humidity: 65%, temperature: 23 ± 2 °C, 12 h light-12 h dark cycle) for three days before the test, fed with food and water ad arbitrium. All animal studies were performed according to the requirement of the National Act on the Use of Experimental Animal (China). The experimental protocol was approved by the Committee on Animal Research, Lianyungang City.
HPLC System and Mobile Phase
Chromatographic analysis was performed on Waters 2695 HPLC system equipped with diode array detector (2996) and Empower software (Waters, Milford, MA, USA). A Waters Sunfire™ C18 reversed-phase column (5-µm particles, 250 mm × 4.6 mm) was used for separation and quantification. Chromatograms were monitored at 254 nm and the temperature of column was kept at 35 °C. C18 cartridge columns, purchased from Waters (Waters, Milford, MA, USA), were used for samples preparation. The mobile phase for HPLC analysis consisted of methanol (component A) and 0.15% formic acid solution (component B, pH 2.7) with a flow rate of 1.0 mL/min, and the change of gradient was different for plasma and tissue samples. For plasma samples, the initial elution condition was 10:90 (A–B, v/v) and held for 5 min, linearly changed to 20:80 (A–B, v/v) at 10 min, to 30:70 (A–B, v/v) at 15 min, to 55:45 (A–B, v/v) at 23 min, to 65:45 (A–B, v/v) at 30 min, and to 70:30 (A–B, v/v) at 35 min, successively, then held 70:30 (A–B, v/v) until 40 min. For tissue samples, the initial mobile phase composition condition was A–B 10:90 (v/v). This was changed linearly to A–B 35:65 (v/v) at 15 min and held at this composition until 25 min, the composition was changed linearly to A–B 60:40 (v/v) at 40 min.
Plasma Analysis Experiment
18 rats were used for plasma samples collection and ten rats were free of TFX to collect blank plasma. Blood was collected from the retrobulbar capillary plexus at 5, 10, 15, 20, 25, 30, 40, 60, 120, 180 and 240 min after oral administration. Blank samples were collected at the same time points, and five duplicates were obtained at every time interval. Plasma was separated from blood samples by centrifugation at 3500 rpm (10 min), and stored at −20 °C until analysis. A 200 µL plasma sample was removed to a 1.5 mL eppendorf tube and spiked with 5 µL of IS, with adjusting pH to 3.0 using 20 µL 1% phosphoric acid solution. The mixed solution was vortexed about 60 seconds and removed to SPE cartridge, which was eluted by water (0.4 mL) and methanol (0.4 mL) successively. The methanol fraction was evaporated to dryness under a stream of nitrogen at 40 °C. The residue was reconstituted in 200 µL of methanol and stored at 4 °C for 30 min, then centrifuged (12000 rpm) for 10 minand and 20 µL of the sample was injected into HPLC system for analysis.
Diuretic Activity Study
50 rats were divided into five groups randomly, two groups of animal were taken as blank control group (water) and positive control group (furosemide), and three groups were oral administration the mixture of tamarixetin and kaempferide (weight ratio consistent with the proportion of TFX) at three different doses. Before the experiment, all rats were dosed 40 mL/kg normal saline solution orally, 20 min later, five groups of animal were administrated 2.3, 4.6 and 9.2 mg/kg mixture, 10 mL/kg water, 2.0 mg/kg furosemide, respectively. The urine samples were collected during the following time range 0–1, 1–3, 3–5, 5–7, 7–9, 9–11, 11–15 and 15–24 h after oral administration, and the actual volume of each urine sample was recorded. The urine of each group had been mixed in one test tube for ion analysis and pH study.
Tissue Distribution Study
Tissues of 20 rats were removed at 10, 30, 60 and 90 min after dosing TFX, washed with physiological saline solution and blotted dry twice, finally weighed and stored at −20°C until disposal (within 24 h). Blank tissues were collected from rats free of TFX and processed as tissue samples. 0.5 g tissues (heart, liver, spleen, lung, kidney and brain) were shredded in ice-bath, and then homogenized in 4 mL ice-cold 1% phosphoric acid normal saline solution. The homogenate was added 10 µL IS and vortex-mixed for 60 seconds. The supernatant, prepared by centrifugation at 6000 rpm (10 min), was extracted by C18 cartridge and eluted with water (0.6 mL) and methanol (0.6 mL) successively. The methanol fraction was evaporated to dryness under a stream of nitrogen at 40 °C. The residue was redissolved in 100 µL of methanol, and then stored 30 min at 4 °C. A 10 µL aliquot was injected into HPLC system after centrifugation at 12000 rpm for 10 min.
HPLC Method Validation
Calibration curves were constructed by plotting peak area versus concentrations in the standard samples. The limit of detection (LOD) and limit of quantitation (LOQ) were determined to evaluate the sensitivity, defined as the concentration that produced a signal-to-noise ratio of 3:1 and 10:1, respectively. Intra- and inter-day precision, assessed by relative standard deviation (RSD) and mean concentration, were measured by performing replicate analysis (n = 5) for each concentration within one day and three continual days. The recoveries from biological samples were calculated by comparing peak areas extracted from biological samples with those of the same quantities added to the mobile phase. The freeze-thaw stability was tested after frozen at −20 °C for 24 h and completely thawed at room temperature. The long-time stability and short-time stability was assessed within the 30 days (−20 °C) and 24 h storage periods (room temperature). All samples were tested at three concentration levels and repeated at least three times in method validation section.
Results
Method Validation
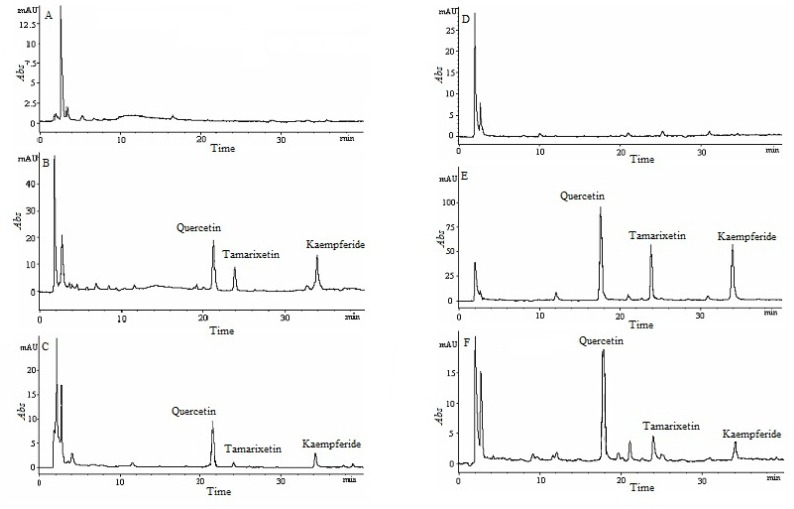
Extract recovery, selectivity, sensitivity, stability, precision and accuracy studies were carried out for HPLC method validation. The extraction recoveries of tamarixetin, kaempferide and IS in plasma and tissues were more than 86.4% (Table 1 and 2), which guaranteed that the measured concentration approach the real one. After removing interferences from the biological samples by solid-phase extraction, the endogenous components were disappeared at the retention time of analytes in plasma and tissues analysis (Figure 1). The well-defined analyte peaks demonstrated that acceptable selectivity was obtained by using this method. The measured mean concentration was very close to the added concentration (plasma samples: >87.1%, tissue samples: >79.3%), the RSD of analytes were less than 8.90% (intra-day, plasma samples: <6.75%, tissue samples: <8.90%) and 9.74% (inter-day, plasma samples: <8.69%, tissue samples: <9.74%), respectively (Table 1 and 3). That indicated the developed HPLC method had good precision and accuracy for plasma and tissue samples. Table 4 showed the correlation coefficient of all calibration curves was more than 0.991 (plasma samples: >0.998, tissue samples: >0.991), and the LOD and LOQ of this method were 0.06 and 0.16 µg/mL, which ensured the reliability of the experimental results. The recoveries of the freeze (−20°C)-thaw stability after three cycles, the short-time stability after 24 h stored at room temperature, the long-time stability after 30 days stored at −20°C were more than 85.6% (Table 5 and 6). These results indicated that all biological samples were stable after three freeze-thaw cycles, short-time and long-time storage period, with a reduction of less than15%.
Table 1.
Intra- and inter-day accuracy and precision and recovery for kaempferide and tamarixetin in rat plasma
| Spiked concentration (µg/mL) |
Intra-day (n = 5) | Inter-day (n = 5) | Recovery (%) (n = 5) |
RSD (%) |
||
| Measured concentration (µg/mL) |
RSD (%) | Measured concentration (µg/mL) |
RSD (%) | |||
| kaempferide | ||||||
| 700 | 706 ± 9.48 | 1.34 | 698 ± 14.3 | 2.05 | 100 ± 1.27 | 1.25 |
| 140 | 134 ± 6.86 | 5.11 | 134 ± 7.48 | 5.60 | 95.4 ± 2.19 | 2.30 |
| 14 | 12.9 ± 0.87 | 6.75 | 12.2 ± 1.06 | 8.69 | 89.6 ± 6.74 | 7.52 |
| tamarixetin | ||||||
| 800 | 801 ± 15.6 | 1.95 | 785 ± 19.5 | 2.48 | 99.5 ± 2.61 | 2.62 |
| 160 | 162 ± 10.6 | 6.56 | 157 ± 7.14 | 4.56 | 98.4 ± 4.06 | 4.13 |
| 16 | 15.6 ± 0.947 | 6.07 | 15.1 ± 0.831 | 5.51 | 96.2 ± 6.25 | 6.50 |
| quercetin | ||||||
| 1100 | 98.7 ± 1.36 | 1.38 | ||||
| 275 | 98.3 ± 1.79 | 1.82 | ||||
| 11 | 97.6 ± 2.94 | 3.01 | ||||
Table 2.
Recovery for kaempferide, tamarixetin and quercetin in rat tissues (n = 5)
| Recovery (%) and RSD |
Spiked concentration (µg/mL) | |||||||
| Tissues | quercetin | tamarixetin | kaempferide | |||||
| 275 | 800 | 160 | 16 | 700 | 140 | 14 | ||
| Heart | Recovery | 87.7 ± 1.97 | 93.7±1.61 | 88.6±2.35 | 89.1±1.24 | 93.5±2.13 | 89.3±1.87 | 89.5±2.57 |
| RSD | 2.24 | 1.72 | 2.65 | 1.39 | 2.28 | 2.09 | 2.87 | |
| Liver | Recovery | 89.7 ± 3.15 | 92.6±0.94 | 91.5±2.84 | 88.2±2.06 | 90.6±1.48 | 90.5±2.39 | 88.9±3.47 |
| RSD | 3.52 | 1.02 | 3.10 | 2.34 | 1.63 | 2.64 | 3.90 | |
| Spleen | Recovery | 90.6 ± 2.68 | 92.2±2.18 | 91.1±1.26 | 90.8±3.28 | 91.5±3.24 | 91.8±2.24 | 87.4±2.26 |
| RSD | 2.95 | 2.36 | 1.38 | 3.61 | 3.54 | 2.44 | 2.59 | |
| Lung | Recovery | 88.4 ± 2.84 | 87.6±2.54 | 86.5±2.96 | 86.4±1.42 | 87.2±2.73 | 87.5±2.83 | 87.1±2.36 |
| RSD | 3.29 | 2.93 | 3.46 | 1.65 | 3.13 | 3.23 | 2.71 | |
| Kidney | Recovery | 88.1 ± 3.54 | 91.8±2.18 | 89.6±3.58 | 86.6±2.85 | 87.5±2.64 | 88.7±2.34 | 87.6±3.52 |
| RSD | 4.01 | 2.37 | 4.00 | 3.29 | 3.13 | 2.98 | 4.02 | |
| Brain | Recovery | 88.2±2.97 | 89.6±3.15 | 87.8±2.43 | 87.7±3.57 | 88.6±2.31 | 87.6±3.61 | 87.8±2.16 |
| RSD | 3.38 | 3.52 | 2.77 | 4.07 | 2.61 | 4.12 | 2.46 | |
Figure 1.
Chromatographic profiles of tissue and plasma samples: blank heart (A); blank heart spiked with tamarixetin, kaempferide and quercetin (B); heart obtain at 30 min after administration (C); blank plasma (D); blank plasma spiked with tamarixetin, kaempferide and quercetin (E); plasma sample obtain at 30 min after administration (F).
Table 3.
Intra- and inter-day accuracy and precision in tissues (n = 5)
| Compounds | Spiked concentration (µg/mL) |
Intra-/inter-day | Measured concentration (µg/mL) and RSD (%) | |||||
| Heart | Liver | Spleen | Lung | Kindey | Brain | |||
| 800 | Intra-day | 784±20.2 2.58 |
792±19.6 2.48 |
804±23.3 2.90 |
765±18.3 2.39 |
773±26.2 3.39 |
796±23.1 2.90 |
|
| Inter-day | 778±25.4 3.26 |
783±21.5 2.75 |
791±16.8 2.12 |
785±20.3 2.59 |
762±22.8 2.99 |
779±21.7 2.79 |
||
| Tamarixetin | 160 | Intra-day | 146±6.44 4.41 |
152±10.5 6.91 |
158±7.64 4.84 |
137±5.38 3.93 |
144±6.21 4.31 |
154±9.83 6.38 |
| Inter-day | 151±7.56 5.01 |
141±12.8 8.89 |
150±6.89 4.59 |
144±6.47 4.49 |
143±5.71 3.99 |
161±8.90 5.53 |
||
| 16 | Intra-day | 13.4±1.03 7.69 |
14.2±0.940 6.62 |
15.2±1.33 8.75 |
12.8±0.87 6.80 |
13.6±1.21 8.90 |
14.6±0.977 6.69 |
|
| Inter-day | 14.9±0.972 6.52 |
13.8±1.09 7.90 |
14.5±1.12 7.72 |
13.1±1.04 7.94 |
14.2±1.32 9.30 |
13.9±1.25 8.99 |
||
| 700 | Intra-day | 675±17.5 2.59 |
683±20.0 2.93 |
699±24.1 3.45 |
674±21.4 3.18 |
679±18.2 2.68 |
688±21.2 3.08 |
|
| Inter-day | 688±14.6 2.12 |
684±21.5 3.14 |
674±20.8 3.09 |
661±22.3 3.37 |
676±17.4 2.57 |
678±22.5 3.32 |
||
| Kaempferide | 140 | Intra-day | 123±5.60 4.55 |
134±8.66 6.46 |
136±6.75 4.96 |
128±6.84 5.34 |
127±7.05 5.55 |
136±8.95 6.58 |
| Inter-day | 127±6.99 5.50 |
131±7.00 5.34 |
140±10.8 7.71 |
122±9.32 7.64 |
124±6.38 5.15 |
128±10.1 7.89 |
||
| 14 | Intra-day | 11. 1±0.785 7.07 |
12.8±1.07 8.36 |
13.4±1. 19 8.88 |
11.2±0.704 6.29 |
12.5±1.07 8.56 |
13.2±1.10 8.33 |
|
| Inter-day | 11.6±1.13 9.74 |
12.2±1.05 8.61 |
12. 9±0.912 7.07 |
11.4±0.883 7.75 |
11.6±0.983 8.47 |
12.7±0.894 7.04 |
||
Table 4.
Calibration curves for kaempferide and tamarixetin in plasma and tissues. (Y, peak area ratio (analyte/IS); X, concentration of the tamarixetin and kaempferide (µg/mL); LOQ, S/N = 10:1; LOD, S/N = 3:1.)
| Compounds | Standard curves | r | Test range (µg/mL) |
LOD (µg/mL) |
LOQ (µg/mL) |
| kaempferide (in plasma) | Y=0.00276X − 0.0448 | 0.999 | 14–1400 | 0.12 | 0.24 |
| tamarixetin (in plasma) | Y=0.00269X − 0.0219 | 0.998 | 16–800 | 0.08 | 0.16 |
| kaempferide(in heart) | Y=0. 00336X+0.0411 | 0.994 | 0.28–280 | 0.10 | 0.20 |
| tamarixetin(in heart) | Y=0. 00352X+0.0341 | 0.993 | 0.32–160 | 0.06 | 0.16 |
| kaempferide (in liver) | Y=0. 00343X+0.0423 | 0.996 | 0.28–280 | 0.10 | 0.20 |
| tamarixetin (in liver) | Y=0. 00347X+0.0402 | 0.996 | 0.32–160 | 0.06 | 0.16 |
| kaempferide (in spleen) | Y=0. 00341X+0.0435 | 0.995 | 0.28–280 | 0.10 | 0.24 |
| tamarixetin (in spleen) | Y=0. 00354X+0.0369 | 0.996 | 0.32–160 | 0.06 | 0.20 |
| kaempferide (in lung) | Y=0. 00332X+0.0431 | 0.993 | 0.28–280 | 0.10 | 0.20 |
| tamarixetin (in lung) | Y=0. 00338X+0.0472 | 0.991 | 0.32–160 | 0.06 | 0.16 |
| kaempferide (in kidney) | Y=0. 00329X+0.0443 | 0.994 | 0.28–280 | 0.10 | 0.20 |
| tamarixetin (in kidney) | Y=0. 00342X+0.0360 | 0.995 | 0.32–160 | 0.06 | 0.20 |
| kaempferide (in brain) | Y=0. 00343X+0.0414 | 0.995 | 0.28–280 | 0.10 | 0.24 |
| tamarixetin (in brain) | Y=0. 00337X+0.0318 | 0.993 | 0.32–160 | 0.06 | 0.16 |
(Y, peak area ratio (analyte/IS); X, concentration of the tamarixetin and kaempferide (µg/mL); LOQ, S/N = 10:1; LOD, S/N = 3:1.)
Table 5.
Stability data for the tamarixetin and kaempferide in rat plasma (n = 3)
| Compounds | Spiked concentration (µg/mL) |
Measured concentration (µg/mL) | ||||||||
| Short-time 4 hours |
12 hours | 24 hours | Long-time 1 day |
6 days | 30 days | Freeze-thaw 1 cycle |
2 cycles | 3 cycles | ||
| Tamarixetin | 800 160 16 |
791±10.3 155±5.06 14.7±0.700 |
770±14.7 151±4.37 14.4±0.787 |
742±17.3 146±5.65 14.0±0.668 |
792±17.5 154±1.15 15.1±0.634 |
781±19.6 153±3.90 15.0±1.22 |
777±18.6 151±4.35 14.7±0.534 |
796±14.8 153±4.53 14.9±0.708 |
778±16.8 149±3.65 14.6±0.845 |
758±15.6 147±6.16 14.1±0.827 |
| Kaempferide | 700 140 14 |
681±12.7 132±2.98 12.8±0.614 |
677±14.8 133±4.35 12.6±0.914 |
645±15.3 127±6.55 12.2±0.848 |
694±11.4 133±7.24 12.8±1.02 |
685±9.88 130±6.34 12.4±0.959 |
674±12.4 126±8.08 12.2±0.852 |
693±178 129±8.97 12.5±0.584 |
671±11.5 126±7.60 12.1±0.898 |
663±17.2 123±6.01 12.1±1.08 |
Table 6.
Stability data for the tamarixetin and kaempferide in rat tissues (n = 3)
| Compounds | Spiked concentration (µg/mL) |
Time piont |
Measured concentration (µg/mL) | |||||
| Heart | Liver | Spleen | Lung | Kindey | Brain | |||
| 3 cycles |
734±18.9 | 746±24.7 | 722±25.2 | 714±17.6 | 738±19.7 | 724±17.0 | ||
| 800 | 30 days |
750±18.8 | 741±21.5 | 738±24.7 | 693±20.3 | 734±19.2 | 717±26.1 | |
| 24 h | 730±24.7 | 726±21.4 | 714±31.7 | 660±42.6 | 705±27.7 | 682±18.9 | ||
| 3 cycles | 147±6.11 | 132±7.76 | 142±8.45 | 143±6.53 | 147±5.56 | 142±5.37 | ||
| Tamarixetin | 160 | 30 days |
142±7.60 | 146±5.44 | 146±7.16 | 137±5.73 | 143±6.82 | 140±6.88 |
| 24 h | 137±5.90 | 140±7.71 | 140±7.82 | 141±6.05 | 146±10.7 | 138±6.95 | ||
| 3 cycles | 14.4±0.678 | 14.0±0.616 | 14.4±0.442 | 13.9±0.671 | 14.4±0.879 | 13.9±0.901 | ||
| 16 | 30 days |
14.3±0.547 | 14.1±0.896 | 14.5±0.685 | 13.8±0.647 | 14.0±0.666 | 14.2±0.760 | |
| 24 h | 13.8±0.725 | 13.7±0.582 | 13.9±0.751 | 14.1±0.733 | 13.7±0.607 | 13.9±0.972 | ||
| 3 cycles |
650±13.2 | 638±21.6 | 627±22.9 | 624±23.4 | 639±24.1 | 625±24.6 | ||
| 700 | 30 days |
654±21.8 | 634±17.4 | 640±23.0 | 615±26.0 | 648±24.4 | 622±23.2 | |
| 24 h | 639±24.3 | 622±31.9 | 666±32.7 | 612±27.0 | 627±26.7 | 600±34.0 | ||
| 3 cycles |
126±4.75 | 125±5.10 | 129±4.61 | 124±6.26 | 125±5.03 | 125±5.82 | ||
| Kaempferide | 140 | 30 days |
125±5.42 | 127±4.75 | 128±5.94 | 123±5.36 | 124±5.96 | 123±7.08 |
| 24 h | 123±.61 | 122±8.32 | 124±7.95 | 121±7.55 | 121±6.56 | 121±6.29 | ||
| 3 cycles |
12.4±0.598 | 12.4±0.609 | 12.3±0.768 | 12.5±0.784 | 12.2±0.889 | 12.1±0.820 | ||
| 14 | 30 days |
12.5±0.715 | 12.5±0.792 | 12.3±0.736 | 12.3±0.997 | 12.3±0.776 | 12.3±0.738 | |
| 24 h | 12.1±0.822 | 12.0±0.885 | 12.2±1.02 | 12.0±0.942 | 12.2±0.836 | 12.0±0.931 | ||
The present results demonstrated a reliable and reproducible HPLC method for simultaneous determination of tamarixetin and kaempferide in rat plasma and tissues after oral administration of TFX. Due to the high sensitivity and good selectivity, the HPLC results could be utilized successfully for Xiheliu on MT theory study in rats.
Plasma Analysis
Tamarixetin and kaempferide could be quantitative analysised using the established HPLC method, and Table 7 showed their concentration in plasma after oral administration. The concentration of tamarixetin and kaempferide were approximately the highest level within 25 min, suggesting they were absorbed very quickly. Because oral administration was the main way for TCM dosing and the functional compounds of TCM should be absorbed into the blood after oral administration, the result suggested tamarixetin and kaempferide may be the most important active constituent of Xiheliu, and even the material basis.
Table 7.
Concentration of tamarixetin and kaempferide in rat plasma after oral administion of TFX (n = 6)
| Compounds | Measured concentration (µg/mL) | ||||||||||
| 5 min | 10 min | 15 min | 20 min | 25 min | 30 min | 40 min | 60 min | 120 min | 180 min | 240 min | |
| Tamarixetin | 0.2 | 1.4 | 2.1 | 2.2 | 3.1 | 2.5 | 1.6 | 1.3 | 0.90 | 0.70 | 0.54 |
| Kaempferide | 0.3 | 1.3 | 1.7 | 2.5 | 2.3 | 1.8 | 1.9 | 1.4 | 1.2 | 0.85 | 0.78 |
Diuretic Activity
Diuresis was regarded as the most important effect of Xiheliu in Compendium of Materia Medica, so the diuretic activity would be the basic precondition for the material basis of Xiheliu. Table 8 and 9 revealed that the mixture of tamarixetin and kaempferide had diuretic activity after oral administration, and could accelerate the urine excretion lastingly. Compared with the positive control group, high dose group was collected more urine and without the disorder of ion. Further diuretic activity of single compound was studied, and the volume of urine excretion was not significant increased.
Table 8.
Urine volume of after oral administration of TFX (n = 10)
| Groups | Dosage (mg/kg) |
Volume of urine (mL) | Total volume (mL) |
pH | |||||||
| 0–1 h | 1–3 h | 3–5 h | 5–7 h | 7–9 h | 9–11 h | 11–15 h | 15–24 h | ||||
| Control | - | 3.85 | 6.58 | 6.45 | 5.96 | 5.87 | 5.32 | 9.64 | 15.87 | 59.5 | 7.16 |
| Low dose | 2.3 | 4.01 | 6.83 | 6.51 | 5.84 | 5.96 | 5.66 | 10.35 | 17.12 | 62.3 | 6.84 |
| Medium dose | 4.6 | 5.67* | 8.12** | 8.07* | 7.46* | 7.28 | 6.39 | 11.24 | 18.09 | 72.3** | 6.76 |
| High dose | 9.2 | 5.56* | 8.27** | 8.11** | 7.38* | 7.36* | 6.74 | 10.97 | 18.53 | 72.9** | 6.65 |
| Furosemide | 2.0 | 11.2** | 12.7** | 9.18* | 7.84* | 6.35 | 4.32 | 7.63 | 10.97 | 70.2* | 6.88 |
p<0.05
p<0.01
Table 9.
Concentration of Na+, K+ and Cl− ions in urine samples (n = 10)
| Groups | Dosage ( mg/kg) | Na+ (mmol/L) | K+ (mmol/L) | Cl− (mmol/L) | Na+/ K+ |
| Control | - | 88.6 | 40.2 | 86.4 | 1.32 |
| Low dose | 2.3 | 92.1 | 50.1** | 96.4* | 1.09 |
| Medium dose | 4.6 | 94.3* | 48.6* | 102 | 1.17 |
| High dose | 9.2 | 97.6* | 49.2** | 104* | 1.16 |
| Furosemide | 2.0 | 99.6** | 32,5* | 122** | 1.86* |
p<0.05
p<0.01
Tissue Distribution
In MT theory, heart, liver, spleen, lung, kidney and brain were the most important target tissues of TCM. In this work, all those tissue samples were washed and dried twice, which effectively avoided the false positive results arising for the blood or content in tissues, to study the distribution of tamarixetin and kaempferide. At 30 min after oral administration of TFX to rats, the highest level of two analytes was observed in lung, heart and liver, and at 60 min the highest tissues were kidney, heart and lung. Tamarixetin and kaempferide were few even below the LOQ in all collected tissues after 90 min, there was no long-term accumulation after administration. Table 10 showed the concentrations of the tamarixetin and kaempferide in rat tissues at 10, 30 and 60 min after oral dose of TFX.
Table 10.
Concentration of tamarixetin and kaempferide in tissues (n = 5)
| Compounds | Liver (µg/mL) |
Heart (µg/mL) |
Spleen (µg/mL) |
Lung (µg/mL) |
Kidney (µg/mL) |
Brain (µg/mL) |
| tamarixetin (at 10 min) | 0.57 | 1.15 | 0.32 | 1.04 | 0.56 | 0.28 |
| kaempferide (at 10 min) | 0.32 | 0.76 | 0.24 | 0.85 | 0.15 | 0.21 |
| tamarixetin (at 30min) | 1.26 | 1.64 | 0.37 | 1.71 | 0.97 | 0.45 |
| kaempferide (at 30min) | 0.84 | 1.08 | 0.26 | 1.07 | 0.28 | 0.14 |
| tamarixetin (at 60 min) | 0.24 | 0.77 | 0.45 | 0.73 | 0.81 | 0.62 |
| kaempferide (at 60 min) | 0.14 | 0.61 | 0.18 | 0.60 | 0.72 | 0.34 |
Discussion
At present, the lack of material basis became the main barrier in TCM theories study (Zhang et al., 2009). The procedure of this research contains two steps: first, define the material basis of Xiheliu on MT theory; second, study its distribution in vivo and compare the result with Xiheliu on MT to assess the feasibility. An effective method for MT theory study was established in this work, and will serve more pertinency for the application of TCM.
In order to ensure the experimental results reliable, a sensitivity HPLC method was developed for quantitative analysis of plasma and tissues. In this method, biological sample pretreatment by solid-phase extraction resulted in an excellent recovery. Endogenous substance peaks were separated from the analyte peaks and acceptable selectivity was obtained by changing the analytic parameters. All biological samples were kept frozen for no longer than 24 h before pretreatment, and good stability of analytes (>86.3%) ensured the veracity of the results. The values of accuracy and precision were within recommended limits and the sensitivity and reproducibility were satisfactory for both analytes. The reliable and reproducible HPLC method made further experimental MT study possible.
Due to most TCM have pharmacological activity after oral administration, the material basis should be in blood after oral dosed. It was found that tamarixetin and kaempferide had the highest concentration in plasma by the established HPLC method. This result illustrated tamarixetin and kaempferide were absorbed easily after oral administration, and the material basis of Xiheliu on MT may be one or both of them.
The biological activity research carried out would be helpful to determine the material basis exactly. Diuresis was regarded as the most important effect of Xiheliu in Compendium of Materia Medica, so the material basis of Xiheliu must had the diuretic activity. The mixture of tamarixetin and kaempferide could last accelerating the urine excretion and was not caused the disorder of ion in rat comparing with furosemide, which demonstrated the mixture had good diuretic activity. However, significant diuretic activity was not proved to own by any one of them in further diuretic experiment. Therefore, tamarixetin and kaempferide, were regarded as the material basis of Xiheliu.
Tamarixetin and kaempferide were the major active constituents of Tamarix chinensis (Zhang et al., 1991). Tamarixetin had been found to have antibacterial (Sultanova et al., 2001), superoxide anion scavenging (Fazilatum et al., 2005), free radical scavenging (Nessa et al., 2004), hepatic protective (Yannai et al., 1998) and antioxidant activity (von Moltke, et al., 2004). Kaempferide was reported had the peroxynitrite free radical scavenging (Calgarotto et al., 2007), antitrypanosomal and antileishmanial (Tasdemir et al., 2006), antioxidant and antiradical activities (Burda and Oleszek, 2001). These reports were consistent with the effect description of Xiheliu in Compendium of Materia Medica and Chinese Pharmacopeia. Taking into account the reported activities, tamarixetin and kaempferide embodied almost all effects of Xiheliu, and then they were designated as the material basis of Xiheliu. If tamarixetin and kaempferide were the real material basis of Xiheliu, then the MT theory study base on them should embody the selectivity of Xiheliu in vivo. The result of tissue distribution showed that the highest level of tamarixetin and kaempferide were determined in heart, lung, kidney and liver. This indicated the heart, lung, kidney and liver were the most important targeted tissues of tamarixetin and kaempferide. Xiheliu was recorded on heart, lung and kidney tropism successively in Compendium of Materia Medica, which not the same as the tissue distribution result exactly. Considered the liver was the most important blood-supply tissue, the tissue distribution of tamarixetin and kaempferide were consistent with the Xiheliu on MT and can be used to clarify the mechanism of MT theory powerfully.
This work investigated Xiheliu on MT in rats by analyzing the material basis and its tissue distribution, and the result implied that tamarixetin and kaempferide were the material basis of Xiheliu on MT and the tissue distribution of material basis was an effective method for study the TCM on MT. In comparison with previously reported method, the significant advantages of this present method are that it is effective and credible.
Acknowledgements
Financial support provided by the Natural Science Foundation of Huaihai Institute of Technology (No. 10HS009, KX10051) is gratefully acknowledged.
References
- 1.Blasa M, Angelino D, Gennari L, Ninfali P. The cellular antioxidant activity in red blood cells (CAA-RBC): A new approach to bioavailability and synergy of phytochemicals and botanical extracts. Food Chem. 2011;125:685–691. [Google Scholar]
- 2.Burda S, Oleszek W. Antioxidant and antiradical activities of flavonoids. J Agric Food Chem. 2001;49:2774–2779. doi: 10.1021/jf001413m. [DOI] [PubMed] [Google Scholar]
- 3.Calgarotto AK, Miotto S, Honorio KM, da Silva ABF, Marangoni S, Silva JL, Comar M, Jr, Oliverira KMT, da Silva SL. A multivariate study on flavonoid compounds scavenging the peroxynitrite free radical. J Mol Struct: THEOCHEM. 2007;808:25–33. [Google Scholar]
- 4.Chinese pharmacopeia committee, editor. Chinese Pharmacopeia. Chemical Industry Press; 2010. p. 187. [Google Scholar]
- 5.Fazilatun N, Nornisah M, Zhari I. Superoxide radical scavenging properties of extracts and flavonoids isolated from the leaves of Blumea balsamifera. Pharm Biol. 2005;43:15–20. [Google Scholar]
- 6.Galeotti F, Barile E, Curir P, Dolci M, Lanzotti V. Flavonoids from carnation (Dianthus caryophyllus) and their antifungal activity. Phytochem Lett. 2008;1:44–48. [Google Scholar]
- 7.Huang LM, Tang SH. Research on the origins and content of meridian tropism theory. J Trad Chin Med. 2009;50:680–682. [Google Scholar]
- 8.Jiang YQ, Zuo CX. Studies of chemical constituents from Tamarix Chinese Lour. Acta Pharmaceutica Sinica. 1988;23:749–755. [PubMed] [Google Scholar]
- 9.Klocke JA, Van Wagenen B, Balandrin MF. The ellagitannin geraniin and its hydrolysis products isolated as insect growth inhibitors from semi-arid land plants. Phytochem. 1986;25:85–91. [Google Scholar]
- 10.Nessa F, Ismail Z, Mohamed N, Haris MRHM. Free radical-scavenging activity of organic extracts and of pure flavonoids of Blumea balsamifera DC leaves. Food Chem. 2004;88:243–252. [Google Scholar]
- 11.Sultanova N, Makhmoor T, Abilov ZA, Parween Z, Omurkamzinova VB, Atta-ur-Rahman, Iqbal Choudhary M. Antioxidant and antimicrobial activities of Tamarix ramosissima. J Ethnopharmacol. 2001;78:201–205. doi: 10.1016/s0378-8741(01)00354-3. [DOI] [PubMed] [Google Scholar]
- 12.Tasdemir D, Kaiser M, Brun R, Yardley V, Schmidt TJ, Tosun F, Rüedi P. Antitrypanosomal and antileishmanial activities of flavonoids and their analogues: in vitro, in vivo, structure-activity relationship, and quantitative structure-activity relationship studies. Antimicrob Agents Chemother. 2006;50:1352–1364. doi: 10.1128/AAC.50.4.1352-1364.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Moltke LL, Weemhoff JL, Bedir E, Khan IA, Harmatz JS, Goldman P, Greenblatt DJ. Inhibition of human cytochromes P450 by components of Ginkgo biloba. J Pharm Pharmacol. 2004;56:1039–1044. doi: 10.1211/0022357044021. [DOI] [PubMed] [Google Scholar]
- 14.Wu HY. Arthritis treatment with Xiheliu. Jiangxi J Trad Chin Med. 1996;S2:94. [Google Scholar]
- 15.Xu SN. Investing modern research on meridian-reaching actions of traditinal Chinese medicinal herbs. Chin Pharmacol Bull. 2004;20:598–600. [Google Scholar]
- 16.Yang LL, Yen KY, Kiso Y, Hikino H. Antihepatotoxic actions of formosan plant drugs. J Ethnopharmacol. 1987;19:103–110. doi: 10.1016/0378-8741(87)90142-5. [DOI] [PubMed] [Google Scholar]
- 17.Yannai S, Day AJ, Williamson G, Rhodes MJ. Characterization of flavonoids as monofunctional or bifunctional inducers of quinone reductase in murine hepatoma cell lines. Food Chem Toxicol. 1998;36:623–630. doi: 10.1016/s0278-6915(98)00022-2. [DOI] [PubMed] [Google Scholar]
- 18.Zhang XY, Ling LQ, Wang HK. Studies of chemical constituents from Xiheliu. Chin Trad Herbal Drug. 1991;22:299–300. [Google Scholar]
- 19.Zhang YL, Wang Y, Qiao YJ. The Material Basis of TCM Properties Based on Pharmacophore. World Sci Technol: Modern Trad Chin Med Materia Med. 2009;11:735–738. [Google Scholar]
- 20.Zhao RZ, Sun SY, Chen FK, Ba SF. Research on the pharmacological effect of Xiheliu. Chin Trad Herbal Drug. 1995;26:85. [Google Scholar]
- 21.Zhao Y, Li Y, Wang X, Sun WJ. The experimental study of Cortex Eucommiae on meridian tropsim: The distribution study of aucubin in rat tissues. J Pharm Biomed Anal. 2008;46:368–373. doi: 10.1016/j.jpba.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 22.Zhao ZJ, Hu HX, Zhang XX. Study on the Modernization of the Meridian Affinity Doctrine. J Beijing Univ Trad Chin Med. 2002;25:5–7. [Google Scholar]
- 23.Zhao ZJ, Wei C. The present research state and prospect of doctrine of channel distribution of Chinese medicine. China J Trad Chin Med Pharm. 2003;18:40–46. [Google Scholar]
- 24.Zhou J. New understanding of the basic theory of traditional Chinese medicine. Chin J Integr Med. 2009;15:7–12. doi: 10.1007/s11655-009-0007-y. [DOI] [PubMed] [Google Scholar]