Abstract
The regulation of energy intake is a complex process involving the integration of homeostatic signals and both internal and external sensory inputs. To better understand the neurobiology of this process and how it may be dysfunctional in obesity, this study examined activity of the brain’s “default network” in reduced-obese (RO) as compared to lean individuals. The default network is a group of functionally connected brain regions thought to play an important role in internally directed cognitive activity and the interplay between external and internal sensory processing. Functional magnetic resonance imaging was performed in 24 lean and 18 RO individuals in the fasted state after 2 days of eucaloric energy intake and after 2 days of 30% overfeeding in a counterbalanced design. Scanning was performed while subjects passively viewed images of food and nonfood objects. Independent component analysis was used to identify the default network component. In the eucaloric state, greater default network activity was observed in RO compared to lean individuals in the lateral inferior parietal and posterior cingulate cortices. Activity was positively correlated with appetite. Overfeeding resulted in increased default network activity in lean but not RO individuals. These findings suggest that the function of the default network, a major contributor to intrinsic neuronal activity, is altered in obesity and/or obese-prone individuals. Future studies of the network’s function and its relationship to other brain networks may improve our understanding of the mechanisms and treatment of obesity.
Introduction
Obesity is associated with multiple health problems, including diabetes, hypertension, cancer, and coronary artery disease, and as such is a serious and growing public health concern. The obese state results from a chronic positive energy balance, due in part to excessive energy intake relative to metabolic needs. The regulation of energy intake is dependent upon the integration of both internal factors, such as homeostatic signals and cognitive state, and external factors, including social context and the availability of food (1–4). The process by which the human brain integrates these signals to produce maladaptive eating behaviors in obese individuals is, however, largely unknown.
Functional neuroimaging, which allows in vivo measurement of neuronal responses, can be used to further our understanding of the neurobiology of food intake behavior. To date, imaging studies have identified complex networks of brain regions involved in food intake behavior, including the prefrontal cortex, orbitofrontal cortex, inferior temporal cortex, insula, striatum, amygdala, hippocampus, and hypothalamus (3,5–10). These networks are consistent with multiple cognitive processes, including sensory processing and interception, attention, motivation, and reward processing (11–13).
To date, however, relatively few studies have used functional imaging to directly investigate the neuronal processes that may be altered in obesity. Using positron emission tomography, satiety-related differences in regional cerebral blood flow in obese compared to lean individuals have been observed in the prefrontal, limbic, and insular cortices (14–17). Our previous functional magnetic resonance imaging study of the response to visual food cues found blunted responses in the insula and inferior visual cortex in reduced-obese (RO), relative to lean individuals (18). This study also found that downregulation of these responses in lean subjects by 2 days of overfeeding did not occur in RO subjects.
To improve our understanding of the neuronal mechanisms underlying obesity, it is useful to expand the examination of neuronal processes beyond those that evaluate responses specific to tasks thought to be important in intake behavior, such as the response to visual food cues. The “default network,” also known as the “default mode network,” is a functionally connected network of brain regions that includes the posterior cingulate cortex, cuneus/precuneus, medial prefrontal cortex, medial temporal lobe, and inferior parietal cortices (19). Activity in the default network is thought to reflect a baseline state of brain function, in which subjects are not focused on the external environment, but rather are focused on their internal mental state, which may include various forms of spontaneous cognition, self-reflective thought or attention to internal stimuli (19). Although first widely reported as network of brain regions that “disengages” or “deactivates” when people engage in goal-directed tasks, the default network now has been robustly detected both during the resting state and across a variety of cognitive tasks.
The default network is a major contributor to the intrinsic neuronal activity that accounts for 60–80% of the brain’s energy use (20). Considering that energy metabolism is significantly altered in obesity, understanding the potential differences in default network function between obese and lean individuals may yield valuable clues about neurobiological mechanisms of obesity. As such, the goals of this study were (i) to identify differences in default network between lean and RO individuals, and (ii) to examine the effect of overfeeding on default network activity in both groups. We also examined the relationship between default network and measures of appetite. Given our prior findings showing altered response to food as compared to nonfood objects and blunted changes in these responses to over-feeding in obesity, we hypothesized that elements of the default network would be altered in RO individuals, and also show less response to overfeeding, compared to lean individuals.
Methods and Procedures
Subjects
Lean (BMI 19–23 kg/m2) and overweight/obese (BMI 27–32 kg/m2) healthy, right-handed individuals aged 25–45 were recruited and screened. Lean subjects had no family history of obesity and were weight stable by self-report for >10 years. Eligible subjects were free of metabolic and psychiatric disease and eating disorders. Obese participants entered a weight loss program with a goal weight loss of 8–10% of their initial body weight. This was accomplished by on-going supervision and intervention by the University of Colorado Denver Clinical Translational Research Center research dieticians. The primary goal was weight loss and not a specific macronutrient composition or caloric prescription. Counseling was individualized in order to find the best plan for each participant. Once the weight loss was achieved, the RO subjects were maintained at this new reduced weight for 8 weeks prior to studies being performed. Individuals unable to achieve a minimum 5% weight loss and/or maintain their weight loss were excluded. Actual weight loss was 8.0 ± 0.9% (mean ± s.d.) of initial body weight. Twenty-four lean individuals (12 women, 12 men) and 18 RO individuals (10 women, 8 men) were studied (Table 1).
Table 1.
Subject characteristics (number or mean ± s.d.)
| Lean | Reduced-obese | |
|---|---|---|
| N (M/W) | 24 (12/12) | 18 (8/10) |
| Age (years) | 34.7 ± 5.4 | 35.2 ± 5.7 |
| BMI (kg/m2) | 21.6 ± 1.7 | 27.5 ± 2.6* |
| Body fat (%) | 19.8 ± 6.8 | 31.9 ± 7.0* |
| Premeal hunger | 77.8 ± 11.8 | 80.2 ± 13.8 |
| Premeal appetitea | 80.5 ± 12.3 | 77.8 ± 12.9 |
| Postmeal satiety | 73.5 ± 19.9 | 77.1 ± 11.5 |
M, men; W, women.
Appetite: prospective food consumption.
P < 0.05 for lean compared to reduced-obese.
Study design
Subjects were studied on two occasions (eucaloric and overfed) in a randomized crossover design. In women, study periods were performed in the follicular phase of their menstrual cycle. At least 1 month separated the two diet conditions. Each study period included a 3-day run-in diet phase and a 2-day “controlled” diet phase. The run-in diet phase was to ensure energy and macronutrient balance. Estimates of daily energy needs were made using several factors: (i) usual intake via 3-day food diary, (ii) the Harris–Benedict equation, (iii) baseline measurements of resting metabolic rate by hood indirect calorimetry (2900 metabolic cart; Sensormedics, Yorba Linda, CA) plus an activity factor, and (iv) lean body mass. On one occasion (eucaloric), subjects were maintained on the eucaloric diet for 2 more days. On another occasion, subjects were overfed by 30% above eucaloric needs (overfeeding) for 2 days. The macronutrient composition of both diets was 50% carbohydrate, 30% fat, and 20% protein. Additional details of the study design have been published previously (13).
Functional magnetic resonance imaging
Imaging was performed the morning after the second day of the controlled diet in the overnight fasted state. Imaging studies were performed using a GE 3.0 T MR scanner. Prior to functional imaging, a high-resolution, T1-weighted 3D anatomical scan was acquired for each subject. Functional images were then acquired with an echo-planar (single- shot) gradient-echo T2* blood oxygenation level dependant imaging contrast technique, with TR = 2000 ms, TE = 30 ms, 642 matrix, 240 mm2 FOV, 28 axial slices angled parallel to the planum sphenoidale, 4 mm thick, 0 mm gap. Additionally, one inversion-recovery echo-planar- image (TI = 505 ms) volume was acquired to improve coregistration between the echo-planar images and gray matter templates used in preprocessing. Head motion was minimized with a VacFix head-conforming vacuum cushion (Par Scientific A/S, Odense, Denmark).
Functional imaging was performed while the subjects were presented visual stimuli using a projector and screen system. Visual stimuli consisted of three different categories: neutral nonfood objects (O), foods of high hedonic value (H), and foods of neutral hedonic or utilitarian value (U). Two 8-min runs were performed with each run consisting of a pseudo randomized block design with eight blocks of pictures of H, eight blocks of U, and eight blocks of O. Each block consisted of 10 stimuli shown for 2 s each for a total of 20 s per block or 240 scans per run. Subjects were asked lie quietly and to view the images. Results and additional methods from this functional magnetic resonance imaging task have been described previously (13). For the default network analysis described here, data were collapsed across all task conditions (i.e., all 480 scans were used for the independent component analysis described below).
Behavioral measurements
Before and after each meal during the controlled diet, subjects rated their hunger, fullness, and prospective food consumption (appetite) on visual analogue scales as described by Rolls (21). Hunger was rated on a 100-mm line preceded by the question, “How hungry do you feel right now?” and anchored by “not at all hungry” and “extremely hungry” on the right. Fullness was rated by the question, “How full do you feel right now?” with the anchors “not at all” and “extremely.” Prospective consumption was rated using the question, “How much food do you think you could eat right now?” anchored by “nothing at all” and “a large amount.”
Functional magnetic resonance imaging data analysis
The first four image volumes from each run were excluded for saturation effects.
Data were analyzed using SPM5 (Wellcome Department of Imaging Neuroscience, London, UK). Functional data from each subject were realigned to the first volume. The realigned images were then normalized to Montreal Neurological Institute space using the unified segmentation algorithm (22) (default parameters) on the coregistered inversion-recovery echo-planar image and applying the resultant estimated linear and nonlinear warp parameters to the echo-planar imaging data. The resliced, normalized data had a 3 × 3 × 3 mm voxel size. Data were then smoothed with a 6 mm FWHM Gaussian kernel. After accounting for reslicing during preprocessing steps, the final smoothness of the data was approximately three times the acquisition voxel size. Data from two additional subjects, one lean and one RO, were excluded do to excess head motion (>1.5 mm) during scanning.
Group independent component analyses was conducted using the GIFT toolbox, (http://icatb.sourceforge.net) (23). Data were processed separately for the lean and RO groups. For each group, the dimensionality of the data from each subject was reduced using principle component analysis, whereby the more than 99% of the variance in the data was represented by a reduced number of data points to minimize the computational load (23). Data were then concatenated into an aggregate data set. A modified minimum description length algorithm (24) was used to determine the number of spatially independent sources, estimated to be 28 for the lean group and 32 for the RO group. The independent sources were then estimated with an independent component analysis using the infomax algorithm (25). Individual subject independent component analysis data sets were then back reconstructed. The default network component was identified by selecting the component with the highest spatial correlation to a default network mask (26). The mask included the lateral posterior parietal cortex, precuneus, posterior cingulate cortex, frontal pole, and occipitotemporal junction, as defined anatomically from the WFU Pickatlas (http://www.fmri.wfubmc.edu). The anatomical mask was smoothed with a 6 mm FWHM kernel to match the functional data. For both groups, only one component significantly correlated with the mask (r = 0.54, P = 0.007 for the lean group, r = 0.51, P = 0.03 for the RO group). For each subject, default mode components from each run of the task were then converted to z values and averaged to produce one default mode component which was then entered into second-level analyses. The term “activity” is used in this manuscript to reflect the signal strength or amplitude of the component of interest, identified by independent component analysis and template matching.
Responses were examined in two additional resting state networks to determine if effects observed in the default network were specific to that network. To examine activity in the posterior visual resting state network, an anatomically based template for component extraction was created as described above. The template included the lingual and middle occipital gyri that have been shown to be the dominant contributors to this network (27). The motor-sensory resting state network also was examined. The template used to identify this component included the cingulate gyri, precentral gryi, superior temporal gyri, thalamus and hippocampus, identified in De Luca et al. as the primary nodes of the network.
Component maps were evaluated across the entire brain on a voxel-wise basis with second-level parametric models in SPM5. The comparison of lean to RO subjects in the eucaloric state was performed with a t-test. Comparisons across both group and diet condition were evaluated with directional contrasts (SPM t-contrasts) in the context of a 2 × 2 repeated measures ANOVA. All imaging results were considered significant at a whole-brain level if they exceeded a voxel-wise threshold of P < 0.01, corrected for multiple comparisons using the false discovery rate technique (28).
Results
Eucaloric state
Figure 1a shows default network activity in both lean and RO individuals in the eucaloric state. In both groups, the network was observed in the posterior cingulate/retrosplenial cortex, lateral inferior parietal cortex, and medial prefrontal cortex, consistent with the known major nodes of the network (19). The primary finding of this study is that relative to lean individuals, RO individuals showed greater activity in posterior aspects of the default network (Figure 1b) in the eucaloric state. The differences were centered in the posterior cingulate gyrus (t = 4.0, P < 0.01, corrected; x = −6, y = −42, z = 33) and the left lateral inferior parietal cortex (t = 4.9, P < 0.008, corrected, x = −54, y = −66, z = 39). No differences were observed showing greater activity in lean compared to RO individuals. Also, no differences were observed in posterior visual or motor-sensory resting state networks.
Figure 1.
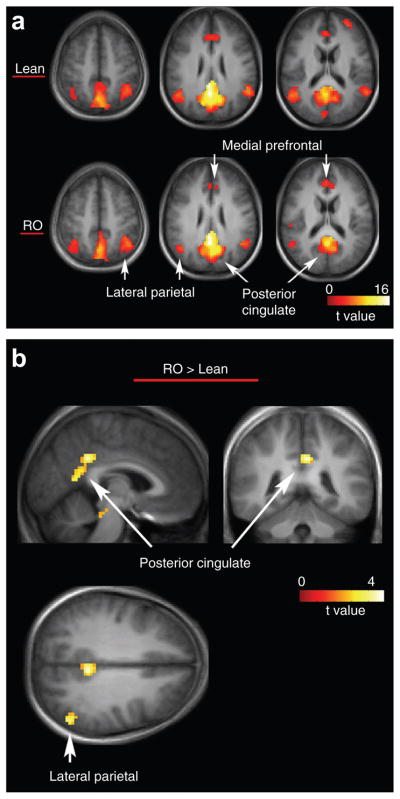
The default network in both lean and reduced-obese (RO) individuals and differences between the groups. (a) Default network activity in lean (top) and RO (bottom) individuals (b) greater network activity in reduced-obese, compared to lean individuals is shown. Statistical maps thresholded at P < 0.01 for visualization. Data are shown in the radiologic convention (R on L) on a group average anatomical image.
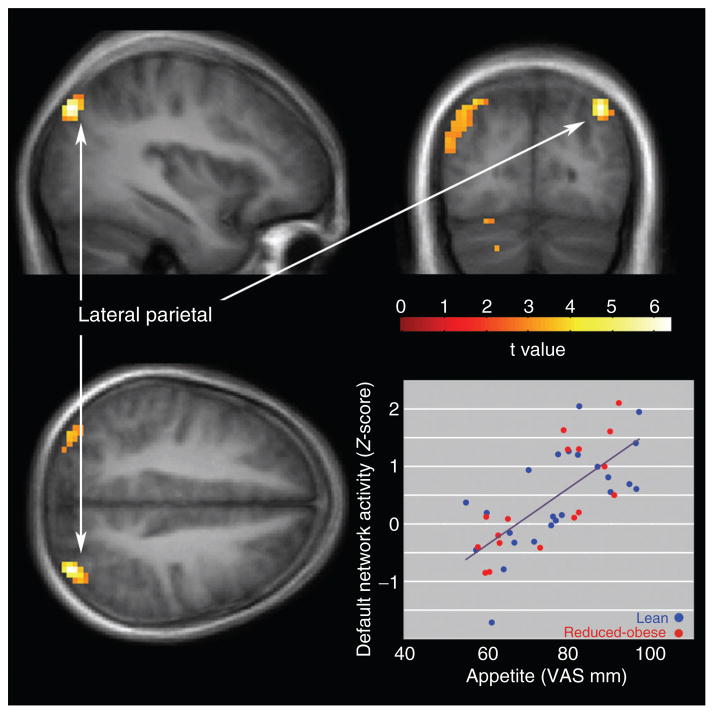
Across both lean and RO individuals, default network activity in the left parietal cortex was positively correlated with appetite (Figure 2) in the eucaloric state. The correlation was significant across all subjects (R2 = 0.51, t = 6.38, P < 0.003, corrected, x = −36, y = −78, z = 42). Significant correlations also were observed in the left lateral parietal cortex separately for both lean individuals (R2 = 0.44, t = 4.18, P < 0.001) and RO individuals (R2 = 0.62, t = 4.99, P < 0.001). No relationship between appetite measures and posterior cingulate default network activity was observed. Similarly, no significant correlations were observed between default network activity and ratings of hunger or satiety, or with measures of task-related activity reported previously (13).
Figure 2.
Positive correlation across all subjects between appetite and default network activity in the lateral parietal cortex. Statistical maps thresholded at P < 0.01 for visualization. Data are shown in the radiologic convention (R on L) on a group average anatomical image.
Eucaloric vs. overfed state
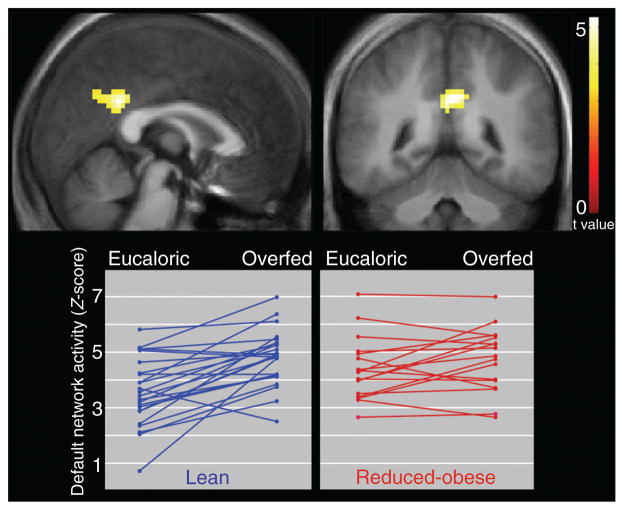
Compared to the eucaloric state, the overfed state was associated with greater default network posterior cingulate activity across all subjects (t = 3.94, P < 0.01, corrected, x = 0, y = −45, z = 27). This effect was driven entirely by the lean individuals (t = 5.25, P < 0.01, corrected, x = −6, y = −45, z = 33; Figure 3). A comparison of the overfed state only did not reveal any significant group differences between lean and RO individuals.
Figure 3.
Compared to the eucaloric state, overfeeding was associated with greater posterior cingulate default network activity in thin individuals. Statistical maps thresholded at P < 0.01 for visualization. Data are shown in the radiologic convention (R on L) on a group average anatomical image. Plots show individual data points.
Discussion
The present study was performed to examine the default mode network in lean individuals screened to be resistant to weight gain and obesity as compared to RO individuals, who are prone to weight gain/regain. The results of this study demonstrate that there is increased activity of the default network in RO as compared to lean individuals and that overfeeding results in increased activation of the network in lean individuals. In addition, default network activity in the parietal cortex is associated with measures of appetite.
The primary finding of this study was increased default network activity within the posterior cingulate and lateral inferior parietal cortices in RO, compared to lean individuals in the eucaloric state. While no other studies to date have examined the default network in relation to body weight or obesity, many prior findings have implicated the posterior cingulate and inferior parietal cortices during food intake–related behavior.
In lean individuals, the inferior parietal cortex has been shown to respond to the sight of food compared to nonfood objects (3,7,13,29–31) and to the taste of flavored liquids compared to water (7). Overfeeding leading to satiety has been found to either decrease (3,14) or increase (32) this response to visual food cues. In obese individuals, the inferior parietal cortex has been shown to respond to food pictures to a similar degree as lean individuals (18,31). In the context of food-related tasks, the function of the inferior parietal lobe is likely to be related to visual attention (33) and/or episodic memory retrieval (34). Within the default network, activity in the inferior parietal cortex has been suggested to be involved in the use of episodic memories to construct mental models or in decision making (19,35,36). In essence, this region is likely involved in cognitive processes in which past experiences in the form of episodic memory are drawn upon to think about similar present or future conditions.
Activation of the posterior cingulate cortex also has been observed in response to visual food cues (8,13,30,37) and to the taste (38,39) and smell of food (40). During attention tasks, the posterior cingulate plays a role in allocating neural resources based on the input from the limbic system and the motivational salience of visual targets, i.e., directing resources to pictures of food in hungry subjects (41). Within the default network, the posterior cingulate cortex is involved in the integration and coordination of the subsystems involved in self-referential thoughts and episodic memory (19,35). Thus, this region is thought to be a central hub in the default network, likely playing a major role in integrating information from other node components.
Given the likely involvement of the lateral inferior parietal and posterior cingulate nodes of the default network in self-referential thought, mental models and the integration of episodic memory, it is possible to speculate on the functional significance of over-activity of these regions in RO individuals in the baseline “eucaloric” state. One possible explanation is that compared to lean individuals, RO individuals under eucaloric conditions are more engaged in processing information about their internal states. Cognitive tasks that involve the processing of external visual, auditory or somatosensory information consistently have been shown to deactivate areas within the default network (42–45). Moreover, activity within the areas of the default network has been shown to negatively correlate with activity within task-specific regions during processing of external sensory information (43–46). As such, greater activation of the default network by RO individuals may reflect increased attention to their internal states such appetite or other processing of gut signals or food-related cognitive factors. The observed positive correlation between parietal default network activity and measured appetite across both lean and RO individuals supports this hypothesized relationship. These effects may have been especially pronounced in the present study because all subjects were scanned in the fasted state.
A possible divergence in functions of different areas of the default network was observed in the response to overfeeding, as compared to the eucaloric diet. While posterior cingulate activity was similar across diet conditions in RO individuals, this activity increased in lean individuals when overfed as compared to eucaloric conditions. In fact, in the overfed state, posterior cingulate activity was similar between lean and RO individuals. This pattern, a significant change in response in lean but not RO individuals after overfeeding, is identical but opposite in direction to our previous findings that food image–specific responses in the inferior visual cortex were attenuated after overfeeding in lean but not RO individuals (13) and is consistent with the frequently observed anticorrelation between task-related responses and default network activity (46). It is possible that posterior cingulate default network activity is elevated in lean individuals after overfeeding due to responsive sensing of alterations in energy balance, leading to a shift in the balance between external and internal processing, a process that may be altered in RO individuals. Another view is that RO individuals may exhibit a reduced dynamic range or inflexibility of the posterior cingulate to external vs. internal awareness, compared with lean individuals.
In summary, this study is the first to examine differences in the brain’s default network in obesity. In the eucaloric state, greater activity in RO, compared to lean individuals was observed in the lateral inferior parietal and posterior cingulate cortices. Lateral parietal activity correlated positively with appetite. Upon overfeeding, posterior cingulate default network activity increased in lean but not RO individuals. As such, activity in lean individuals in the overfed state was similar to activity in RO individuals across both the eucaloric and overfed state. These findings suggest that function of the default network is altered in obesity and/or obese-prone individuals. Future studies of the network’s function and its relationship to other brain networks may improve our understanding of the mechanisms and treatment of obesity.
Acknowledgments
This study was supported by NIH/NCRR Colorado CTSI Grant Number UL1 RR025780, NIH/NIDDK Clinical Nutrition Research Unit Grant Number DK48520, and NIH/NCRR Grant Numbers RR016185, R01DK072174 and R01DK089095.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
References
- 1.Baskin DG, Figlewicz Lattemann D, Seeley RJ, et al. Insulin and leptin: dual adiposity signals to the brain for the regulation of food intake and body weight. Brain Res. 1999;848:114–123. doi: 10.1016/s0006-8993(99)01974-5. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz MW. Central nervous system regulation of food intake. Obesity (Silver Spring) 2006;14 (Suppl 1):1S–8S. doi: 10.1038/oby.2006.275. [DOI] [PubMed] [Google Scholar]
- 3.Cornier MA, Von Kaenel SS, Bessesen DH, Tregellas JR. Effects of overfeeding on the neuronal response to visual food cues. Am J Clin Nutr. 2007;86:965–971. doi: 10.1093/ajcn/86.4.965. [DOI] [PubMed] [Google Scholar]
- 4.Berthoud HR. Neural control of appetite: cross-talk between homeostatic and non-homeostatic systems. Appetite. 2004;43:315–317. doi: 10.1016/j.appet.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Porubská K, Veit R, Preissl H, Fritsche A, Birbaumer N. Subjective feeling of appetite modulates brain activity: an fMRI study. Neuroimage. 2006;32:1273–1280. doi: 10.1016/j.neuroimage.2006.04.216. [DOI] [PubMed] [Google Scholar]
- 6.Simmons WK, Martin A, Barsalou LW. Pictures of appetizing foods activate gustatory cortices for taste and reward. Cereb Cortex. 2005;15:1602–1608. doi: 10.1093/cercor/bhi038. [DOI] [PubMed] [Google Scholar]
- 7.Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC. Cerebral processing of food-related stimuli: effects of fasting and gender. Behav Brain Res. 2006;169:111–119. doi: 10.1016/j.bbr.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Killgore WD, Young AD, Femia LA, et al. Cortical and limbic activation during viewing of high-versus low-calorie foods. Neuroimage. 2003;19:1381–1394. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- 9.St-Onge MP, Sy M, Heymsfield SB, Hirsch J. Human cortical specialization for food: a functional magnetic resonance imaging investigation. J Nutr. 2005;135:1014–1018. doi: 10.1093/jn/135.5.1014. [DOI] [PubMed] [Google Scholar]
- 10.Beaver JD, Lawrence AD, van Ditzhuijzen J, et al. Individual differences in reward drive predict neural responses to images of food. J Neurosci. 2006;26:5160–5166. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schur EA, Kleinhans NM, Goldberg J, et al. Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. Int J Obes (Lond) 2009;33:653–661. doi: 10.1038/ijo.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watts AG. Understanding the neural control of ingestive behaviors: helping to separate cause from effect with dehydration-associated anorexia. Horm Behav. 2000;37:261–283. doi: 10.1006/hbeh.2000.1581. [DOI] [PubMed] [Google Scholar]
- 13.Cornier MA, Salzberg AK, Endly DC, et al. The effects of overfeeding on the neuronal response to visual food cues in thin and reduced-obese individuals. PLoS ONE. 2009;4:e6310. doi: 10.1371/journal.pone.0006310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gautier JF, Chen K, Salbe AD, et al. Differential brain responses to satiation in obese and lean men. Diabetes. 2000;49:838–846. doi: 10.2337/diabetes.49.5.838. [DOI] [PubMed] [Google Scholar]
- 15.Gautier JF, Del Parigi A, Chen K, et al. Effect of satiation on brain activity in obese and lean women. Obes Res. 2001;9:676–684. doi: 10.1038/oby.2001.92. [DOI] [PubMed] [Google Scholar]
- 16.DelParigi A, Chen K, Salbe AD, et al. Persistence of abnormal neural responses to a meal in postobese individuals. Int J Obes Relat Metab Disord. 2004;28:370–377. doi: 10.1038/sj.ijo.0802558. [DOI] [PubMed] [Google Scholar]
- 17.DelParigi A, Chen K, Salbe AD, et al. Successful dieters have increased neural activity in cortical areas involved in the control of behavior. Int J Obes (Lond) 2007;31:440–448. doi: 10.1038/sj.ijo.0803431. [DOI] [PubMed] [Google Scholar]
- 18.Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Tregellas JR. Sex-based differences in the behavioral and neuronal responses to food. Physiol Behav. 2010;99:538–543. doi: 10.1016/j.physbeh.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 20.Raichle M. A paradigm shift in functional brain imaging. J Neurosci. 2009;29:12729. doi: 10.1523/JNEUROSCI.4366-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rolls BJ. Carbohydrates, fats, and satiety. Am J Clin Nutr. 1995;61:960S–967S. doi: 10.1093/ajcn/61.4.960S. [DOI] [PubMed] [Google Scholar]
- 22.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 23.Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li YO, Adali T, Calhoun VD. Estimating the number of independent components for functional magnetic resonance imaging data. Hum Brain Mapp. 2007;28:1251–1266. doi: 10.1002/hbm.20359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7:1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- 26.Garrity AG, Pearlson GD, McKiernan K, et al. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 27.De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage. 2006;29:1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 28.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 29.Wang GJ, Volkow ND, Telang F, et al. Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage. 2004;21:1790–1797. doi: 10.1016/j.neuroimage.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 30.Führer D, Zysset S, Stumvoll M. Brain activity in hunger and satiety: an exploratory visually stimulated FMRI study. Obesity (Silver Spring) 2008;16:945–950. doi: 10.1038/oby.2008.33. [DOI] [PubMed] [Google Scholar]
- 31.McCaffery JM, Haley AP, Sweet LH, et al. Differential functional magnetic resonance imaging response to food pictures in successful weight-loss maintainers relative to normal-weight and obese controls. Am J Clin Nutr. 2009;90:928–934. doi: 10.3945/ajcn.2009.27924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tataranni PA, Gautier JF, Chen K, et al. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci USA. 1999;96:4569–4574. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nachev P, Husain M. Disorders of visual attention and the posterior parietal cortex. Cortex. 2006;42:766–773. doi: 10.1016/s0010-9452(08)70415-5. [DOI] [PubMed] [Google Scholar]
- 34.Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci (Regul Ed) 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage. 2008;42:1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 36.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siep N, Roefs A, Roebroeck A, et al. Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res. 2009;198:149–158. doi: 10.1016/j.bbr.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 38.Gautier JF, Chen K, Uecker A, et al. Regions of the human brain affected during a liquid-meal taste perception in the fasting state: a positron emission tomography study. Am J Clin Nutr. 1999;70:806–810. doi: 10.1093/ajcn/70.5.806. [DOI] [PubMed] [Google Scholar]
- 39.DelParigi A, Chen K, Salbe AD, Reiman EM, Tataranni PA. Sensory experience of food and obesity: a positron emission tomography study of the brain regions affected by tasting a liquid meal after a prolonged fast. Neuroimage. 2005;24:436–443. doi: 10.1016/j.neuroimage.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 40.Bragulat V, Dzemidzic M, Bruno C, et al. Food-related odor probes of brain reward circuits during hunger: a pilot FMRI study. Obesity (Silver Spring) 2010;18:1566–1571. doi: 10.1038/oby.2010.57. [DOI] [PubMed] [Google Scholar]
- 41.Mohanty A, Gitelman DR, Small DM, Mesulam MM. The spatial attention network interacts with limbic and monoaminergic systems to modulate motivation-induced attention shifts. Cereb Cortex. 2008;18:2604–2613. doi: 10.1093/cercor/bhn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Binder JR, Frost JA, Hammeke TA, et al. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- 43.McKiernan KA, D’Angelo BR, Kaufman JN, Binder JR. Interrupting the “stream of consciousness”: an fMRI investigation. Neuroimage. 2006;29:1185–1191. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greicius MD, Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cogn Neurosci. 2004;16:1484–1492. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- 45.Tian L, Jiang T, Liu Y, et al. The relationship within and between the extrinsic and intrinsic systems indicated by resting state correlational patterns of sensory cortices. Neuroimage. 2007;36:684–690. doi: 10.1016/j.neuroimage.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 46.Fox MD, Snyder AZ, Vincent JL, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]