Abstract
Drug-associated cues can elicit relapse to drug seeking after abstinence. Studies with extinction–reinstatement models implicate dopamine (DA) in the nucleus accumbens shell (NAshell) and dorsolateral caudate-putamen (dlCPu) in cocaine seeking. However, less is known about their roles in cue-induced opiate seeking after prolonged abstinence. Using a morphine self-administration and abstinence–relapse model, we explored the roles of NAshell and dlCPu DA and the D1/D2-like receptor mechanisms underlying morphine rewarding and/or seeking. Acquisition of morphine self-administration was examined following 6-Hydroxydopamine hydrobromide (6-OHDA) lesions of the NAshell and dlCPu. For morphine seeking, rats underwent 3 weeks’ morphine self-administration followed by 3 weeks’ abstinence from morphine and the training environment. Prior to testing, 6-OHDA, D1 antagonist SCH23390, or D2 antagonist eticlopride was locally injected; then rats were exposed to morphine-associated contextual and discrete cues. Results show that acquisition of morphine self-administration was inhibited by NAshell (not dlCPu) lesions, while morphine seeking was attenuated by lesions of either region, by D1 (not D2) receptor blockade in NAshell, or by blockade of either D1 or D2 receptors in dlCPu. These data indicate a critical role of dopaminergic transmission in the NAshell (via D1-like receptors) and dlCPu (via D1- and D2-like receptors) in morphine seeking after prolonged abstinence.
Keywords: Abstinence, D1-like, D2-like, dorsolateral caudate-putamen (dlCPu), morphine, nucleus accumbens shell (NAshell), seeking, self-administration
Introduction
Relapse to drug seeking is a major impediment in the treatment of drug addiction. Stimuli or cues associated with previous drug intake can provoke drug craving in addicts following abstinence (O’Brien, 2005; Sell et al., 2000), or drug-seeking behavior in animals after either an extinction training (Bossert et al., 2007, 2009; De Wit and Stewart, 1981) or a long period of abstinence (Gal and Gyertyan 2006).
Previous studies of cocaine seeking have focused on the dopaminergic (DAergic) system in the nucleus accumbens (NAc) and the dorsolateral striatum (dlCPu, dorsolateral caudate-putamen). Given that drug addiction is hypothesized as a transition from NAc-dependent goal-directed drug taking to dlCPu-dependent habitual drug seeking (Belin and Everitt, 2008; Everitt et al., 2008; Holmes and Clemens, 2011), it is not surprising that animal and human studies revealed differential roles for DA in NAc shell (NAshell) (Anderson et al., 2003, 2006; Schmidt and Pierce, 2006; Shalev et al., 2002) and that in dlCPu (Ito et al., 2002; Volkow et al., 2006) in cocaine seeking. Although the neural substrates for relapse of cocaine seeking is relatively well elucidated for conditioned cued reinstatement (See, 2002, 2005), the mechanism underlying opiate seeking remains poorly understood.
Unlike cocaine directly increasing DA levels by reuptake blockade or reverse transport (Jones et al., 1995), opiates (heroin and morphine) indirectly increase DA release in striatum by disinhibiting GABA interneurons in the ventral tegmental area (VTA) (Johnson and North, 1992). As a result, repeated opiate and cocaine administration differentially affects neuroplasticity. For example, the former decreases GABAergic synaptic transmission in VTA whereas the latter increases it (Liu et al., 2005; Madhavan et al., 2010). Thus, the roles of ventral and dorsal striatal DA in cue-induced opiate seeking might differ from that of cocaine (Badiani et al., 2011; Shalev et al., 2002). Existing studies on the roles of DA in the NAc and dlCPu in opiate reward and seeking show several discrepancies and gaps. For instance, extracellular DA in the NAshell is elevated by opiate self-administration and drug-paired cues (Bassareo et al., 2007; Lecca et al., 2007; Pontieri et al., 1995), whereas Gerrits and colleagues reported that NAc is not involved in the acquisition of heroin self-administration (Gerrits and Van Ree, 1996; Gerrits et al., 1994). Although recent evidence suggests that DA transmission via D1 receptors in the NAshell and dlCPu plays an important role in reinstatement induced by heroin-associated context (Bossert et al., 2007, 2009), the role of D2 receptors in these area requires further investigation. In addition, it is noteworthy that most evidence regarding the roles of NAshell and dlCPu in drug seeking derives from studies with the extinction–reinstatement model, whereas human addicts rarely undergo extinction training. Furthermore, few studies have compared the roles of the dorsal and ventral striatal DA system in opiate reward and seeking behaviors.
To address these issues, the present study examined the effect of lesions of the DAergic system in NAshell and dlCPu on reward learning in a morphine self-administration paradigm, as well as that on drug seeking induced by contextual and discrete cues after prolonged abstinence in a clinically relevant abstinence–relapse model (Gal and Gyertyan, 2006). Given that the literature suggests a more important role of NAshell DA than core DA in responding to opiate or associated cues (Bassareo et al., 2007; Lecca et al., 2007; Pontieri et al., 1995), we chose NAshell for comparison with dlCPu. Since DA transmission is mainly mediated via D1- and D2-like receptors (Edwards et al., 2007; Jackson and Westlind-Danielsson, 1994), whose cellular and behavioral effects can be dissociated (Liu and Weiss, 2002; Sun and Rebec, 2005; Tobin et al., 2009), the D1 receptor antagonist SCH23390, or the D2 receptor antagonist eticlopride were microinjected into either the NAshell or dlCPu to investigate the possibly dissociable roles of D1 and D2 in each site in morphine-seeking behavior.
Materials and methods
Animals
Adult male Sprague-Dawley rats weighing 250 g on arrival (Vital River Laboratory Animal Technology Co., LTD, Beijing, China) were housed individually in a temperature- and humidity-controlled vivarium with a reversed 12-h light/dark cycle (lights on at 7:00 P.M.) and ad libitum access to food and water, except during nose-poke training for food pellets. Each rat was handled 3 min per day for 7 days. All behavioral tests occurred during the dark phase. Experiments were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978) and the Regulations for the Administration of Affairs Concerning Experimental Animals (China, 1988), and all procedures were approved by the Ethics Committee of the Institute of Psychology, Chinese Academy of Sciences.
Drugs
Morphine hydrochloride was purchased from Northeast Pharmaceutical Group Co., LTD (Shenyang, China). Other agents, including 6-OHDA (6-hydroxydopamine hydrobromide), desipramine hydrochloride, SCH23390 (D1 receptor antagonist), and eticlopride (D2 receptor antagonist) were purchased from Sigma-Aldrich Co., LLC (St Louis, MO, USA).
Apparatus
The rats were trained and tested for morphine self-administration in standard operant chambers (Med Associates, Inc., St. Albans, VT, USA), which were placed in a light- and sound-attenuating cubicle. Each chamber contained two nose-poke holes (ENV-114M, Med Associates), located 5 cm above the grid floor, and an LED light as a cue. A house light (ENV-215M, Med Associates) was mounted on the opposite wall. Drug solution was delivered through polyethylene tubing, protected by a leash assembly (PHM-120, Med Associates), and suspended through the ceiling of the chamber from a fluid swivel (PHM-115, Med Associates). Drug was delivered by a 10 mL syringe in an infusion pump (PHM-100, Med Associates). Experimental sessions were controlled and recorded using MED PC Software IV (Med Associates).
Surgery
Rats’ body weight was restricted to 85–90% of the freely feeding weight, and trained to nose-poke for 45 mg food pellets (FR1, daily 1 h/session or 50 pellets). After obtaining 50 food pellets in three consecutive sessions, rats again received an unrestricted food access. Rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.; Sigma-Aldrich) before implantation with jugular catheters and intracranial guide cannula. Rats were implanted with chronically indwelling intravenous catheters similar to previous surgical procedures (Capriles et al., 2003; Hellemans et al., 2002). Briefly, a silicone catheter was inserted 35 mm into the right jugular vein and delicately anchored to the vein with silk suture. The other end of the catheter passed subcutaneously to exit into a 22-gauge connector (Plastics One, Roanoke, VA, USA) mounted on the skull (and covered with a plastic cap when not in use for drug infusions). Immediately after catheter surgery, animals were placed in a stereotaxic frame (Stoelting, Wood Dale, IL, USA) and implanted with stainless steel guide cannula (26 gauge, Plastics One) just dorsal to the following brain areas: dlCPu (+1.2, ±3.6, −3.2) and NAshell (+1.4, ±0.8, −5.5). Coordinates (in mm for anterior–posterior, medial–lateral, and dorsal–ventral) were based on a rat brain atlas (Paxinos and Watson, 1986). Cannulas were secured to the skull using jewelers’ screws and dental acrylic. After surgery, a stylet was inserted into the cannula to prevent blockage. The catheters were flushed daily with 0.2 mL saline–heparin (30 U/mL) solution to maintain patency. To prevent infection, rats were treated post-surgically with Timentin (20 mg in 0.2 mL) for 7 days. Patency of catheters was tested using an ultra-short-acting barbiturate, Brevital (methohexital sodium, 10 mg/mL, 2 mg/rat), when is necessary. Generally, a total loss of muscle tone within 3 s after a Brevital injection indicates the patency of a catheter. All rats were allowed to recover for at least 7 days. A total of nine rats in different groups did not maintain good catheter-patency for all 21 training sessions, and data from those rats were not used.
Intracranial microinjections
Rats received bilateral infusions of 6-OHDA (experiments 1 and 2) and SCH23390 or eticlopride (experiments 3 and 4). In experiments 1 and 2, rats were treated with 15 mg/kg desipramine (to protect noradrenergic terminals) before receiving 6-OHDA (free base, 8 μg in 1 μL ascorbic saline per side) or vehicle (0.9% ascorbic saline) at a rate of 0.5 μL/min in the NAshell or dlCPu, 7 days prior to morphine self-administration training (experiment 1) or morphine-seeking test (experiment 2). This type of 6-OHDA lesion provides a selective and circumscribed destruction of DA axons and terminals within the striatum (Debeir et al., 2005; Kirik et al., 1998). In experiments 3 and 4, D1 receptor antagonist SCH23390 (0.1or 1 μg in 0.5 μL per side), D2 receptor antagonist eticlopride (0.2 or 2 μg in 0.5 μL per side), or saline (0.5 μL), were locally injected into the NAshell (experiment 3) or dlCPu (experiment 4) 10 min prior to the drug-seeking test. Dosages and injection volume of these antagonists were based on our pilot studies as well as data reported from previous studies on drug reward and seeking (Anderson et al., 2003; Bachtell et al., 2005; Bossert et al., 2009; Sun and Rebec, 2005).
All drugs were injected by a 10 μL Hamilton syringe driven by a Microinjection pump (Cole Parmer, IITC, Life Sci. Instruments, CA, USA), via polyethylene tubing attached to a 30-G stainless steel needle extending 2.0 mm below the tip of an implanted guide cannula in the NAshell or dlCPu. The needle remained in place for 5 min to prevent reflux and to allow for drug diffusion. Microinjection sites were confirmed on 40 μm cresyl violet stained sections.
Behavioral procedures
Morphine self-administration training, abstinence and seeking
Rats self-administered morphine in 21 daily 3 h sessions. Morphine hydrochloride was dissolved in saline and infused in a volume of 0.1 mL over 5 s at a dose of 0.3 mg/kg (first 14 sessions) and 0.1 mg/kg (last 7 sessions) per infusion under a fixed-ratio 1 (FR1), 20 s timeout reinforcement schedule. According to previous work (Bossert et al., 2007, 2009), the active nose-poke responses and infusions would be increased significantly when drug dose decreased, to indicate a stable drug self-administration. Each session start was signaled by illumination of the house light. Nose-poking in the active hole resulted in an infusion of morphine and a presentation of a compound tone-light cue (5 s activation of the white stimulus light inside the active nose-poke hole and a tone generator, 2 kHz, 70 dB, 10 dB above ambient noise) for 5 s, followed by a 20 s timeout period. During infusion and timeout, the house light was off, and responding during the timeout period was recorded but had no programmed consequences. Responses in the inactive hole were counted but had no scheduled consequences during the full session.
After 21 self-administration sessions, all rats experienced forced abstinence for 3 weeks without extinction training as described previously (Gal and Gyertyan, 2006). Briefly, during the abstinence period, they were transported to another room, different from that for self-administration training, for 1 h every day. This procedure was designed to minimize the potential impact of dishabituation on morphine seeking in the subsequent tests.
On the 22nd day after the last self-administration session, a 1 h relapse test session similar to previous procedures (Gal and Gyertyan, 2006) was conducted in the self-administration chamber to assess the context and discrete cue-induced morphine seeking, under conditions identical to those of training except that the nose-poke responding was not reinforced by morphine. All cues previously associated with morphine infusions (contextual and infusion-contingent discrete tone–light cues, sound of the infusion pump, house-light, time out period) were present but no syringe was put in the infusion pump.
Locomotor activity
This test was designed to exclude possible effects of motor impairment caused by the 6-OHDA lesion or DA receptor antagonists treatment (Fuchs et al., 2007; See et al., 2007). It was measured in a novel environment after the final test of self-administration or morphine-seeking behavior. In experiments 1 and 2, 6-OHDA- and sham-treated rats were placed directly into a Plexiglas open-field apparatus (width × length × height, 45 × 45 × 45 cm). In experiments 3 and 4, rats were infused with SCH23390 (1 μg/side), eticlopride (2 μg/side), or saline 10 min before testing. A ceiling-mounted video camera captured rats’ movements and relayed the data to a computer for subsequent software-based analysis of the time-dependent horizontal activity.
Histological examination
After completion of the behavioral tests, rats were anesthetized deeply with sodium pentobarbital (90 mg/kg, i.p.; Sigma-Aldrich) and perfused with 4% paraformaldehyde. Brains were removed, post-fixed overnight, then cryoprotected by immersion in 30% sucrose solution in 0.1 M phosphate buffer (PB) at 4°C until they sank. Serial coronal sections (40 μm) were cut on a freezing microtome.
To identify 6-OHDA lesion areas in rats in experiments 1 and 2, tyrosine hydroxylase (TH) immunohistochemistry and verification were performed as previously reported (Faure et al., 2005). Sections were collected in a 0.01 M phosphate-buffered saline (PBS), pH 7.4, and pretreated with 3% hydrogen peroxide for 10 min. After carefully washing in 0.01M PBS with 0.3% Triton X-100 (PBS-Tx), they were incubated in 5% normal goat serum in PBS-Tx for 30 min at room temperature, then in PBS-Tx containing the rabbit polyclonal TH (ab6211, 1:5000, Abcam Inc, Cambridge, MA, USA) antibody overnight at 4°C. After rinsing with PBS-Tx, sections were then incubated in secondary antibody, goat anti-rabbit IgG (pv6001, Zhongshan-Golden Bridge Biotech Co. Ltd., Beijing, China) at room temperature for 30 min. Tissues were washed in 0.1 M PBS for three times for 10 min before being developed with 0.06% 3,3-diaminobenzidine (DAB) for 5 min at room temperature. Sections were rinsed in PBS, 95% ethanol (2× for 2 min), and 100% ethanol (3× for 15 s) and were dehydrated in xylene (3× for 5 min). Slides were mounted and sealed with Permount for examination under a light microscope. Loss of DA innervation was measured with TH labeling of DA fibers as reported previously (Faure et al., 2005). Maximum and minimum sizes of lesions were reported on schematic sections of the atlas (Paxinos and Watson, 1986) (see Figure 1). 6-OHDA injected in NAshell led to dopaminergic deafferentation in the medial NAc (mNAc), including mainly the NAshell and part of the core.
Figure 1.
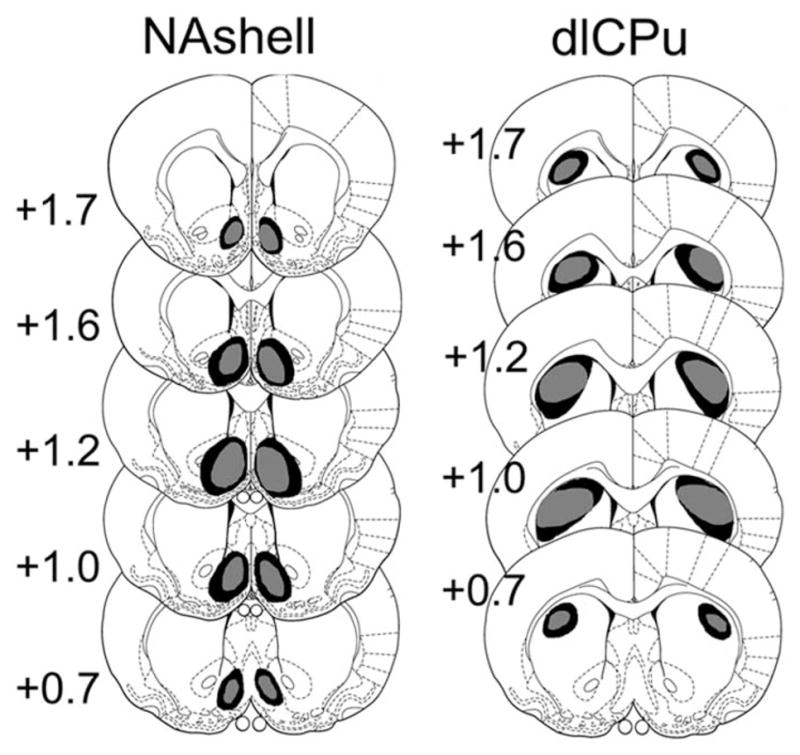
Coronal sections (adapted from Paxinos G and Watson C (1986) The Rat Brain in Stereotaxic Coordinates. 2nd ed. Academic Press with permission from Elsevier) representing the extent of dopaminergic deafferentation observed after bilateral infusions of 6-OHDA in various brain regions. TH immunoreactivity reveals a drastic loss of dopaminergic processes in NAshell and dlCPu. The histological reconstruction reveals the largest (Max; darker) and smallest (Min; lighter) lesion demarcation. Numbers to the left of the sections indicate anteroposterior (AP) distance from bregma in millimeters.
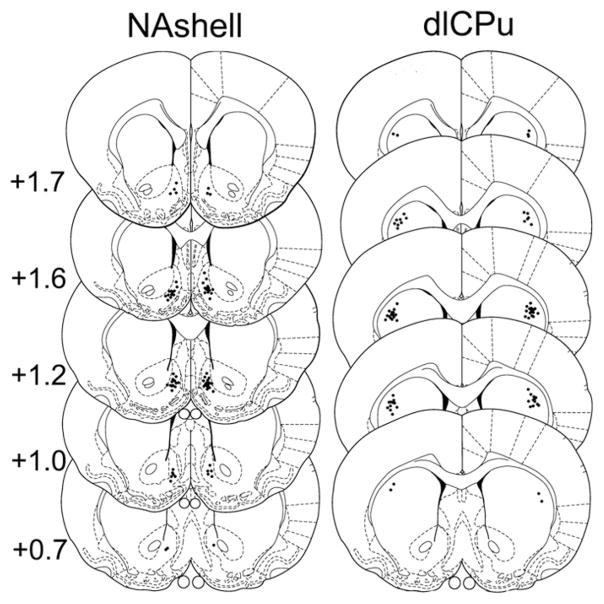
In experiments 3 and 4, tissue was stained with cresyl violet to confirm injection sites. Sections were thawed 10 min at room temperature and then placed in 0.5% cresyl violet (Sigma-Aldrich, St Louis, MO, USA) in distilled water for 20 min. All sections were rinsed with water, dehydrated with ethanol, cleared with xylene and sealed with Permount and coverslips, and examined under a light microscope. Only rats with correct injection sites were used for statistical analysis (Figure 2).
Figure 2.
Coronal sections (adapted from Paxinos G and Watson C (1986) The Rat Brain in Stereotaxic Coordinates. 2nd ed. Academic Press with permission from Elsevier) with graphical illustration of cannula tip placements in the various brain regions. Numbers to the left of the sections indicate anteroposterior (AP) distance from bregma in millimeters
Statistical analysis
Data are represented as mean ± S.E.M. and were analyzed with the statistical program SigmaStat v 3.5 (Systat Software Inc., San Jose, CA, USA). Comparisons were considered statistically significant at p<0.05. A two-way repeated measure analysis of variance (ANOVA) was performed for morphine self-administration (active or inactive nose-pokes, infusions) and locomotor activity. The within-subject factor was ‘test sessions’ or ‘time courses’, while the between-subject factor was ‘treatment’ (‘6-OHDA’ versus ‘sham’, ‘antagonist’ versus ‘saline’). Post-hoc Tukey test followed ANOVA when appropriate. For morphine seeking, nose-poke data (active or inactive) were analyzed separately by Student’s t-test (‘6-OHDA’ versus ‘sham’) or one-way ANOVA between ‘treatment’ (‘antagonist’ versus ‘saline’), with a Tukey post-hoc test to compare different treatment groups.
Results
Experiment 1, acquisition of morphine self-administration after 6-OHDA lesions in mNAc or dlCPu
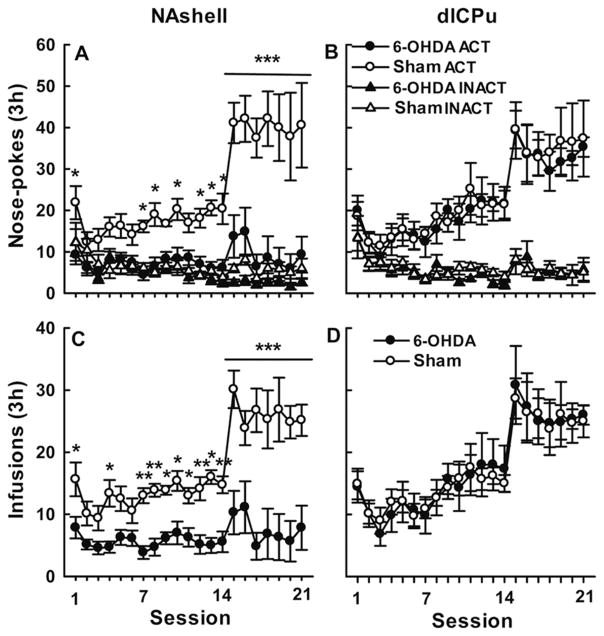
To investigate the effect of 6-OHDA on the acquisition of morphine self-administration, the rats were injected with 6-OHDA and saline into the mNAc (two groups; n=7–8) or dlCPu (two groups; n=7–9), seven days before self-administration training. Figure 3A depicts nose-pokes during the 3 h training sessions by rats with 6-OHDA and sham infusions in mNAc. Two-way repeated-measures ANOVA of active-site nose-pokes showed significant ‘group × session’ interaction [F(20,249)=3.856, p<0.001] and main effect of group and session [group, F(1,13)=45.342, p<0.001; session, F(20,249)=6.468, p<0.001]. A post-hoc test showed that the number of active nose-pokes was significantly reduced by mNAc 6-OHDA in sessions 1, 7, 8, 10, 12–14 (p<0.05), and 18–21 (p<0.001). When morphine dose was reduced from 0.3 to 0.1 mg/kg per infusion, rats receiving saline increased in number of active nose pokes (p<0.05), indicating a reliable morphine self-administration, but those given 6-OHDA did not.
Figure 3.
Effects of 6-OHDA lesions in NAshell or dlCPu on self-administration. Shown are nosepoke responses (ACT=active and INACT=inactive) and morphine infusions over 21 days of training (means ± S.E.M, 7–9 rats per group). Morphine doses were 0.3 mg/kg per infusion for days 1–14, and 0.1 mg/kg per infusion for days 15–21. Injections of 6-OHDA were made 7 days before training. Only lesions of the NAshell reduced active nose-poke responding and infusions (significantly different from sham infusion in respective sessions: *p<0.05, **p<0.01, ***p<0.001).
The same tests on dlCPu-treated rats (Figure 3B) revealed no significant interaction [F(20,262)=0.206, p>0.05] or group differences [F(1,14)=0.217, p>0.05], but only a significant session effect [F(20,262)=11.083, p<0.001]. Post-hoc test showed that reducing the morphine dose for the dlCPu-treated rats caused an increase in number of active nose-pokes in both sham and 6-OHDA groups (p<0.05), with no significant group differences in any session (all p>0.05).
A similar pattern appeared with regard to morphine infusions (Figure 3C and 3D). Again, there was a significant ‘group × session’ interaction in mNAc-treated rats [F(20,249)=3.706, p<0.001] but not in dlCPu treated rats [F(20,262)=0.160, p>0.05]. Main effects of group and session were significant in mNAc rats [group, F(1,13)=39.644, p<0.001; session, F(20,249)=7.218, p<0.001] but dlCPu-treated rats showed only a significant session effect [F(20,262)=14.828, p<0.001]. Post-hoc analysis of infusion numbers showed that rats given mNAc 6-OHDA reduced their infusions overall compared with sham controls, and also failed to increase infusions when morphine dose was reduced (Figure 3C), indicating failure to acquire stable morphine self-administration. In contrast, rats given 6-OHDA in dlCPu did not differ from sham controls in any session.
These data suggest that destroying DAergic innervation by injecting 6-OHDA into the mNAc, but not dlCPu, impaired the acquisition of morphine self-administration behavior. The effect on self-administration did not result from the movement impairment, since the 6-OHDA and sham groups did not differ in locomotor activity (Figure 4) or in numbers of nose-pokes in the inactive hole [mNAc: F(1,13)=4.25, p>0.05; dlCPu: F(1,14)=0.322, p>0.05], as shown in Figure 3.
Figure 4.
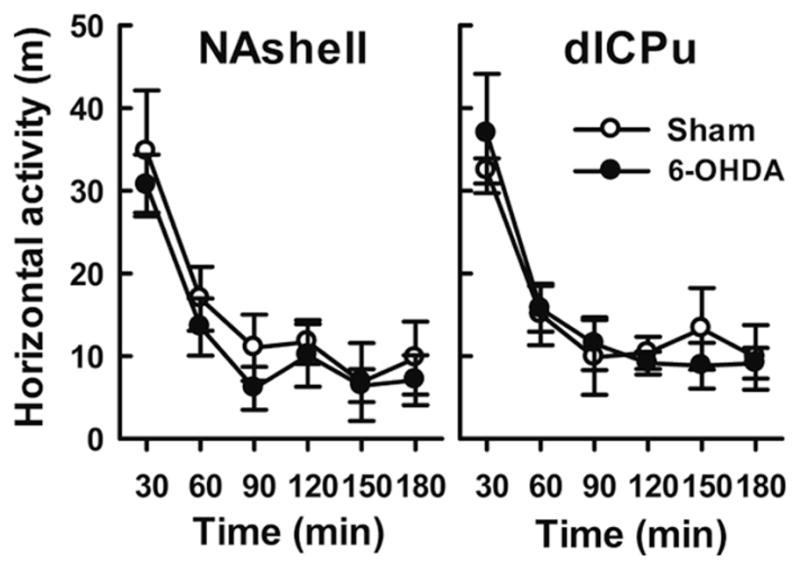
6-OHDA lesions of NAshell or dlCPu on locomotor in a novel environment. Locomotor activity is shown in horizontal distance traveled (m) (mean ± S.E.M) in 3 h for the rats treated with sham or 6-OHDA infusions in the NAshell (left panel) or dlCPu (right panel). No significant differences were seen between lesions versus sham control group.
Experiment 2, cue-induced morphine seeking after 6-OHDA lesions in the mNAc or dlCPu
Four new groups of rats undergoing morphine self-administration (7–8 per group) were matched for their active responses and morphine intake during training. At the end of training, the number of infusions per 3 h session averaged 52 ± 5.3. Number of active and inactive nose-pokes on the last day of training was 63 ± 6.4 and 6.8 ± 1.5, respectively. Two weeks later (7 days before a seeking test), saline or 6-OHDA was injected into the NAshell (two groups) or dlCPu (two groups). The sham-treated rats showed robust cue-induced drug-seeking behavior after 3 week forced morphine abstinence. This behavior, however, was attenuated in rats subjected to 6-OHDA lesions of the mNAc or dlCPu (Figure 5). Compared with sham controls, significantly fewer active nose-pokes were demonstrated by rats receiving 6-OHDA in the mNAc or dlCPu (both p<0.01). In contrast, ‘inactive responding’ was not affected by 6-OHDA injections in either locus (both p>0.05). Further, all rats could discriminate between the active and inactive nose-pokes and preferred to respond on the previously drug-paired hole (mNAc and dlCPu: p<0.001 in both 6-OHDA and sham group). In addition, locomotor activity did not differ between groups (Figure 6).
Figure 5.
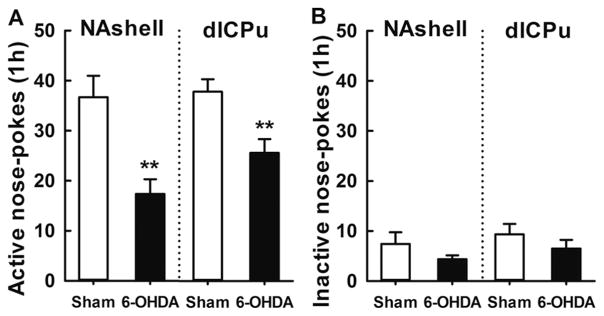
Effect of 6-OHDA lesions in NAshell or dlCPu on cue-morphine seeking. Shown are the nose-poke responses (active and inactive) (mean ± S.E.M) during a morphine-seeking test administered 7 days after 6-OHDA treatment (6–9 per group). Lesions of the NAshell (left panel) and dlCPu (right panel) reduced active nose-poke responding on the test day (significantly different from sham group: **p<0.01). Inactive nose-pokes did not differ between 6-OHDA and sham group.
Figure 6.
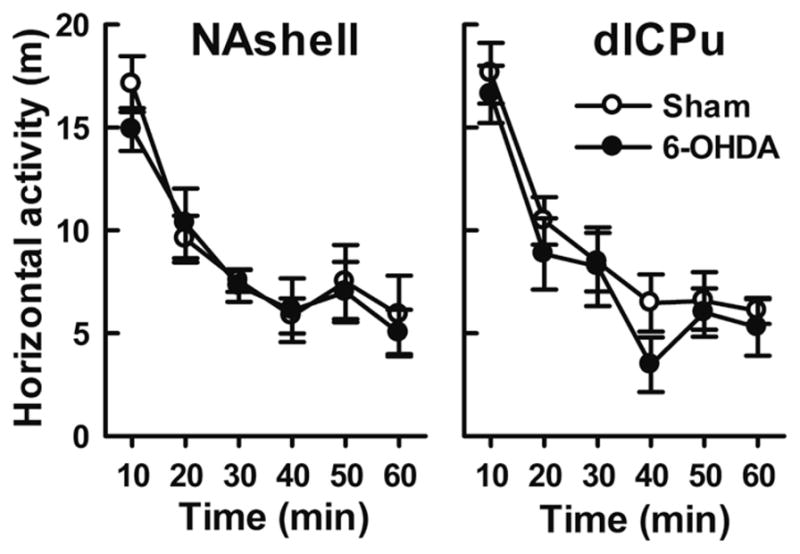
6-OHDA lesions of NAshell or dlCPu on locomotor activity novel environment. Locomotor activity is showed in horizontal distance traveled (m) (mean ± S.E.M) in 1 h for rats treated with sham or 6-OHDA infusions in the NAshell (left panel) or dlCPu (right panel). No significant differences were seen between lesions versus sham group.
Experiments 3 and 4, morphine seeking after blockade of D1- or D2-like receptors in NAshell and dlCPu
Since the lesions indicated that DAergic transmission in both the NAshell and dlCPu is involved in cue-induced morphine seeking, we performed further experiments to test the roles of D1 and D2 receptors. After abstinence for three weeks, five groups of rats (n=6–8) were received NAshell injections of the D1 antagonist SCH23390 (0.1 or 1 μg/side), the D2 antagonist eticlopride (0.2, or 2 μg/side), or saline. Another five groups (n=7–8) were used for equivalent experiments on dlCPu (all groups were matched for active responses and morphine intake during training).
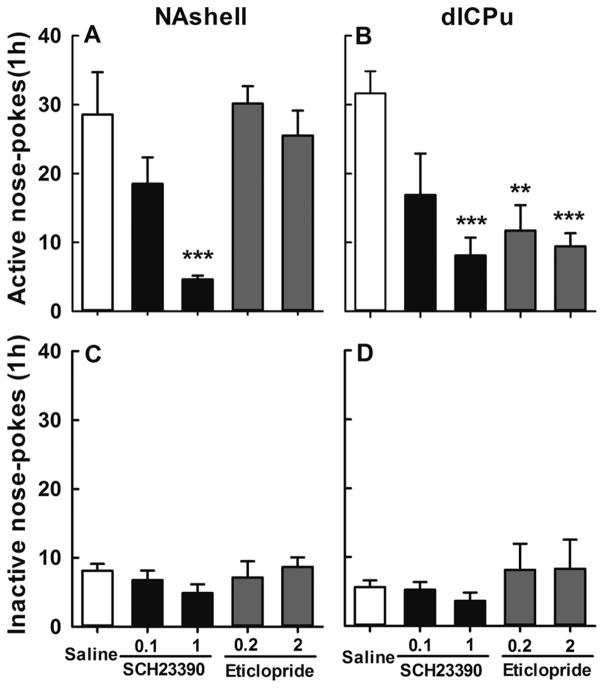
At the end of the training phase for the NAshell experiments, the number of infusions per 3 h session averaged 41 ± 3.5 overall, while the numbers of active and inactive nose-pokes were, respectively, 60 ± 4.2 and 4.6 ± 0.8. This robust seeking behavior was attenuated by pretreatment with D1 antagonist (Figure 7A). One-way ANOVA of active nose-pokes revealed a significant effect of SCH23390 dose [F(4,33)=7.58, p<0.001]. A Tukey post-hoc test showed that only high-dose treatment (1 μg/side) reduced the number of active responses (p<0.01). On the other hand, even a high dose of the D2 antagonist, eticlopride, failed to affect morphine seeking (p>0.05 for 0.2 or 2 μg/side).
Figure 7.
Effects of D1 and D2 receptor antagonism in NAshell dlCPu on cue-induced morphine seeking. Data (mean ± S.E.M) represent active and inactive nose-poke responses during a morphine-seeking test. SCH23390 (0.1, 1 μg/side) or eticlopride (0.2, 2 μg/side) was injected into NAshell or dlCPu 10 min beforehand (6–9 per group with equal levels of active nose-pokes and drug infusions during training). SCH23390 in NAshell (left panel), and SCH23390 or Eticlopride in dlCPu (right panel) dose-dependently reduced active nose-poke responding on the test day (significantly different from sham infusion, **p<0.01, ***p<0.01). Inactive nose-pokes did not differ among groups.
Additional experiments were carried out with a fresh set of rats to examine effects of DA receptor antagonists in dlCPu. These experiments yielded similar results as that in NAshell except that, strikingly, D2 blockade in the dorsal striatal region proved as effective as D1 blockade, or even more so. Again, rats (n=6–8) were matched for responses and infusions at the end of self-administration training (38.2 ± 3.0 infusions, 53 ± 4.2 active responses, and 7.1 ±1.6 inactive responses). Robust drug-seeking behavior was again present after the 3 week abstinence, but both antagonists attenuated this behavior (Figure 7B). One-way ANOVA of active nose-pokes revealed significant effects of DA antagonist and dose [F(4,31)=4.870, p<0.01]. Post-hoc analysis by Tukey test showed that both SCH23390 (1 μg/side) and eticlopride (0.2 and 2 μg/side) decreased the number of active nose-poke responses significantly below the saline control level (p<0.01). The lower doses of SCH23390, however, did not significantly affect morphine seeking (p>0.05).
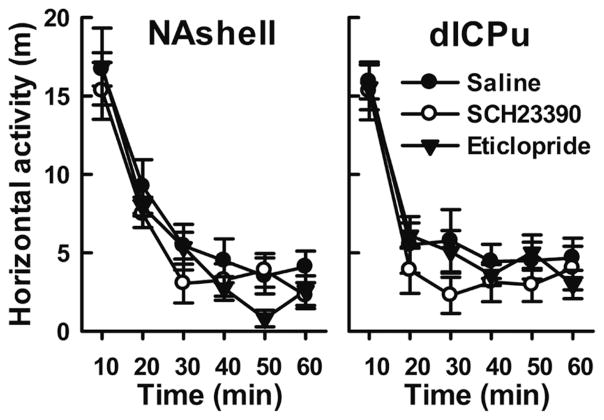
In contrast with morphine-seeking behavior, inactive responding was not affected by either dose of either antagonist at either of the tested loci (Figure 7C and D, all p>0.05). Thus, the rats were clearly able to discriminate between nose-pokes and preferred to respond on the previously drug-paired active nose-poke. Furthermore, none of them displayed non-specific effects such as reduced locomotor activity (Figure 8). Taken together the results indicate that cue-induced morphine-seeking behavior was selectively reduced in a dose-dependent manner by D1 but not D2 receptor antagonism in NAshell, and by both types of antagonists in dlCPu.
Figure 8.
Effect of infusing DA antagonist into NAshell or dlCPu on locomotor activity in a novel environment. Locomotor activity is shown in horizontal distance traveled (m) after bilateral injection of saline or DA antagonist (SCH23390, 1 μg/side; eticlopride, 2 μg/side) in the NAshell (left panel) or the dlCPu (right panel). No significant differences were seen between the drug and saline control groups.
Discussion
We found that cue-induced morphine seeking was attenuated by DAergic denervation of the medial NAc (mNAc) and dlCPu with 6-OHDA, by blockade of D1-like receptors with SCH23390 in the NAshell, and by blockade of D1- and D2-like receptors with SCH23390 and eticlopride, respectively, in the dlCPu. This finding indicates a critical role of DAergic transmission in the NAshell (via D1 receptors) and dlCPu (via D1 and D2 receptors) in opiate seeking after prolonged abstinence. Interestingly, blockade of D2 receptors in the NAshell and that in the dlCPu differentially affected opiate seeking, with D2 being important in the dlCPu but not in the NAshell. The injection volume and dosage of DA antagonist in the NAshell and dlCPu were chosen based on previous studies (Anderson et al., 2003, 2006; Bachtell et al., 2005; Bossert et al., 2009; Laviolette et al., 2008) that proved this treatment not to affect the adjacent regions. Therefore, it is less likely that the results here were induced by SCH23390 or eticlopride diffused to NAc core dorsomedial or medial-lateral caudate putamen.
DA transmission is crucial for opiate-seeking behavior induced by drug-priming and stress. For example, heroin-priming-induced reinstatement can be mimicked by the DA reuptake inhibitor GBR-12909 or D2 receptor agonist quinpirole and bromocriptine; meanwhile, priming- or foot-shock-induced reinstatement can be attenuated by systematic antagonism of the D1, D2, or mixed receptors (De Vries et al., 1999, 2002; Ettenberg et al., 1996; Shaham and Stewart, 1996; Wise et al., 1990). However, the roles of striatal DA or of its receptors in opiate seeking induced by drug-associated discrete or contextual cues have not been fully elucidated (Shalev et al., 2002). Here, our results indicate that DA in both the mNAc and dlCPu is critical for morphine seeking induced by mixed discrete and contextual cues after abstinence, extending the previous findings on DA mechanisms in priming- or stress-induced opiates seeking, as well as those on cue-induced cocaine seeking (for review see Shaham et al., 2003; Shalev et al., 2002). Although mNAc 6-OHDA lesions include part of the NAc core in the current study, the literature suggested that the NAshell DA is more important for cue-induced morphine seeking (Bassareo et al., 2007; Bossert et al., 2007; Ghitza et al., 2003). It is also unlikely that the motor deficits can account for the decreased seeking behavior (active nose-poke responding) observed following our treatment with 6-OHDA or DA D1/D2 antagonists, because this treatment in NAshell or dlCPu fails to affect locomotor activity in a novel environment and the inactive nose-poke responses in the seeking test. Further experiments training rats for a non-drug reinforcer (i.e. food self-administration) would verify the specificity of the experimental treatment on drug seeking, and will be used in our future studies.
Several lines of evidence have underscored the importance of dlCPu and DA in cue-induced cocaine seeking with reversible inactivation (See et al., 2007), in vivo micro-dialysis (Ito et al., 2002) or DA receptor antagonism (Vanderschuren et al., 2005). However, relevant studies for cue-induced opiate seeking have lagged behind. The present study revealed a critical role of dlCPu DA, and both D1- and D2-like receptors in cue-induced morphine seeking by DAergic lesions and receptor blockade, extending findings of a previous reversible inactivation study (Rogers et al., 2008) by elucidating the DA receptor mechanisms. These findings complement earlier studies implicating dlCPu D1-like receptors in context-induced heroin seeking in a reinstatement model (Bossert et al., 2009), suggesting that the dorsal striatal DA (via D1 and D2 receptor) is important for cue-induced opiate relapse in the abstinence–relapse model. Therefore, dlCPu DA may be involved in opiates and psycho-stimulants relapse behavior.
In addition, our data implicate ventral striatal DA and D1 receptors in cue-induced morphine seeking. This is consistent with findings in priming-induced morphine seeking (Wang et al., 2003), context-induced heroin seeking (Bossert et al., 2007) and discrete cue-induced cocaine seeking (Alleweireldt et al., 2002) derived from extinction–reinstatement studies. Although reinstatement studies on cocaine seeking suggest an important role of the NAc core relative to the shell (Bachtell et al., 2005; Fuchs et al., 2004, 2008b; Owesson-White et al., 2009; Suto et al., 2009), NAshell may be more crucial for cue-induced opiate seeking, which is supported by reports that morphine-paired conditioned cues elicit incentive reactions, DA release, and Fos expression in the NAshell but not in the core (Bassareo et al., 2007; Harris and Aston-Jones, 2003).
However, several cocaine-seeking studies suggested that NAshell might not participate in regulation of cue-induced seeking behaviors. Inactivation of NAshell did not affect cue-induced cocaine seeking (See et al., 2007); exposure of drug-associated cues did not affect DA release in the NAshell (Ito et al., 2000; Neisewander et al., 1996). Three explanations may account for this discrepancy. Firstly, DA antagonism and reversible inactivation in the same brain areas possibly have different effects on drug seeking (Anderson et al., 2003; Mcfarland and Kalivas, 2001). Secondly, our training conditions (3 weeks of self-administration, 3 h/session) may have been too brief for the morphine-seeking behavior to shift fully from NAc-dependent ‘goal directed’ to dlCPu-dependent ‘habitual seeking’ (Canales, 2005), because the dorsal striatum became progressively more engaged in drug seeking as the drug experience increased (Porrino et al., 2004; Zapata et al., 2010). Nevertheless, further studies are necessary to elucidate the roles of ventral and dorsal striatal pathways in opiate seeking after self-administration training with short and long access to drug. Furthermore, the neural mechanisms for opiate and cocaine seeking may differ (Badiani et al., 2011). For example, in rats, exposure to cocaine and morphine inversely affect the dendritic branching and spine density in the NAc (Robinson and Kolb, 2004) and the GABAergic synaptic transmission in the VTA (Liu et al., 2005; Madhavan et al., 2010). Such dissociable changes may contribute to a role of NAshell DA in morphine (not cocaine) seeking.
Unlike the observed effectiveness of solely D1 receptor in the NAshell, we found that both D1 and D2 receptors in the dlCPu were involved in morphine seeking. This provides evidence that dlCPu D2 receptors mediate conditioned opiate seeking, extending previous findings that D1 receptors in NAc and dlCPu mediate reinstatement of heroin seeking induced by contextual or discrete cues (Bossert et al., 2007, 2009) and that systematic blockade of D2-like receptors attenuates opiate seeking (De Vries et al., 2002; Wise et al., 1990). D1 and D2 receptors interact synergistically within the striatum to stimulate both forms of output and to control behaviors (Lahoste et al., 2000), and thus antagonizing one of the two kinds of receptors has been reported to attenuate the stimulation of the other (Charntikov et al., 2011; Waszczak et al., 2002). On the other hand, the D2 antagonist eticlopride may have enhanced glutamate release by antagonizing inhibitory receptors on corticostriatal terminals (Yamamoto and Davy, 1992). In any case, our results suggest that activation of D2-like DA receptors mediates mixed cues-induced morphine seeking in a region-specific manner within the mesocorticolimbic and nigrostriatal DA terminal regions.
In addition, we provide further support for accumbal DA regulating opiate incentive learning. Selective 6-OHDA lesions of mNAc DA terminals impaired the acquisition of stable morphine self-administration. Such effect is less likely attributable to 6-OHDA lesions that possibly expand to the core, because 6-OHDA lesions or DA receptor antagonism predominantly in the NAc core did not impair the acquisition of opiate self-administration or conditioned place preference (Fenu et al., 2006; Gerrits and Van Ree 1996; Gerrits et al., 1994) and opiate did not increase DA levels in NAc core (Lecca et al., 2007; Pontieri et al., 1995). Moreover, microdialysis data show that DA in the NAshell rather than the core is responsive to i.v. morphine and self-administered heroin (Lecca et al., 2007; Pontieri et al., 1995). Taken together, the current finding implies NAshell DA in mediating opiate incentive learning. Nevertheless, further studies should directly compare the exact role of DA in the NAshell versus that in the core in opiate reinforcement or seeking. Furthermore, a critical role for NAshell DA in opiate incentive learning is consistent with literature reporting that blocking or depleting NAc DA transmission impairs the acquisition of opiate-induced drug-seeking behaviors such as morphine- or heroin-induced conditioned place preference and heroin self-administration (Fenu et al., 2006; Singer and Wallace, 1984; Spyraki et al., 1983). Interestingly, local 6-OHDA lesions in NAc or systematic DA receptors antagonism reportedly have no effect on the maintenance of morphine and heroin self-administration after its acquisition (Dworkin et al., 1988; Ettenberg et al., 1982; Pettit et al., 1984). Therefore, NAc DA may be more important for the acquisition, rather than maintenance, of opiate self-administration, as hypothesized by Di Chiara and colleagues following their seminal studies on the effects of drugs of abuse on NAc shell DA (Di Chiara, 1998, 1999, 2002; Di Chiara et al., 1999, 2004). Although studies on acquisition of heroin self-administration reported some inconsistency (Gerrits and Van Ree, 1996; Gerrits et al., 1994), this discrepancy may mainly result from the injection cite centered to NAc core (Gerrits’ studies) versus shell (our study). Besides, the non-DAergic mechanisms mediating opiate reinforcement may also contribute to this (Xi and Stein, 2002). Heroin passes the blood–brain barrier more rapidly, and thus has likely more potent non-DA reinforcing effects than morphine (Oldendorf et al., 1972; Sawynok, 1986). Differences in behavioral procedures may also explain the discrepancy between Gerrits’ results and ours. That is, Gerrits’ studies included a test session prior to each self-administration session. Direct comparison of DAergic and non-DAergic mechanisms of opiate reward learning behaviors would deepen our understanding the acquisition of opiates addiction.
It is noteworthy that the present study utilized an abstinence–relapse model rather than the widely used extinction–reinstatement model (Fuchs et al., 2008a), which reportedly involves different neural substrates (Fuchs et al., 2006; Neisewander et al., 2000; Zavala et al., 2007). Although the latter is particularly useful for assessing incentive motivation of various drug-associated cues (Shaham et al., 2003), human drug users seldom, if ever, undergo such extinction training sessions in clinical settings. Therefore assessing drug seeking after a drug-free period in the abstinence model (Gal and Gyertyan, 2006) may better capture the neural mechanisms of cue-induced drug relapse in humans (Reichel and Bevins, 2009). It is necessary to fully explore and compare the mechanisms of cue-induced opiate seeking using the extinction–reinstatement and abstinence–relapse models in future studies.
In conclusion, the current study provides evidence for a critical role of the dorsal and ventral striatum while differentiating the roles of D1 and D2 receptors in relapse to opiate seeking after prolonged abstinence. Since drug seeking is mediated by multiple DA terminal regions including the frontal cortex, hippocampus, amygdala and ventral pallidum (Mclaughlin and See, 2003; Neisewander et al., 2000; Rogers et al., 2008), it remains to be determined how greatly DA transmission in these regions contributes to drug seeking in the abstinence model and in the reinstatement model. Further research will be necessary to investigate the relationship between the ventral and dorsal striatum and that of the two regions with other brain structures in relapse, in order to fully understand the mechanism of relapse. An immediate target may be the cortex that projects widely and diffusely across the striatum (Haber et al., 2006). Taking advantages of different animal models, we may develop behavioral and pharmacological interventions that alter dorsal or ventral striatal activity, which may ultimately lead to new ways of treating addiction to opiates and other drugs of abuse.
Acknowledgments
Funding
This work was supported by the National Basic Research Program of China (2009CB522002); Knowledge Innovation Program of Chinese Academy of Sciences (KSCX2-EW-R-12); National Natural Science Foundation Grants of China (31170988); and Chinese Academy of Sciences Visiting Professorships for Senior International Scientists Program (2010T1S13).
Footnotes
Reprints and permission: sagepub.co.uk/journalsPermissions.nav
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Alleweireldt AT, Weber SM, Kirschner KF, et al. Blockade or stimulation of D1 dopamine receptors attenuates cue reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2002;159:284–293. doi: 10.1007/s002130100904. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Bari AA, Pierce RC. Administration of the D1-like dopamine receptor antagonist Sch-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:132–138. doi: 10.1007/s00213-002-1298-5. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Schmidt HD, Pierce RC. Administration of the D2 dopamine receptor antagonist sulpiride into the shell, but not the core, of the nucleus accumbens attenuates cocaine priming-induced reinstatement of drug seeking. Neuropsychopharmacology. 2006;31:1452–1461. doi: 10.1038/sj.npp.1300922. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Whisler K, Karanian D, et al. Effects of intranucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacology (Berl) 2005;183:41–53. doi: 10.1007/s00213-005-0133-1. [DOI] [PubMed] [Google Scholar]
- Badiani A, Belin D, Epstein D, et al. Opiate versus psychostimulant addiction: the differences do matter. Nature Rev Neurosci. 2011;12:685–700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V, De Luca MA, Di Chiara G. Differential impact of Pavlovian drug conditioned stimuli on in vivo dopamine transmission in the rat accumbens shell and core and in the prefrontal cortex. Psychopharmacology (Berl) 2007;191:689–703. doi: 10.1007/s00213-006-0560-7. [DOI] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, et al. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Wihbey KA, Pickens CL, et al. Role of dopamine D(1)-family receptors in dorsolateral striatum in context-induced reinstatement of heroin seeking in rats. Psychopharmacology (Berl) 2009;206:51–60. doi: 10.1007/s00213-009-1580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canales JJ. Stimulant-induced adaptations in neostriatal matrix and striosome systems: transiting from instrumental responding to habitual behavior in drug addiction. Neurobiol Learn Mem. 2005;83:93–103. doi: 10.1016/j.nlm.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, et al. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- China State Council. Regulations for the administration of affairs concerning experimental animals. Beijing, China: The State Science and Technology Commission; 1988. [Google Scholar]
- Charntikov S, Der-Ghazarian T, Herbert MS, et al. Importance of D1 and D2 receptors in the dorsal caudate-putamen for the locomotor activity and stereotyped behaviors of preweanling rats. Neuroscience. 2011;183:121–133. doi: 10.1016/j.neuroscience.2011.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, et al. Dopaminergic mechanisms mediating the incentive to seek cocaine and heroin following long-term withdrawal of iv drug self-administration. Psychopharmacology (Berl) 1999;143:254–260. doi: 10.1007/s002130050944. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, et al. Relapse to cocaine- and heroin-seeking behavior mediated by dopamine D2 receptors is time-dependent and associated with behavioral sensitization. Neuropsychopharmacology. 2002;26:18–26. doi: 10.1016/S0893-133X(01)00293-7. [DOI] [PubMed] [Google Scholar]
- De Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Debeir T, Ginestet L, Francois C, et al. Effect of intrastriatal 6-OHDA lesion on dopaminergic innervation of the rat cortex and globus pallidus. Exp Neurol. 2005;193:444–454. doi: 10.1016/j.expneurol.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. A motivational learning hypothesis of the role of mesolimbic dopamine in compulsive drug use. J Psychopharmacol. 1998;12:54–67. doi: 10.1177/026988119801200108. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. Eur J Pharmacol. 1999;375:13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, et al. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Bassareo V, et al. Drug addiction as a disorder of associative learning. Role of nucleus accumbens shell/extended amygdala dopamine. Ann N Y Acad Sci. 1999;877:461–485. doi: 10.1111/j.1749-6632.1999.tb09283.x. [DOI] [PubMed] [Google Scholar]
- Dworkin SI, Guerin GF, Co C, et al. Lack of an effect of 6-hydroxydopamine lesions of the nucleus accumbens on intravenous morphine self-administration. Pharmacol Biochem Behav. 1988;30:1051–1057. doi: 10.1016/0091-3057(88)90138-4. [DOI] [PubMed] [Google Scholar]
- Edwards S, Whisler KN, Fuller DC, et al. Addiction-related alterations in D1 and D2 dopamine receptor behavioral responses following chronic cocaine self-administration. Neuropsychopharmacology. 2007;32:354–366. doi: 10.1038/sj.npp.1301062. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Macconell LA, Geist TD. Effects of haloperidol in a response-reinstatement model of heroin relapse. Psychopharmacology (Berl) 1996;124:205–210. doi: 10.1007/BF02246658. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Pettit HO, Bloom FE, et al. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology (Berl) 1982;78:204–209. doi: 10.1007/BF00428151. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, et al. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lon B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure A, Haberland U, Conde F, et al. Lesion to the nigrostriatal dopamine system disrupts stimulus-response habit formation. J Neurosci. 2005;25:2771–2780. doi: 10.1523/JNEUROSCI.3894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenu S, Spina L, Rivas E, et al. Morphine-conditioned single-trial place preference: role of nucleus accumbens shell dopamine receptors in acquisition, but not expression. Psychopharmacology (Berl) 2006;187:143–153. doi: 10.1007/s00213-006-0415-2. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, et al. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, et al. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2004;176:459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Lasseter HC, Ramirez DR, et al. Relapse to drug seeking following prolonged abstinence: the role of environmental stimuli. Drug Discov Today Dis Models. 2008a;5:251–258. doi: 10.1016/j.ddmod.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Ramirez DR, Bell GH. Nucleus accumbens shell and core involvement in drug context-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2008b;200:545–556. doi: 10.1007/s00213-008-1234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal K, Gyertyan I. Dopamine D3 as well as D2 receptor ligands attenuate the cue-induced cocaine-seeking in a relapse model in rats. Drug Alcohol Depend. 2006;81:63–70. doi: 10.1016/j.drugalcdep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Gerrits MA, Ramsey NF, Wolterink G, et al. Lack of evidence for an involvement of nucleus accumbens dopamine D1 receptors in the initiation of heroin self-administration in the rat. Psychopharmacology (Berl) 1994;114:486–494. doi: 10.1007/BF02249340. [DOI] [PubMed] [Google Scholar]
- Gerrits MA, Van Ree JM. Effect of nucleus accumbens dopamine depletion on motivational aspects involved in initiation of cocaine and heroin self-administration in rats. Brain Res. 1996;713:114–124. doi: 10.1016/0006-8993(95)01491-8. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Fabbricatore AT, Prokopenko V, et al. Persistent cue-evoked activity of accumbens neurons after prolonged abstinence from self-administered cocaine. J Neurosci. 2003;23:7239–7245. doi: 10.1523/JNEUROSCI.23-19-07239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, et al. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Enhanced morphine preference following prolonged abstinence: association with increased fos expression in the extended amygdala. Neuropsychopharmacology. 2003;28:292–299. doi: 10.1038/sj.npp.1300037. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Shaham Y, Olmstead MC. Effects of acute and prolonged opiate abstinence on extinction behaviour in rats. Can J Exp Psychol. 2002;56:241–252. doi: 10.1037/h0087400. [DOI] [PubMed] [Google Scholar]
- Holmes NM, Clemens KJ. Multiple interpretations of cocaine-seeking behavior after prolonged self-administration training. J Neurosci. 2011;31:3935–3936. doi: 10.1523/JNEUROSCI.6354-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, et al. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, et al. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci. 2002;22:6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DM, Westlind-Danielsson A. Dopamine receptors: molecular biology, biochemistry and behavioural aspects. Pharmacol Ther. 1994;64:291–370. doi: 10.1016/0163-7258(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Garris PA, Wightman RM. Different effects of cocaine and nomifensine on dopamine uptake in the caudate-putamen and nucleus accumbens. J Pharmacol Exp Ther. 1995;274:396–403. [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Bjorklund A. Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol. 1998;152:259–277. doi: 10.1006/exnr.1998.6848. [DOI] [PubMed] [Google Scholar]
- Lahoste GJ, Henry BL, Marshall JF. Dopamine D1 receptors synergize with D2, but not D3 or D4, receptors in the striatum without the involvement of action potentials. J Neurosci. 2000;20:6666–6671. doi: 10.1523/JNEUROSCI.20-17-06666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Lauzon NM, Bishop SF, et al. Dopamine signaling through D1-like versus D2-like receptors in the nucleus accumbens core versus shell differentially modulates nicotine reward sensitivity. J Neurosci. 2008;28:8025–8033. doi: 10.1523/JNEUROSCI.1371-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca D, Valentini V, Cacciapaglia F, et al. Reciprocal effects of response contingent and noncontingent intravenous heroin on in vivo nucleus accumbens shell versus core dopamine in the rat: a repeated sampling microdialysis study. Psychopharmacology (Berl) 2007;194:103–116. doi: 10.1007/s00213-007-0815-y. [DOI] [PubMed] [Google Scholar]
- Liu QS, Pu L, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027–1031. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Weiss F. Reversal of ethanol-seeking behavior by D1 and D2 antagonists in an animal model of relapse: differences in antagonist potency in previously ethanol-dependent versus nondependent rats. J Pharmacol Exp Ther. 2002;300:882–889. doi: 10.1124/jpet.300.3.882. [DOI] [PubMed] [Google Scholar]
- Madhavan A, Bonci A, Whistler JL. Opioid-induced GABA potentiation after chronic morphine attenuates the rewarding effects of opioids in the ventral tegmental area. J Neurosci. 2010;30:14029–14035. doi: 10.1523/JNEUROSCI.3366-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcfarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mclaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Guide for the care and use of laboratory animal resources. Bethesda, MD, USA: National Institutes of Health; 1978. [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, et al. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, O’ell LE, Tran-Nguyen LT, et al. Dopamine overflow in the nucleus accumbens during extinction and reinstatement of cocaine self-administration behavior. Neuropsychopharmacology. 1996;15:506–514. doi: 10.1016/S0893-133X(96)00097-8. [DOI] [PubMed] [Google Scholar]
- O’Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry. 2005;162:1423–1431. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- Oldendorf WH, Hyman S, Braun L, et al. Blood-brain barrier: penetration of morphine, codeine, heroin, and methadone after carotid injection. Science. 1972;178:984–986. doi: 10.1126/science.178.4064.984. [DOI] [PubMed] [Google Scholar]
- Owesson-White CA, Ariansen J, Stuber GD, et al. Neural encoding of cocaine-seeking behavior is coincident with phasic dopamine release in the accumbens core and shell. Eur J Neurosci. 2009;30:1117–1127. doi: 10.1111/j.1460-9568.2009.06916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2. Sydney; Orlando: Academic Press; 1986. [Google Scholar]
- Pettit HO, Ettenberg A, Bloom FE, et al. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology (Berl) 1984;84:167–173. doi: 10.1007/BF00427441. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci U S A. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, et al. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Bevins RA. Forced abstinence model of relapse to study pharmacological treatments of substance use disorder. Curr Drug Abuse Rev. 2009;2:184–194. doi: 10.2174/1874473710902020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151:579–588. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawynok J. The therapeutic use of heroin: a review of the pharmacological literature. Can J Physiol Pharmacol. 1986;64:1–6. doi: 10.1139/y86-001. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. Cooperative activation of D1-like and D2-like dopamine receptors in the nucleus accumbens shell is required for the reinstatement of cocaine-seeking behavior in the rat. Neuroscience. 2006;142:451–461. doi: 10.1016/j.neuroscience.2006.06.004. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- See RE, Elliott JC, Feltenstein MW. The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology (Berl) 2007;194:321–331. doi: 10.1007/s00213-007-0850-8. [DOI] [PubMed] [Google Scholar]
- Sell LA, Morris JS, Bearn J, et al. Neural responses associated with cue evoked emotional states and heroin in opiate addicts. Drug Alcohol Depend. 2000;60:207–216. doi: 10.1016/s0376-8716(99)00158-1. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Effects of opioid and dopamine receptor antagonists on relapse induced by stress and re-exposure to heroin in rats. Psychopharmacology (Berl) 1996;125:385–391. doi: 10.1007/BF02246022. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, et al. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Singer G, Wallace M. Effects of 6-OHDA lesions in the nucleus accumbens on the acquisition of self injection of heroin under schedule and non schedule conditions in rats. Pharmacol Biochem Behav. 1984;20:807–809. doi: 10.1016/0091-3057(84)90204-1. [DOI] [PubMed] [Google Scholar]
- Spyraki C, Fibiger HC, Phillips AG. Attenuation of heroin reward in rats by disruption of the mesolimbic dopamine system. Psychopharmacology (Berl) 1983;79:278–283. doi: 10.1007/BF00427827. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. The role of prefrontal cortex D1-like and D2-like receptors in cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2005;177:315–323. doi: 10.1007/s00213-004-1956-x. [DOI] [PubMed] [Google Scholar]
- Suto N, Ecke LE, Wise RA. Control of within-binge cocaine-seeking by dopamine and glutamate in the core of nucleus accumbens. Psychopharmacology (Berl) 2009;205:431–439. doi: 10.1007/s00213-009-1553-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin S, Newman AH, Quinn T, et al. A role for dopamine D1-like receptors in acute food deprivation-induced reinstatement of heroin seeking in rats. Int J Neuropsychopharmacol. 2009;12:217–226. doi: 10.1017/S1461145708008778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Luo F, Ge X, et al. Effect of 6-OHDA lesions of the dopaminergic mesolimbic system on drug priming induced reinstatement of extinguished morphine CPP in rats. Beijing Da Xue Xue Bao. 2003;35:449–452. [PubMed] [Google Scholar]
- Waszczak BL, Martin LP, Finlay HE, et al. Effects of individual and concurrent stimulation of striatal D1 and D2 dopamine receptors on electrophysiological and behavioral output from rat basal ganglia. J Pharmacol Exp Ther. 2002;300:850–861. doi: 10.1124/jpet.300.3.850. [DOI] [PubMed] [Google Scholar]
- Wise RA, Murray A, Bozarth MA. Bromocriptine self-administration and bromocriptine-reinstatement of cocaine-trained and heroin-trained lever pressing in rats. Psychopharmacology (Berl) 1990;100:355–360. doi: 10.1007/BF02244606. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Stein EA. GABAergic mechanisms of opiate reinforcement. Alcohol Alcohol. 2002;37:485–494. doi: 10.1093/alcalc/37.5.485. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Davy S. Dopaminergic modulation of glutamate release in striatum as measured by microdialysis. J Neurochem. 1992;58:1736–1742. doi: 10.1111/j.1471-4159.1992.tb10048.x. [DOI] [PubMed] [Google Scholar]
- Zapata A, Minney VL, Shippenberg TS. Shift from goal-directed to habitual cocaine seeking after prolonged experience in rats. J Neurosci. 2010;30:15457–15463. doi: 10.1523/JNEUROSCI.4072-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, Biswas S, Harlan RE, et al. FOS and Glutamate AMPA receptor subunit coexpression associated with cue-elicited cocaine-seeking behavior in abstinent rats. Neuroscience. 2007;145:438–452. doi: 10.1016/j.neuroscience.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]