Abstract
Dental plaque critically affects the etiology of caries, periodontitis and periimplantitis. The mechanical removal of plaque can only be performed partially due to limited accessibility. Therefore, plaque still represents one of the major therapeutic challenges. Even though antiseptic mouth rinses reduce the extent of biofilm temporarily, plaque removal remains incomplete and continuous usage can even result in side effects.
Here we tested argon plasma produced by kinpen09 as one option to inactivate microorganisms and to eliminate plaque. S. sanguinis biofilms cultivated in either the European Biofilm Reactor (EUREBI) or in 24 well plates were treated with argon plasma. In both test systems a homogeneous, good analyzable and stable biofilm was produced on the surface of titan plates within 72 h (>6,9 log10 CFU/ml). Despite the significantly more powerful biofilm production in EUREBI, the difference of 0.4 log10 CFU/ml between EUREBI and the 24 well plates was practically not relevant. For that reason both test models were equally qualified for the analysis of efficacy of cold atmospheric pressure plasma.
We demonstrate a significant reduction of the biofilm compared to the control in both test models. After plasma application of 180 s the biofilm produced in EUREBI or in 24 well plates was decreased by 0.6 log10 CFU/ml or 0.5 log10 CFU/ml, respectively. In comparison to recently published studies analyzing the efficacy of kinpen09, S. sanguinis produces a hardly removable biofilm.
Future investigations using reduced distances between plasma source and biofilm, various compositions of plasma and alternative plasma sources will contribute to further optimization of the efficacy against S. sanguinis biofilms.
Keywords: S. sanguinis biofilm, biofilm reactor, microtiter plate biofilm model, cold atmospheric pressure plasma, argon plasma, kinpen09
Abstract
Die dentale Plaque beeinflusst entscheidend die Ätiologie von Karies, Parodontitis und Periimplantitis. Die Plaque stellt nach wie vor eine therapeutische Herausforderung dar, weil ihre Eliminierung durch mechanische Reinigung aufgrund der schweren Zugänglichkeit nur unvollständig gelingt. Antiseptische Mundspülungen können zwar über die Senkung der Bakterienzahl das Plaquewachstum hemmen, allerdings ohne dabei die Plaque selbst zu eliminieren. Darüber hinaus sind nur wenige antiseptische Wirkstoffe aufgrund möglicher Nebenwirkung für die Daueranwendung geeignet.
Als mögliche Option zur Inaktivierung der Mikroorganismen in der Plaque bei gleichzeitiger Plaqueelimination wurde die Anwendung von Argonplasma, erzeugt mit dem kinpen09, an S. sanguinis Biofilmen, kultiviert im Europäischen Biofilmreaktor (EUREBI) bzw. in der 24-Well-Platte, untersucht. In beiden Modellen konnte auf Titanplättchen innerhalb von 72 h ein homogener, stabiler, gut analysierbarer Biofilm (>6,9 log10 KbE/ml) erzeugt werden. Trotz der signifikant stärkeren Biofilmbildung im EUREBI war die erzielte Differenz von 0,4 log10 KbE/ml ohne praktische Relevanz, so dass beide Prüfmodelle gleichermaßen zur Erprobung der Wirksamkeit von Plasmaquellen geeignet sind.
Verglichen mit der Kontrolle war in beiden Biofilmmodellen nach der längsten gewählten Einwirkungszeit von 180 s eine signifikante Reduktion des Biofilms um 0,6 log10 KbE/ml bzw. 0,5 log10 KbE/ml erreichbar. Im Vergleich zur Wirksamkeit des kinpen09 in anderen Studien bildet S. sanguinis offensichtlich einen schwierig eliminierbaren Biofilm aus, wobei die Reifungszeit des Biofilms sowie andere Plasmazusammensetzungen und Behandlungseinstellungen die vergleichsweise geringe Effektivität beeinflusst haben dürften.
Um die Wirksamkeit gegen S. sanguinis Biofilme zu verbessern, sind weitere Untersuchungen mit reduziertem Abstand zwischen Plasmaquelle und Biofilm, veränderter Zusammensetzung des Plasmas sowie mit anderen Plasmaquellen erforderlich.
Introduction
Dental plaque not only affects the etiology of caries and periodontitis [1], [2], [3], [4], [5], [6], [7], but it can also induce perimucositis or periimplantitis, and thereby jeopardize positive long-term effects of dental implants [8]. Thus it is crucial to remove biofilms from dental implants, as done in periodontal treatments, in order to prevent or treat periimplant infections [9], [10], [11].
For many decades scientists have searched for efficient ways to remove, delete or prevent dental plaque [12]. The most common practice to remove plaque is mechanical cleaning [5], that can be improved by combination with chemical agents, sonication and the application of magnetization force or an electric field [7], [13]. However, all combinations have their flaws. Mechanical cleaning can only partially remove dental plaque due to the limited accessibility of various biofilms [13], [14]. The mechanical removal of plaque from implants by curets or sonication damages the abutments [15]. Antiseptic mouthrinses can cause problems after long-term usage [16]. Side effects such as unpleasant taste, discoloration of teeth or desquamation und painful mucosa are described for chlorhexidine [17], the gold standard of plaque control [13], [18]. Furthermore, chlorhexidine caused pre-malignant alterations in animal experiments [19].
Therefore, it is essential to develop novel techniques for plaque removal. One of the most recent innovations is cold atmospheric pressure plasma [20]. The possibility of removing biofilms and inactivating microorganisms in biofilms with cold atmospheric pressure plasma, so-called tissue-tolerable plasma, could improve the removal of plaque in the mouth, particularly from gingival pockets hardly to access [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31]. Cold atmospheric pressure plasma could also enhance elimination of biofilms during surgery of infected implants [32]. Furthermore, plasma can be considered supportive for the treatment of periimplantitis [31], since it stimulates growth of osteoblasts and improves the reossification process of dental implants.
Many in vitro models for testing the efficacy against biofilms are expensive, complicated and do not provide sufficiently reproducible results [33]. In addition, variations in culture systems and bacterial species make the direct comparison between different studies difficult [34]. For that reason it is important to develop novel in vitro models generating reproducible, good analyzable and homogeneous biofilms.
One goal of this study was the comparison of the European Biofilm Reactor (EUREBI), an advancement of the CDC reactor [35], with the conventional 24 well plate with respect to biofilm production. EUREBI possesses three inlets for different nutrient solutions instead of only two as the CDC reactor. The friction of the stirrer between glass bottom and impeller is decreased in EUREBI due to its flat bottom. Therefore the speed of stirring can be controlled more precisely. Per cycle 40 instead of 24 test samples can be applied. Since the mechanically exposed pieces are made of stainless steel, the service life (durability) of EUREBI is extended. Furthermore, the Luerlock-System allows installation of sterile tube systems. The entire EUREBI can be completely cleaned and autoclaved.
We assume that EUREBI is the more effective system to produce biofilms. In both test systems biofilm production was analyzed on titan surfaces, because this material is widely used in the field of implantology [8]. The primary colonizer of dental plaque chosen for the present study was the bacterial species S. sanguinis [1], [2], [12], [36], [37], [38], [39], [40], [41]. Providing that both test systems, EUREBI and 24 well plates, were capable of stable biofilm production, the purpose of this work was the analysis of the efficacy of cold atmospheric pressure plasma against those stable biofilms and to compare the two biofilm reactors.
Material and methods
Testorganism
S. sanguinis DSM 20068 was cultivated in brain heart infusion (BHI) (BBL™, Becton Dickinson GmbH, Heidelberg, Germany) supplemented with 1% sucrose (Merck, Darmstadt, Germany). One inoculation loop of cells was re-suspended in 100 ml BHI. The liquid culture was incubated at 37° C for 48 h.
EUREBI biofilm model and biofilm evaluation
100 ml of the 48 h liquid culture and 550 ml of fresh BHI were added to the biofilm reactor. Afterwards, 5 titanium discs (15 mm diameter, 1 mm thickness, Institut Straumann AG, Basel, Switzerland) were fixed in one reactor bar and placed in the reactor. The reactor was coated for 24 h. Flow of the liquid culture was started and controlled by Infusomat fmS (B. Braun AG, Melsungen, Germany) with 18.6 ml/h. The used medium was transferred out of the reactor system by the Infusomat. In addition the medium was mixed using a magnetic stirrer (IKA RCT basic, IKA®-Werke GmbH & Co. KG, Staufen, Germany) with a speed of 50 rpm and heated on a plate at 37°C. For adjustment of temperature, a measuring sensor was inserted into the reactor (Figure 1 (Fig. 1)). The number of colony forming units (CFU) were determined at 0 h, 24 h, 48 h and 72 h (per exposure time n = 5 discs). After 72 h stable biofilm was established.
Figure 1. Experimental setup. On the left European Biofilm Reactor (EUREBI) with vertical placed titanium discs, on the right infusion bag as storage vessel for liquid culture, in the middle Infusomat to control the flow of liquid culture.

In order to analyze the biofilm culture the titanium discs were washed one time with 0.89% NaCl solution to remove not adherent cells and placed into a 24 well plate. 1 ml of 0.89% NaCl solution was added to each well.
The biofilm was removed by treatment in an ultrasonic bath (Branson 2510, 130 W, 42 kHz, Mississauga, Canada) for 30 minutes. Serial dilutions of 100 µl of re-suspended biofilm solutions were transferred to 900 µl fresh 0.89% NaCl solution. An aliquot portion of 100 µl from serial dilutions 10–3, 10–4 and 10–5 was plated on Columbia sheep blood agar (BBL™, Becton Dickinson GmbH, Heidelberg, Germany) and incubated at 37°C for 48 h. The CFU were determined with a colony counter (Bibby Scientific Ltd, Stone, UK), calculated in accordance with DIN EN 1040 [42] and expressed in the common logarithmic scale as CFU/ml. The reduction factor (RF) was calculated by subtraction of the treated samples from the mean of untreated control samples. Statistical analyses were performed by the Wilcoxon-Mann-Whitney test with Bonferroni correction for α=0.05 (Statview 5.0, SAS Institute GmbH, Heidelberg, Germany).
For photometric detection the biofilm-covered discs were colored with 0.1% crystal violet solution (Carl Roth GmbH, Karlsruhe, Germany). 500 µl solution was pipetted into each well onto the discs. After an incubation period of 15 min, each disc was washed 3 times with 1 ml 0.89% NaCl solution to rinse unbound stain. 500 µl of an ethanol HCl mixture (Merck, Darmstadt, Germany) was pipetted into each well to elute the crystal violet. After an incubation time of 15 min, 200 µl of the eluate were transferred into wells of a 96 well microplate. The extinction was detected at 620 nm (Microplate ELISA-Reader 2020, anthos, Microsystems GmbH, Krefeld, Germany).
Microtiter plate biofilm model
The liquid culture, prepared analog to EUREBI liquid culture, was incubated for 24 h (37°C) instead of 48 h. After 24 h, 1 ml of the liquid solution was pipetted into each well of a 24 well plate (Plastic Techno Products Ltd., Trasadingen, Switzerland). One titanium disc was placed in each well. As negative control 10 titanium discs were not inoculated with the liquid culture, but only covered with BHI. At time point 0 h and after an incubation period of 24 h, 48 h and 72 h, respectively, serial dilutions were made in order to determine the CFU/ml. After 72 h a stable biofilm was established as in EUREBI. Measurement of CFU and photometric detection of the biofilm was performed as described for the EUREBI.
Plasma application
For plasma generation, the plasma jet kinpen09 (Neoplas GmbH, Greifswald, Germany) was used with argon as carrier gas in continuous mode [43] with a gas flow of 5 standard liters/min (slm), a frequency of 1.8 MHz and a voltage of 170 V.
Gas flow was controlled by a flow controller (MKS Instruments, Munich, Germany). Titanium discs covered with the 72 h old biofilm of both models were transferred for plasma treatment into 24 well plates. The plasma source was attached to a computer-controlled x/y/z table and the 24 well plate was positioned below. Additional titanium discs were placed in each well under the exposed disc for storing the samples higher. Therefore the distance between the top disc and the plasma source was 10 mm. The kinpen09 moved meander-like in a diameter of 15 mm over each well (Figure 2 (Fig. 2)). All six titanium discs were treated with plasma for a period of 30 s, 60 s, 90 s, 120 s and 180 s. Six samples were treated only with argon gas as “gas flow” control and 6 untreated samples served as negative control.
Figure 2. Experimental setup: Exposure of plasma jet kinpen09 (Neoplas GmbH, Greifswald, Germany) with argon as carrier gas on titanium plates in the 24 well plate.

Results
EUREBI
After 72 h of cultivation the mean value was 7.3 log10 CFU/ml (±0.24). This demonstrates a homogeneous biofilm. Also with photometric determination a uniform biofilm growth was detected (mean 1.34±0.173).
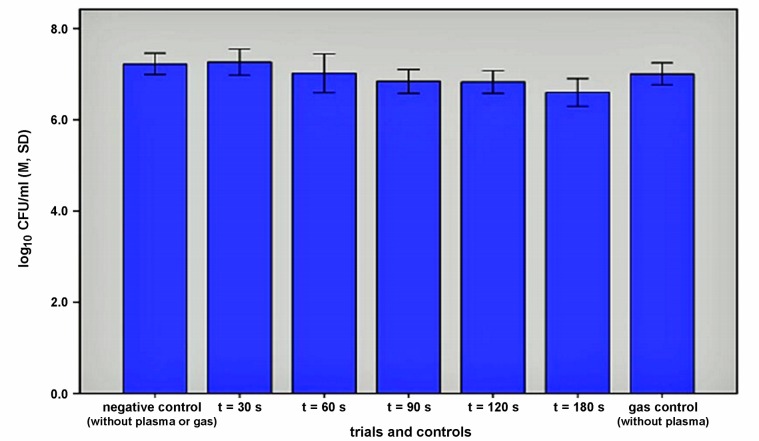
In EUREBI efficacy was shown already after 90 s (p=0.0052). With the application of plasma we achieved a reduction of 0.3 log10 compared to untreated control. After 180 s the reduction was 0.58 log10 (Figure 3 (Fig. 3)).
Figure 3. Efficacy of argon plasma on S. sanguinis biofilms grown in EUREBI: means of CFU/ml (M), n=6 per treatment mode, error bars: standard deviation (SD).
Microtiter plate model
In agreement with the EUREBI results a homogeneous biofilm formation was observed after 72 h (mean 6.9 log10 CFU/ml, ±0.48). Results of the photometric detection method accord with the results of the cultural biofilm analysis (mean of extinction 1.22±0.197).
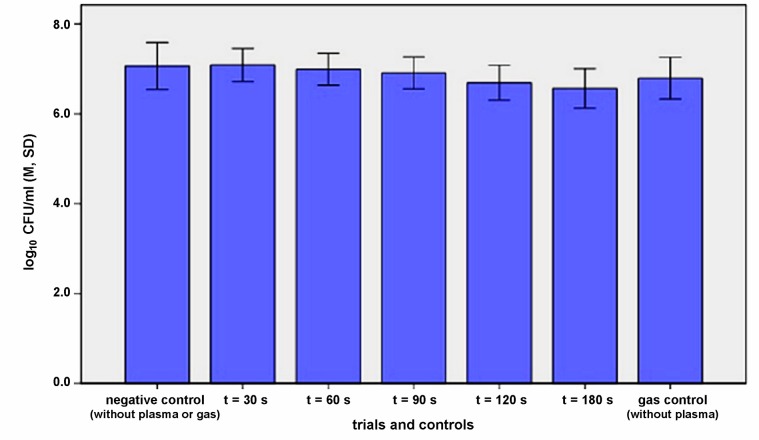
In the microtiter plate model the effectiveness of plasma application was slightly lower than in the EUREBI model, so that the difference to the untreated control was 0.37 log10 CFU/ml after 120 s (p=0.009). After 180 s the reduction reached 0.5 log10 CFU/ml (Figure 4 (Fig. 4)).
Figure 4. Efficacy of argon plasma on the S. sanguinis biofilm produced in 24 well plate: means of CFU/ml (M), n=6 per treatment mode, error bars: standard deviation (SD).
Discussion
Biofilm production
Biofilm reactors simulate the natural salivation due to the change of media [44], [45], [46]. It is feasible to generate a flow-through system by influx and efflux using the continuous flow technique. This is crucial for biofilm production guaranteeing a steady state [47].
The Constant Depth Film Fermenter (CDFF) allows a comparable simulation of biofilm production and excellent reproducibility [14], [48], [49], [50], [51], [52], [53], [54]. A biofilm of a certain thickness can be produced by a permanently installed scraper. However, the stationary phase is only achieved after 100 h [55]. Relatively high costs are another disadvantage of the CDFF [52].
Comparing the EUREBI model with the microtiter plate model the mean extent of biofilm formation was significant different for the cultural and the photometric detection method (Wilcoxon-Mann-Whitney test p≤0.0001 and p=0.0282). Despite the significantly more powerful biofilm production in EUREBI, the difference of 0.4 log10 CFU/ml is from biological perspective not relevant. Due to this fact and the lower experimental effort, the microtiter plate model should be preferred. However using the EUREBI various factors influencing the growth of biofilm can be checked, which can be not modulate with a conventional 24 well plate.
In agreement with results obtained by Duarte et al. [8], Astasov-Frauenhoffer et al. [56] and Hauser-Gerspach et al. [57] S. sanguinis DSM 20068 produced a consistent biofilm on titanium surfaces.
S. sanguinis was chosen as test species because it is not only highly relevant for plaque production, but also attaches to surfaces 10 to 100 times stronger than S. mutans, S. mitis and S. salivarius, and generates stable biofilms [36]. In addition, S. sanguinis has a fast metabolic ratio and therefore rapidly reaches high cell densities [58], [59].
The BHI medium was supplemented with 1% Saccharose in order to improve surface adherence and synthesis of exopolysaccharides [2], [4], [15], [39], [46], [50], [58], [60], [61], [62], [63]. In numerous studies saliva is used as nutrient solution. However, the composition of saliva is complex [7] which makes standardization of this medium very difficult. Also, sterilization of saliva is challenging.
An additional important factor for biofilm growth is the incubation time. Herles et al. [45] and Oliveira et al. [64] showed that the quantity of bacteria increases in up to 72 h of cell culture. During this period deviations in biofilm production were minimal [65]. Wirthin et al. [53] demonstrated that only after 100 h incubation time the steady state was reached. Despite the quantitative increase some authors consider biofilms after 48 h or even less incubation time as sufficient for routine experiments [8], [23], [46], [58], [66], [67]. A cell culture period of 72 hours is advantageous though, since resistance against antimicrobial agents was at its maximum after 72 h [64], [68] and the plaque milieu only becomes caries-producing after 72 h [1], [6], [39], [69], [70].
Since more than 500 species of bacteria are present in dental plaque [71], it is recommended that bacterial suspension used for biofilm production experiments should contain six [72], nine or ten different species [7], [51], [60], [73], [74]. However, Bowden [4] showed that mono- and multispecies biofilms do not differ regarding sequence of biofilm development and cell counts. Costerton et al. [75] accepted both mono- and multispecies biofilms to test the antibiofilm activity. The results of the present study demonstrate that both monospecies biofilm models using S. sanguinis exhibit a strong capability of resistance. Both biofilms tested were only partially reduced by plasma treatment. For that reason, monospecies models are a suitable system to screen the efficacy of argon plasma.
Efficacy of plasma source
In both test models the longest exposition of 180 s resulted in reductions of 0.6 log10 CFU/ml (EUREBI model) and 0.5 log10 CFU/ml (microtiter plate model). We conclude that biofilms generated by both models exhibit comparable stability towards argon plasma. The antimicrobial efficacy determined here is low compared to results of other studies using the same plasma source but different monospecies biofilms [29], [76]. It is important to mention that the reduction of 2–4 log10 CFU/plate depending on the test organism was observed by Daeschlein et al. [76] under different basic conditions. The exposure time was 3 min longer than the time in the present study. Colonies grown on agar plates were investigated instead biofilm. The distance between sample and plasma source was only 1 mm compared with 10 mm in our study. The colonies were exposed to plasma already after 24 h of cultivation instead of 72 h. Koban et al. [15] obtained a reduction factor of 3.2 log10 CFU/ml using S. mutans biofilms treated with kinpen09 for 60 s with a sample-plasma source distance of 7 mm. However, not the entire plate was meander-like treated, but only one isolated point was exposed. Also, the diameter of the titanium plates was only 5 mm instead of 15 mm as in the present study. In agreement with our data, a lower reduction of 0.5 log10 CFU/ml after 2 min exposure was achieved by Koban et al. [30] using kinpen09 and a C. albicans biofilm. According to data in the literature S. sanguinis plays a particular role regarding the resistance towards plasma [25] and other antimicrobial agents like gaseous ozone [57].
In order to optimize removal of S. sanguinis biofilms, a shorter distance between sample and plasma and an oxygen-admixture to argon plasma could be useful, because these factors influence the efficacy [30], [77].
Conclusions
The difference in the biofilm production between EUREBI and microtiter plate was from the biological perspective not relevant. For that reason both test models are suitable for the analysis of efficacy of cold atmospheric pressure plasma.
Further in vitro und in vivo investigations are necessary to evaluate the use of atmospheric pressure plasma for plaque inactivation in dentistry. This will allow identification of influencing factors for optimization of biofilm inactivation. At the same time possible risks for the patient need to be determined, especially mutagenesis and carcinogenesis have to be excluded [78].
Notes
Competing interests
The authors declare that they have no competing interests.
Authorship
The authors I. Koban and R. Matthes contributed equally to this work.
Acknowledgements
This study was conducted within the multi-disciplinary cooperative research program “Campus PlasmaMed”, in particular within the sub-projects “PlasmaDent”, “PlasmaBiozid” as part of the project “PlasmaCure”. This work was supported by a grant from the German Ministry of Education and Research (BMBF, grant No. 13N9779).
References
- 1.Marsh PD, Bradshaw DJ. Microbiological effects of new agents in dentifrices for plaque control. Int Dent J. 1993 Aug;43(4 Suppl 1):399–406. [PubMed] [Google Scholar]
- 2.Scheie AA. Mechanisms of dental plaque formation. Adv Dent Res. 1994 Jul;8(2):246–253. doi: 10.1177/08959374940080021801. [DOI] [PubMed] [Google Scholar]
- 3.Costerton JW. Overview of microbial biofilms. J Ind Microbiol. 1995 Sep;15(3):137–140. doi: 10.1007/BF01569816. Available from: http://dx.doi.org/10.1007/BF01569816. [DOI] [PubMed] [Google Scholar]
- 4.Bowden GH, Hamilton IR. Survival of oral bacteria. Crit Rev Oral Biol Med. 1998;9(1):54–85. doi: 10.1177/10454411980090010401. Available from: http://dx.doi.org/10.1177/10454411980090010401. [DOI] [PubMed] [Google Scholar]
- 5.Gottenbos B, van der Mei HC, Busscher HJ. Models for studying initial adhesion and surface growth in biofilm formation on surfaces. Meth Enzymol. 1999;310:523–534. doi: 10.1016/S0076-6879(99)10040-5. Available from: http://dx.doi.org/10.1016/S0076-6879(99)10040-5. [DOI] [PubMed] [Google Scholar]
- 6.Schierholz JM, Beuth J, König D, Nürnberger A, Pulverer G. Antimicrobial substances and effects on sessile bacteria. Zentralbl Bakteriol. 1999 Apr;289(2):165–177. doi: 10.1016/S0934-8840(99)80101-7. Available from: http://dx.doi.org/10.1016/S0934-8840(99)80101-7. [DOI] [PubMed] [Google Scholar]
- 7.Wilson M. Use of constant depth film fermentor in studies of biofilms of oral bacteria. Meth Enzymol. 1999;310:264–279. doi: 10.1016/S0076-6879(99)10023-5. Available from: http://dx.doi.org/10.1016/S0076-6879(99)10023-5. [DOI] [PubMed] [Google Scholar]
- 8.Duarte PM, Reis AF, de Freitas PM, Ota-Tsuzuki C. Bacterial adhesion on smooth and rough titanium surfaces after treatment with different instruments. J Periodontol. 2009 Nov;80(11):1824–1832. doi: 10.1902/jop.2009.090273. Available from: http://dx.doi.org/10.1902/jop.2009.090273. [DOI] [PubMed] [Google Scholar]
- 9.Klinge B, Gustafsson A, Berglundh T. A systematic review of the effect of anti-infective therapy in the treatment of peri-implantitis. J Clin Periodontol. 2002;29 Suppl 3:213–225. doi: 10.1034/j.1600-051X.29.s3.13.x. Available from: http://dx.doi.org/10.1034/j.1600-051X.29.s3.13.x. [DOI] [PubMed] [Google Scholar]
- 10.Roos-Jansåker AM, Renvert S, Egelberg J. Treatment of peri-implant infections: a literature review. J Clin Periodontol. 2003 Jun;30(6):467–485. doi: 10.1034/j.1600-051X.2003.00296.x. Available from: http://dx.doi.org/10.1034/j.1600-051X.2003.00296.x. [DOI] [PubMed] [Google Scholar]
- 11.Renvert S, Roos-Jansåker AM, Claffey N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: a literature review. J Clin Periodontol. 2008 Sep;35(8 Suppl):305–315. doi: 10.1111/j.1600-051X.2008.01276.x. Available from: http://dx.doi.org/10.1111/j.1600-051X.2008.01276.x. [DOI] [PubMed] [Google Scholar]
- 12.Liljemark WF, Bloomquist CG, Reilly BE, Bernards CJ, Townsend DW, Pennock AT, LeMoine JL. Growth dynamics in a natural biofilm and its impact on oral disease management. Adv Dent Res. 1997 Apr;11(1):14–23. doi: 10.1177/08959374970110010501. Available from: http://dx.doi.org/10.1177/08959374970110010501. [DOI] [PubMed] [Google Scholar]
- 13.Thrower Y, Pinney RJ, Wilson M. Susceptibilities of Actinobacillus actinomycetemcomitans biofilms to oral antiseptics. J Med Microbiol. 1997 May;46(5):425–429. doi: 10.1099/00222615-46-5-425. Available from: http://dx.doi.org/10.1099/00222615-46-5-425. [DOI] [PubMed] [Google Scholar]
- 14.Pratten J, Wills K, Barnett P, Wilson M. In vitro studies of the effect of antiseptic-containing mouthwashes on the formation and viability of Streptococcus sanguis biofilms. J Appl Microbiol. 1998 Jun;84(6):1149–1155. doi: 10.1046/j.1365-2672.1998.00462.x. Available from: http://dx.doi.org/10.1046/j.1365-2672.1998.00462.x. [DOI] [PubMed] [Google Scholar]
- 15.Koban I, Holtfreter B, Hübner NO, Matthes R, Sietmann R, Kindel E, Weltmann KD, Welk A, Kramer A, Kocher T. Antimicrobial efficacy of non-thermal plasma in comparison to chlorhexidine against dental biofilms on titanium discs in vitro - proof of principle experiment. J Clin Periodontol. 2011 Oct;38(10):956–965. doi: 10.1111/j.1600-051X.2011.01740.x. Available from: http://dx.doi.org/10.1111/j.1600-051X.2011.01740.x. [DOI] [PubMed] [Google Scholar]
- 16.Welk A, Splieth CH, Schmidt-Martens G, Schwahn Ch, Kocher T, Kramer A, Rosin M. The effect of a polyhexamethylene biguanide mouthrinse compared with a triclosan rinse and a chlorhexidine rinse on bacterial counts and 4-day plaque re-growth. J Clin Periodontol. 2005 May;32(5):499–505. doi: 10.1111/j.1600-051X.2005.00702.x. Available from: http://dx.doi.org/10.1111/j.1600-051X.2005.00702.x. [DOI] [PubMed] [Google Scholar]
- 17.Sladek RE, Filoche SK, Sissons CH, Stoffels E. Treatment of Streptococcus mutans biofilms with a nonthermal atmospheric plasma. Lett Appl Microbiol. 2007 Sep;45(3):318–323. doi: 10.1111/j.1472-765X.2007.02194.x. Available from: http://dx.doi.org/10.1111/j.1472-765X.2007.02194.x. [DOI] [PubMed] [Google Scholar]
- 18.Gjermo P. Chlorhexidine and related compounds. J Dent Res. 1989;68:1602–1608. [Google Scholar]
- 19.Kramer A. Antiseptika und Händedesinfektionsmittel. In: Korting HC, Sterry W, editors. Verfahren in der Dermatologie - Dermatika und Kosmetika. Berlin: Blackwell; 2001. pp. 273–294. [Google Scholar]
- 20.Emmert S, Isbary G, Kluschke F, Lademann J, Westermann U, Podmele F, Metelmann HR, Daeschlein G, Masur K, von Woedtke T, Weltmann KD. Clinical plasma medicine - position and perspectives in 2012: Clinical Plasma Medicine Core Group. Clin Plasma Med. 2013;1(1):in press. [Google Scholar]
- 21.Fricke K, Koban I, Tresp H, Jablonowski L, Schröder K, Kramer A, Weltmann KD, von Woedtke T, Kocher T. Atmospheric pressure plasma: a high-performance tool for the efficient removal of biofilms. PLoS ONE. 2012;7(8):e42539. doi: 10.1371/journal.pone.0042539. Available from: http://dx.doi.org/10.1371/journal.pone.0042539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee MH, Park BJ, Jin SC, Kim D, Han I, Kim J, Hyun SO, Chung K, Park J. Removal and sterilization of biofilms and planktonic bacteria by microwave-induced argon plasma at atmospheric pressure. New J Phys. 2009;(11):115022. doi: 10.1088/1367-2630/11/11/115022. Available from: http://dx.doi.org/10.1088/1367-2630/11/11/115022. [DOI] [Google Scholar]
- 23.Kamgang JO, Briandet R, Herry JM, Brisset JL, Naïtali M. Destruction of planktonic, adherent and biofilm cells of Staphylococcus epidermidis using a gliding discharge in humid air. J Appl Microbiol. 2007 Sep;103(3):621–628. doi: 10.1111/j.1365-2672.2007.03286.x. Available from: http://dx.doi.org/10.1111/j.1365-2672.2007.03286.x. [DOI] [PubMed] [Google Scholar]
- 24.Yu H, Perni S, Shi JJ, Wang DZ, Kong MG, Shama G. Effects of cell surface loading and phase of growth in cold atmospheric gas plasma inactivation of Escherichia coli K12. J Appl Microbiol. 2006 Dec;101(6):1323–1330. doi: 10.1111/j.1365-2672.2006.03033.x. Available from: http://dx.doi.org/10.1111/j.1365-2672.2006.03033.x. [DOI] [PubMed] [Google Scholar]
- 25.Scholtz V, Julák J, Kríha V, Mosinger J. Decontamination effects of low-temperature plasma generated by corona discharge. Part I: an overview. Prague Med Rep. 2007;108(2):115–127. [PubMed] [Google Scholar]
- 26.Scholtz V, Julák J, Kríha V, Mosinger J, Kopecká S. Decontamination effects of low-temperature plasma generated by corona discharge. Part II: new insights. Prague Med Rep. 2007;108(2):128–146. [PubMed] [Google Scholar]
- 27.Sladek RE, Stoffels E. Deactivation of Escherichia coli by the plasma needle. J Phys D Appl Phys. 2005;38:1716–1721. doi: 10.1088/0022-3727/38/11/012. Available from: http://dx.doi.org/10.1088/0022-3727/38/11/012. [DOI] [Google Scholar]
- 28.Joaquin JC, Kwan C, Abramzon N, Vandervoort K, Brelles-Mari-o G. Is gas-discharge plasma a new solution to the old problem of biofilm inactivation? Microbiology (Reading, Engl) 2009 Mar;155(Pt 3):724–732. doi: 10.1099/mic.0.021501-0. Available from: http://dx.doi.org/10.1099/mic.0.021501-0. [DOI] [PubMed] [Google Scholar]
- 29.Hübner NO, Matthes R, Koban I, Rändler C, Müller G, Bender C, Kindel E, Kocher T, Kramer A. Efficacy of chlorhexidine, polihexanide and tissue-tolerable plasma against Pseudomonas aeruginosa biofilms grown on polystyrene and silicone materials. Skin Pharmacol Physiol. 2010;23 Suppl:28–34. doi: 10.1159/000318265. Available from: http://dx.doi.org/10.1159/000318265. [DOI] [PubMed] [Google Scholar]
- 30.Koban I, Matthes R, Hübner NO, Welk A, Meisel P, Holtfreter B, Sietmann R, Kindel E, Weltmann K-D, Kramer A, Kocher T. Treatment of Candida albicans biofilms with low-temperature plasma induced by dielectric barrier discharge and atmospheric pressure plasma jet. New J Phys. 2010 Jul 1;12(7):073039. doi: 10.1088/1367-2630/12/7/073039. Available from: http://dx.doi.org/10.1088/1367-2630/12/7/073039. [DOI] [Google Scholar]
- 31.Duske K, Koban I, Kindel E, Schröder K, Nebe B, Holtfreter B, Jablonowski L, Weltmann KD, Kocher T. Atmospheric plasma enhances wettability and cell spreading on dental implant metals. J Clin Periodontol. 2012 Apr;39(4):400–407. doi: 10.1111/j.1600-051X.2012.01853.x. Available from: http://dx.doi.org/10.1111/j.1600-051X.2012.01853.x. [DOI] [PubMed] [Google Scholar]
- 32.Kramer A, Hübner NO, Assadian O, Below H, Bender C, Benkhai H, Bröker B, Ekkernkamp A, Eisenbeiß W, Hamman A, Hartmann B, Heidecke CD, Hinz P, Koban I, Koch S, Kocher T, Lademann J, Lademann O, Lerch M, Maier S, Matthes R, Müller G, Partecke I, Rändler C, Weltmann KD, Zygmunt M. Chancen und Perspektiven der Plasmamedizin durch Anwendung von gewebekompatiblen Atmosphärendruckplasmen (Tissue Tolerable Plasmas, TTP) GMS Krankenhaushyg Interdiszip. 2009;4(2):Doc10. doi: 10.3205/dgkh000135. Available from: http://dx.doi.org/10.3205/dgkh000135. [DOI] [Google Scholar]
- 33.Keevil CW, Bradshaw DJ, Dowsett AB, Feary TW. Microbial film formation: dental plaque deposition on acrylic tiles using continuous culture techniques. J Appl Bacteriol. 1987 Feb;62(2):129–138. doi: 10.1111/j.1365-2672.1987.tb02390.x. Available from: http://dx.doi.org/10.1111/j.1365-2672.1987.tb02390.x. [DOI] [PubMed] [Google Scholar]
- 34.McLean RJ, Whiteley M, Hoskins BC, Majors PD, Sharma MM. Laboratory techniques for studying biofilm growth, physiology, and gene expression in flowing systems and porous media. Meth Enzymol. 1999;310:248–264. doi: 10.1016/S0076-6879(99)10022-3. Available from: http://dx.doi.org/10.1016/S0076-6879(99)10022-3. [DOI] [PubMed] [Google Scholar]
- 35.Goeres DM, Loetterle LR, Hamilton MA, Murga R, Kirby DW, Donlan RM. Statistical assessment of a laboratory method for growing biofilms. Microbiology (Reading, Engl) 2005 Mar;151(Pt 3):757–762. doi: 10.1099/mic.0.27709-0. Available from: http://dx.doi.org/10.1099/mic.0.27709-0. [DOI] [PubMed] [Google Scholar]
- 36.Rosan B, Appelbaum B, Campbell LK, Knox KW, Wicken AJ. Chemostat studies of the effect of environmental control on Streptococcus sanguis adherence to hydroxyapatite. Infect Immun. 1982 Jan;35(1):64–70. doi: 10.1128/iai.35.1.64-70.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosan B, Malamud D, Appelbaum B, Golub E. Characteristic differences between saliva-dependent aggregation and adhesion of streptococci. Infect Immun. 1982 Jan;35(1):86–90. doi: 10.1128/iai.35.1.86-90.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fine DH. Chemical agents to prevent and regulate plaque development. Periodontol 2000. 1995 Jun;8:87–107. doi: 10.1111/j.1600-0757.1995.tb00047.x. Available from: http://dx.doi.org/10.1111/j.1600-0757.1995.tb00047.x. [DOI] [PubMed] [Google Scholar]
- 39.Embleton JV, Newman HN, Wilson M. Influence of growth mode and sucrose on susceptibility of Streptococcus sanguis to amine fluorides and amine fluoride-inorganic fluoride combinations. Appl Environ Microbiol. 1998 Sep;64(9):3503–3506. doi: 10.1128/aem.64.9.3503-3506.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolenbrander PE, Andersen RN, Clemans DL, Whittaker CJ, Klier CM. Potential role of functionally similar coaggregation mediators in bacterial succession. In: Newman HN, Wilson M, editors. Dental plaque revisited. Cardiff: BioLine; 1999. pp. 171–186. [Google Scholar]
- 41.Xu P, Alves JM, Kitten T, Brown A, Chen Z, Ozaki LS, Manque P, Ge X, Serrano MG, Puiu D, Hendricks S, Wang Y, Chaplin MD, Akan D, Paik S, Peterson DL, Macrina FL, Buck GA. Genome of the opportunistic pathogen Streptococcus sanguinis. J Bacteriol. 2007 Apr;189(8):3166–3175. doi: 10.1128/JB.01808-06. Available from: http://dx.doi.org/10.1128/JB.01808-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DIN EN 1040:2006-03 Chemical disinfectants and antiseptics - Quantitative suspension test for the evaluation of basic bactericidal activity of chemical disinfectants and antiseptics - Test method and requirements (phase 1); German version EN 1040:2005. CEN/TC 216 - Chemical disinfectants and antiseptics. Berlin: Beuth-Verlag; 2006. pp. 1–42. [Google Scholar]
- 43.Weltmann KD, Kindel E, von Woedtke T, Hähnel M, Stieber M, Brandenburg R. Atmospheric-pressure plasma sources: Prospective tools for plasma medicine. Pure Appl Chem. 2010;82:1223–1237. doi: 10.1351/PAC-CON-09-10-35. Available from: http://dx.doi.org/10.1351/PAC-CON-09-10-35. [DOI] [Google Scholar]
- 44.Rogers AH. Interactions between plaque bacteria: final report on project. Aust Dent J. 1988 Dec;33(6):501–504. doi: 10.1111/j.1834-7819.1988.tb05857.x. Available from: http://dx.doi.org/10.1111/j.1834-7819.1988.tb05857.x. [DOI] [PubMed] [Google Scholar]
- 45.Herles S, Olsen S, Afflitto J, Gaffar A. Chemostat flow cell system: an in vitro model for the evaluation of antiplaque agents. J Dent Res. 1994 Nov;73(11):1748–1755. doi: 10.1177/00220345940730111101. [DOI] [PubMed] [Google Scholar]
- 46.Larsen T, Fiehn NE. Resistance of Streptococcus sanguis biofilms to antimicrobial agents. APMIS. 1996 Apr;104(4):280–284. doi: 10.1111/j.1699-0463.1996.tb00718.x. Available from: http://dx.doi.org/10.1111/j.1699-0463.1996.tb00718.x. [DOI] [PubMed] [Google Scholar]
- 47.Allison D, Maira-Litran T, Gilbert P. Perfused biofilm fermenters. Meth Enzymol. 1999;310:232–248. doi: 10.1016/S0076-6879(99)10021-1. Available from: http://dx.doi.org/10.1016/S0076-6879(99)10021-1. [DOI] [PubMed] [Google Scholar]
- 48.Wilson M, Burns T, Pratten J. Killing of Streptococcus sanguis in biofilms using a light-activated antimicrobial agent. J Antimicrob Chemother. 1996 Feb;37(2):377–381. doi: 10.1093/jac/37.2.377. Available from: http://dx.doi.org/10.1093/jac/37.2.377. [DOI] [PubMed] [Google Scholar]
- 49.Wilson M. Susceptibility of oral bacterial biofilms to antimicrobial agents. J Med Microbiol. 1996 Feb;44(2):79–87. doi: 10.1099/00222615-44-2-79. Available from: http://dx.doi.org/10.1099/00222615-44-2-79. [DOI] [PubMed] [Google Scholar]
- 50.Wilson M, Patel H, Noar JH. Effect of chlorhexidine on multi-species biofilms. Curr Microbiol. 1998 Jan;36(1):13–18. doi: 10.1007/s002849900272. Available from: http://dx.doi.org/10.1007/s002849900272. [DOI] [PubMed] [Google Scholar]
- 51.Dibdin G, Wimpenny J. Steady-state biofilm: practical and theoretical models. Meth Enzymol. 1999;310:296–322. doi: 10.1016/S0076-6879(99)10025-9. Available from: http://dx.doi.org/10.1016/S0076-6879(99)10025-9. [DOI] [PubMed] [Google Scholar]
- 52.Wimpenny JW. Laboratory models of biofilm. In: Newman HN, Wilson M, editors. Dental plaque revisited, oral biofilms in health and disease. Cardiff: BioLine; 1999. pp. 89–110. [Google Scholar]
- 53.Wirthlin MR, Chen PK, Hoover CI. A laboratory model biofilm fermenter: design and initial trial on a single species biofilm. J Periodontol. 2005 Sep;76(9):1443–1449. doi: 10.1902/jop.2005.76.9.1443. Available from: http://dx.doi.org/10.1902/jop.2005.76.9.1443. [DOI] [PubMed] [Google Scholar]
- 54.Coulthwaite L, Verran J. Development of an in vitro denture plaque biofilm to model denture malodour. J Breath Res. 2008 Mar;2(1):017004. doi: 10.1088/1752-7155/2/1/017004. Available from: http://dx.doi.org/10.1088/1752-7155/2/1/017004. [DOI] [PubMed] [Google Scholar]
- 55.Kinniment SL, Wimpenny JW, Adams D, Marsh PD. Development of a steady-state oral microbial biofilm community using the constant-depth film fermenter. Microbiology (Reading, Engl) 1996 Mar;142(Pt 3):631–638. doi: 10.1099/13500872-142-3-631. [DOI] [PubMed] [Google Scholar]
- 56.Astasov-Frauenhoffer M, Braissant O, Hauser-Gerspach I, Daniels AU, Wirz D, Weiger R, Waltimo T. Quantification of vital adherent Streptococcus sanguinis cells on protein-coated titanium after disinfectant treatment. J Mater Sci Mater Med. 2011 Sep;22(9):2045–2051. doi: 10.1007/s10856-011-4377-5. Available from: http://dx.doi.org/10.1007/s10856-011-4377-5. [DOI] [PubMed] [Google Scholar]
- 57.Hauser-Gerspach I, Vadaszan J, Deronjic I, Gass C, Meyer J, Dard M, Waltimo T, Stübinger S, Mauth C. Influence of gaseous ozone in peri-implantitis: bactericidal efficacy and cellular response. An in vitro study using titanium and zirconia. Clin Oral Investig. 2012 Aug;16(4):1049–1059. doi: 10.1007/s00784-011-0603-2. Available from: http://dx.doi.org/10.1007/s00784-011-0603-2. [DOI] [PubMed] [Google Scholar]
- 58.Bowden GH, Li YH. Nutritional influences on biofilm development. Adv Dent Res. 1997 Apr;11(1):81–99. doi: 10.1177/08959374970110012101. Available from: http://dx.doi.org/10.1177/08959374970110012101. [DOI] [PubMed] [Google Scholar]
- 59.Bowden GH. Controlled environment model for accumulation of biofilms of oral bacteria. Meth Enzymol. 1999;310:216–224. doi: 10.1016/S0076-6879(99)10019-3. Available from: http://dx.doi.org/10.1016/S0076-6879(99)10019-3. [DOI] [PubMed] [Google Scholar]
- 60.Sissons CH. Artificial dental plaque biofilm model systems. Adv Dent Res. 1997 Apr;11(1):110–126. doi: 10.1177/08959374970110010201. Available from: http://dx.doi.org/10.1177/08959374970110010201. [DOI] [PubMed] [Google Scholar]
- 61.Wimpenny JW. The validity of models. Adv Dent Res. 1997 Apr;11(1):150–159. doi: 10.1177/08959374970110010601. Available from: http://dx.doi.org/10.1177/08959374970110010601. [DOI] [PubMed] [Google Scholar]
- 62.Pratten J, Wilson M. Antimicrobial susceptibility and composition of microcosm dental plaques supplemented with sucrose. Antimicrob Agents Chemother. 1999 Jul;43(7):1595–1599. doi: 10.1128/aac.43.7.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Der Ploeg JR, Guggenheim B. Deletion of gtfC of Streptococcus mutans has no influence on the composition of a mixed-species in vitro biofilm model of supragingival plaque. Eur J Oral Sci. 2004 Oct;112(5):433–438. doi: 10.1111/j.1600-0722.2004.00158.x. Available from: http://dx.doi.org/10.1111/j.1600-0722.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- 64.Oliveira M, Santos V, Fernandes A, Bernardo F, Vilela CL. Antimicrobial resistance and in vitro biofilm-forming ability of enterococci from intensive and extensive farming broilers. Poult Sci. 2010 May;89(5):1065–1069. doi: 10.3382/ps.2008-00436. Available from: http://dx.doi.org/10.3382/ps.2008-00436. [DOI] [PubMed] [Google Scholar]
- 65.Rändler C, Matthes R, McBain AJ, Giese B, Fraunholz M, Sietmann R, Kohlmann T, Hübner NO, Kramer A. A three-phase in-vitro system for studying Pseudomonas aeruginosa adhesion and biofilm formation upon hydrogel contact lenses. BMC Microbiol. 2010;10:282. doi: 10.1186/1471-2180-10-282. Available from: http://dx.doi.org/10.1186/1471-2180-10-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pratten J, Smith AW, Wilson M. Response of single species biofilms and microcosm dental plaques to pulsing with chlorhexidine. J Antimicrob Chemother. 1998 Oct;42(4):453–459. doi: 10.1093/jac/42.4.453. Available from: http://dx.doi.org/10.1093/jac/42.4.453. [DOI] [PubMed] [Google Scholar]
- 67.Spencer P, Greenman J, McKenzie C, Gafan G, Spratt D, Flanagan A. In vitro biofilm model for studying tongue flora and malodour. J Appl Microbiol. 2007 Oct;103(4):985–992. doi: 10.1111/j.1365-2672.2007.03344.x. Available from: http://dx.doi.org/10.1111/j.1365-2672.2007.03344.x. [DOI] [PubMed] [Google Scholar]
- 68.Anwar H, Costerton JW. Enhanced activity of combination of tobramycin and piperacillin for eradication of sessile biofilm cells of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990 Sep;34(9):1666–1671. doi: 10.1128/AAC.34.9.1666. Available from: http://dx.doi.org/10.1128/AAC.34.9.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Millward TA, Wilson M. The effect of chlorhexidine on Streptococcus sanguis biofilms. Microbios. 1989;58(236-237):155–164. [PubMed] [Google Scholar]
- 70.Marsh PD. Microbiological aspects of the chemical control of plaque and gingivitis. J Dent Res. 1992 Jul;71(7):1431–1438. doi: 10.1177/00220345920710071501. Available from: http://dx.doi.org/10.1177/00220345920710071501. [DOI] [PubMed] [Google Scholar]
- 71.Rosan B, Lamont RJ. Dental plaque formation. Microbes Infect. 2000 Nov;2(13):1599–1607. doi: 10.1016/S1286-4579(00)01316-2. Available from: http://dx.doi.org/10.1016/S1286-4579(00)01316-2. [DOI] [PubMed] [Google Scholar]
- 72.Guggenheim B, Guggenheim M, Gmür R, Giertsen E, Thurnheer T. Application of the Zürich biofilm model to problems of cariology. Caries Res. 2004 May-Jun;38(3):212–222. doi: 10.1159/000077757. Available from: http://dx.doi.org/10.1159/000077757. [DOI] [PubMed] [Google Scholar]
- 73.ten Cate JM, Marsh PD. Procedures for establishing efficacy of antimicrobial agents for chemotherapeutic caries prevention. J Dent Res. 1994 Mar;73(3):695–703. doi: 10.1177/00220345940730031601. [DOI] [PubMed] [Google Scholar]
- 74.Kinniment SL, Wimpenny JW, Adams D, Marsh PD. The effect of chlorhexidine on defined, mixed culture oral biofilms grown in a novel model system. J Appl Bacteriol. 1996 Aug;81(2):120–125. doi: 10.1111/j.1365-2672.1996.tb04488.x. Available from: http://dx.doi.org/10.1111/j.1365-2672.1996.tb04488.x. [DOI] [PubMed] [Google Scholar]
- 75.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. Available from: http://dx.doi.org/10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 76.Daeschlein G, von Woedtke T, Kindel E, Brandenburg R, Weltmann KD, Jünger M. Antibacterial activity of an atmospheric pressure plasma jet against relevant wound pathogens in vitro on a simulated wound environment. Plasma Proc Polym. 2010;7:224–230. doi: 10.1002/ppap.200900059. Available from: http://dx.doi.org/10.1002/ppap.200900059. [DOI] [Google Scholar]
- 77.Hamman A, Hübner NO, Bender C, Ekkernkamp A, Hartmann B, Hinz P, Kindel E, Koban I, Koch S, Kohlmann T, Lademann J, Matthes R, Müller G, Titze R, Weltmann KD, Kramer A. Antiseptic efficacy and tolerance of tissue-tolerable plasma compared with two wound antiseptics on artificially bacterially contaminated eyes from commercially slaughtered pigs. Skin Pharmacol Physiol. 2010;23:328–332. doi: 10.1159/000314724. Available from: http://dx.doi.org/10.1159/000314724. [DOI] [PubMed] [Google Scholar]
- 78.Kramer A, Hübner NO, Weltmann KD, Lademann J, Ekkernkamp A, Hinz P, Assadian O. Polypragmasia in the therapy of infected wounds - conclusions drawn from the perspectives of low temperature plasma technology for plasma wound therapy. GMS Krankenhhyg Interdiszip. 2008;3(1):Doc13. Available from: http://www.egms.de/en/journals/dgkh/2008-3/dgkh000111.shtml. [PMC free article] [PubMed] [Google Scholar]
- 79.Guggenheim B, Giertsen E, Schüpbach P, Shapiro S. Validation of an in vitro biofilm model of supragingival plaque. J Dent Res. 2001 Jan;80(1):363–370. doi: 10.1177/00220345010800011201. Available from: http://dx.doi.org/10.1177/00220345010800011201. [DOI] [PubMed] [Google Scholar]