Abstract
Background: A number of studies have shown that non-critical medical devices can be contaminated with pathogens, including those resistant to antibiotics and thus become a potential vector for transmission. Electrocardiography (ECG) lead wire are non-critical medical device which are always attached on patient skin during their stay in intensive care unit (ICU). In view of the patient’s critical conditions and exposure to invasive procedures, identification and prevention of possible risks are important to prevent infection in ICUs.
Objective: The objective of this study was to determine the presence of bacterial and fungal contamination on cleaned and disinfected reusable ECG lead wires in intensive care units in a hospital.
Methods: A total of 408 cleaned ECG lead wires from 93 bed-side ECG devices and 43 ECG lead wires from 5 portable ECG devices from 4 intensive care units (ICUs) and 1 post-anaesthesia care unit (PACU) were sampled. ECG lead wires were stirred in 0.89% NaCl with added neutralizer for 30 seconds. Samples of the solutions were cultured directly on blood agar. The remaining solution was cultured on blood agar after sterile filtration. The number of colony forming units (CFUs) was counted and the microorganisms were identified.
Results: More than half of examined ECG lead wires (n=232; 51.4%) were contaminated with >30 CFUs/mL sample of bacteria or with risk pathogens. Gram-positive bacteria were the most frequently isolated organisms; particularly, coagulase negative staphylococci (96%) and aerobic spore forming bacteria (71.2%). Compared to ICUs, PACU had significantly lower proportion of contaminated ECG lead wires (p<0.05). The proportion of contaminated ECG lead wires, as well as mean number of cfus per ECG lead wire, was also significantly lower among multi-wire ECG leads compared to single-wire ECG leads.
Conclusions: Manually cleaned ECG lead wires may serve as a vector for transmission of nosocomial pathogens. The current reprocessing technique for ECG lead wires needs to be improved.
Keywords: ECG lead wire, microbial contamination, ICU, PACU
Abstract
Hintergrund: In verschiedenen Studien konnte eine Kontamination unkritischer Medizinprodukte mit Pathogenen einschließlich multiresistenten Erregern nachgewiesen werden, die eine potentielle Quelle Health-Care assoziierter Infektionen darstellen. Ein derartiges unkritisches Medizinprodukt ist das EKG-Gerät. Da es z.B. auf der Intensivtherapiestation in Kontakt zur Haut des Patienten kommt und dadurch Pathogene unter kritischen Bedingungen bzw. im Rahmen invasiver Eingriffe Infektionen auslösen können, muss dieses Risiko ausgeschaltet werden.
Zielsetzung: In der Studie sollte die mikrobielle Kontamination manuell aufbereiteter EKG-Anschlussklemmen auf Intensivtherapiestationen untersucht werden.
Methode: Auf vier Intensivtherapieeinheiten (ITS) und einer Aufwacheinheit (PACU) wurden 408 bzw. 43 manuell aufbereitete EKG-Anschlussklemmen von 93 bettseitigen bzw. von 5 transportablen EKG-Geräten auf bakterielle und fungielle Kontamination untersucht. Nach 30 s Ausschütteln in 0,89%-iger NaCl-Lösung mit Zusatz eines Neutralisators wurden die Flüssigkeit direkt und zusätzlich nach Sterilfiltration auf Blutagar kultiviert, die Anzahl Kolonie bildender Einheiten (KbE) ermittelt und die Erreger differenziert.
Ergebnisse: Mehr als die Hälfte der Anschlussklemmen (51,4%) war mit ≥30 KbE/ ml oder mit kritischen Pathogenen kontaminiert. Am häufigsten wurden Gram-positive Bakterien nachgewiesen, besonders Coagulase negative Staphylokokken (96%) and aerobe Sporenbildner (71,2%). Verglichen mit den ITS war der Anteil kontaminierter Anschlussklemmen auf der PACU signifikant geringer (p<0.05). Der Anteil kontaminierter Anschlussklemmen und die Höhe der Kontamination war signifikant geringer bei Mehrweganschlussklemmen im Vergleich zu single-use Anschlussklemmen.
Schlussfolgerung: Manuell aufbereitete EKG-Anschlussklemmen sind ein Reservoir für nosokomiale Pathogene. In Auswertung der Befunde wurde die manuelle Aufbereitung durch eine maschinelle Aufbereitung ersetzt.
Introduction
Healthcare-associated infections (HAIs) occurred in every healthcare facility and affecting hundreds of million of patients every year worldwide [1]. HAIs develop during the course of health care treatment and resulted in an increased morbidity and mortality among affected patients that generated additional cost to those already incurred by patient’s underlying disease due to prolong duration of hospital stays and additional diagnostic and therapeutic interventions [2].
The most common reported HAIs are associated with the use of critical devices or invasive procedure, e.g. catheter-associated bloodstream infection (CABSI), ventilator-associated pneumonia (VAP), catheter-associated urinary tract infection (CAUTI), and surgical site infection (SSI) [3]. Collectively, these infections accounts for more than 80% of all HAIs [4]. Given the critical conditions and exposure to medical devices, the risk for infection is greater in ICU and increased with duration of stay [5].
Patients in ICU are also exposed to non-critical devices, i.e. objects that come in contact with intact skin. These include blood pressure cuff, stethoscope, pulse oximetry sensor, ultrasound transducers and electrocardiography leads. Intact skin acts is an effective barrier to most microorganisms, therefore, these items have been said to pose virtually no risk for transmission of infectious agents [6]. However, numerous studies have shown that non-critical devices could be contaminated with pathogens, including those resistant to antibiotics and therefore become a potential source of HAIs [7], [8], [9], [10], [11], [12].
Electrocardiographic (ECG) lead wires are a common medical device that come into direct contact with patient’s skin and are considered as non-critical medical devices because they are not directly in contact with patient’s mucosa or blood. The ECGs lead wires are usually decontaminated after patient use, for instance with disinfectant wipes and hangs uncovered near patient bed, ready to use by the new patient. The existence of small ridges and clamps in an ECG lead wires, as well as variation of surface causes difficulty in cleaning and disinfecting the ECG lead wires thoroughly and brings the ECG lead wires under scrutiny as vector for transmission of multidrug-resistant bacterial pathogens [10], [13]. Reports on microbial growth in ECG wires are scarce. A single-centred study found one or more antibiotic-resistant pathogens in 77% of ECG lead wires after they have been manually reprocessed. Of the contaminated lead wires, 67% had Vancomycin-resistant enterococci (VRE), and 12% had Gram-negative bacilli resistant to extended-spectrum β-lactams [13]. An investigation of a 13 month long VRE outbreak in a burn intensive care unit (BICU) identified a VRE isolate with pulsed-field gel electrophoresis (PFGE) from an ECG lead wires attached to a patient whose culture for VRE was negative. Three days after, VRE grew from a culture of burn wound on his neck and also recovered in a rectal culture of the patient which was found to have the same PFGE pattern with VRE isolates from the ECG lead wires and with the isolate of the previous patient who had occupied the room before the VRE outbreak [14].
A multicentre study showed that the presence of bacterial growth on ECG lead wires are also associated with work environment, where ECG lead wires in emergency department had higher percentage of contaminated ECG lead wires compared to operating room and ICU [15]. Intensive care units (ICUs), as well as neonatology unit, are health care settings which have the highest device-associated hospital-associated infections and that poses greater risk to patient safety given the patients critical conditions and exposure to invasive device [16]. These facts pronounce the critical importance of infection control in intensive care units.
The objective of our study was to determine the presence of bacterial and fungal contamination on cleaned disinfected reusable ECG lead wires in intensive care units in a hospital and to analyze the potential risk for HAIs.
Methods
This is a descriptive, cross-sectional study, which was conducted in a tertiary care hospital. The study was conducted between September to October 2012 in four intensive stations for adult patient and one post-anaesthesia care unit (PACU).
There are six different types of ECG lead wires (Drägerwerk AG & Co. KGaA) that were available in the analyzed ICUs, e.g. single wire with 3 and 6 snaps on leads, multi wires with 3 and 5 clips on leads and multi wires portable ECG with 10 banana leads. All ECG lead wires are color coded according to European standard.
The ECG lead wires were routinely cleaned and disinfected after patient use by wiping the lead and the wire with antibacterial wipes (Terralin® liquid, Schülke GmbH, Norderstedt, Germany; Cleanisept®, Dr. Schumacher GmbH, Melsungen, Germany; or Incidin® Plus, Ecolab GmbH, Düsseldorf, Germany). Cleaned ECG leads and wires were left to dry for a minimum of 30 minutes before use. Cleaned, ready to use, reusable ECG lead wires that are hanging or laid on the monitoring equipment were chosen on the basis of availability as samples for this study. A set of ECG lead wires could be taken as samples repeatedly as long as it has been used by a different patient in a different day and has been cleaned subsequently.
After performing hand disinfection and using aseptic gloves, the investigator hold the ECG cable about 15 cm from the lead and stirred a lead for 30 seconds inside a sterile 50 mL tube (Greiner Bio-One GmbH, Frickenhausen, Germany), which contain 20 mL of neutralizer liquid (30 g Tween 80, 30 g Saponin, 1 g Histidin and 1 g Cystein in 1 L Aqua dest). After sampling, the tube was labelled and lead was cleansed again with disinfection wipes that was available in the room. Samples were immediately transferred to the laboratory. A drop of 0.1 mL and 0.5 mL of each sample were spread evenly in two plates of Columbia Agar with 5% sheep blood (Becton Dickinson GmbH, Heidelberg, Germany). Another 10 mL of sample were filtered through a 0.45 µm-pore-size membrane filter (Merck Millipore Corp., Darmstadt, Germany) and the filters were placed on a Columbia blood agar plate. All three blood agar plates were incubated for 36–48 hours at a temperature of 37 ± 1°C. The total number of colony forming units (cfus) were counted and identified with standard microbiological procedures. A senior microbiologist assisted and supervised the culture and identification process. Contaminated lead wires were confirmed when more than 30 cfus of low risk pathogens were identified or when ≥1 cfu of high risk pathogens were identified. Bacteria were defined as high risk pathogen if they have a high probability of causing an infection.
Results
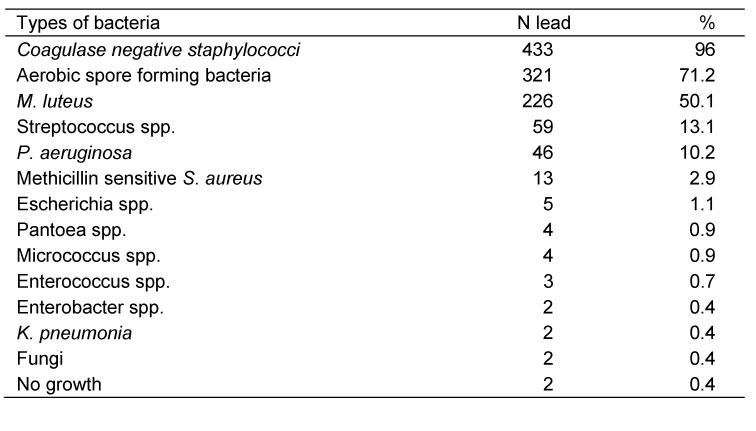
A total of 408 ECG lead wires from 93 bed-side ECG devices and 43 ECG lead wires from 5 portable ECG devices were sampled. All sampled ECG lead wires had been previously manually cleaned and disinfected and were ready to use, however, some of them were visibly dirty and remnants were left on the filter paper. Of 451 lead wires, only 2 lead wires were without contamination as shown in Table 1 (Tab. 1). Gram-positive organisms were the most frequently isolated; particularly, coagulase negative staphylococci (CoNS) and aerobic spore forming bacteria which were isolated in 96% and 71.2% of lead wires, respectively. Pseudomonas aeruginosa accounted for the most common isolated Gram-negative rods, which identified in 10.2% of lead wires, and classified as high risk pathogen in this study. Other isolated pathogens were Streptococcus spp., Escherichia spp., S. aureus, Pantoea spp., Enterococcus spp., Enterobacter spp., and Klebsiella pneumonia. The presence of fungi on ECG lead wires was rare (Table 1 (Tab. 1)). Antibiogram test was performed to 39 of gram-negative isolates and multi-resistant isolates were found in 37 isolates (94.8%).
Table 1. Types of determined bacteria.
More than half of the examined ECG lead wires (51.4%) were contaminated, either by having ≥30 cfus/mL samples of microorganisms or by the presence of ≥1 cfu/mL of high risk pathogens. Of all contaminated lead wires, 53% classified as contaminated because more than 30 cfus/mL samples of microorganisms were isolated, 19.4% because high risk pathogens were isolated and 27.6% of lead wires had both more than 30 cfus/mL samples of microorganisms and high risk pathogens. Median and mean of total number of counted cfus/mL samples was 20 and 169.9, respectively.
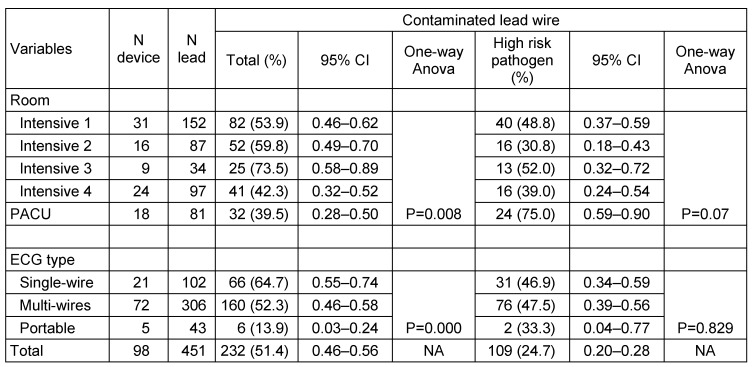
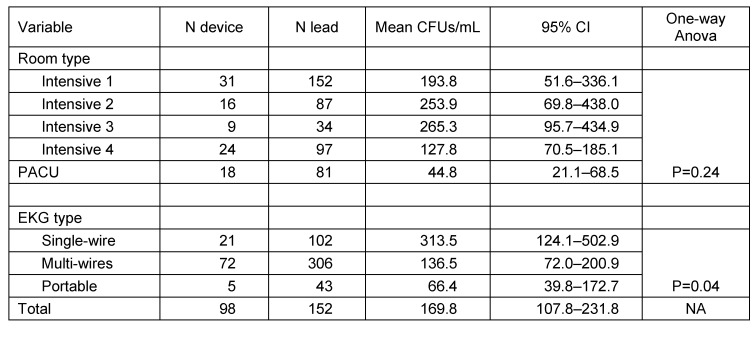
Proportion of contaminated lead wires in different intensive care unit was significantly different and varied from 39.5% in PACU to 73.5% in ICU 3 (p<0.05). However this variation between intensive care unit becomes insignificant when data from PACU were excluded (p=0.49). Even though the proportion of lead wire contamination in PACU was among the lowest compared to other intensive care units, at risk pathogen were identified in 75% of those contaminated. At other intensive care unit, proportion of leads contaminated with high risk pathogen was ranged from 30.8% to 52% of all contaminated leads (Table 2 (Tab. 2)). As well as the proportion of contaminated lead, the mean of counted CFUs/mL per lead wire between intensive care units were not significantly varied (p>0.05) (Table 3 (Tab. 3)).
Table 2. Proportion of contaminated ECG leads.
Table 3. Average number of bacterial colonies.
Interestingly, our study also found that mean CFUs of single wire ECG lead wires was significantly higher compare to multi wires ECG lead wires (mean 313.5 vs. 136.5, p<0.05). Consequently, the proportion of contaminated lead wires was also significantly higher among single-wire compared to multi-wires type leads (64.7% vs. 52.3%, p<0.05). However, both types of leads have similar risk to be occupied by high-risk pathogen. Only five portable ECG devices sampled in this study, four devices equipped with 10 lead wires and one device had 3 lead wires. Proportion of contaminated lead wires and mean of CFUs per lead wire in this group was significantly lower compare to the bed-side type of ECG device (p<0.05).
Analysis of ECG devices as a set of equipment with multiple ECG lead wires revealed that 72 (77.4%) bed-side ECG devices had one or more contaminated lead wires and of those contaminated, 55 (76.4%) had high risk pathogen. Four from five (80%) portable ECG devices were also contaminated.
Discussion
Result of our study supported the findings from previous studies on the potential role of non-critical medical devices, particularly ECG leads as potential vector for transmission of infection. The high proportion of contaminated lead wires was comparable to previous studies which have shown that majority of ECG lead wires were contaminated with bacteria and fungi and that low risk pathogen, i.e. CoNS and aerobic spora forming bacteria, were dominant [13], [15].
There was a significant variation of contaminated lead wires between adult intensive room and post-anaesthesia care unit (PACU), which had the lowest proportion of contaminated lead wires and mean CFUs/mL samples. This may be due to short duration of contact between ECG lead wires with patient’s skin and frequent cleaning times. In PACU, patient stay for about 30–60 minutes before they were transported to intensive care unit or common ward. In accordance to hospital safety procedure, ECG lead wires were directly cleaned with disinfectant wipes before patient use. Due to high mobility of patients in PACU, an ECG lead wire can be cleaned 3 to 5 times a day, compare to only once before patient use in other intensive care units.
ECG lead wire had multiple surfaces with very small nooks and cranny which makes it difficult for cleaning agents, particularly disinfectant wipes, to reach the hidden part and thus leave some parts as a reservoir for microorganism. Therefore, despite frequent cleaning, about 40% of ECG lead wires in PACU were still contaminated with microorganism, including high risk pathogens. The single wire ECG lead in this study was designed with Z-Snap™ zero-insertion force electrode snaps to allow fast and easy application and cleaning with disinfectant wipes [17]. Compared to the multi-wire clips-on type ECG leads, the snaps-on type lead is simpler and has less cleft. However, we found that the mean of cfu/mL in the single wire ECG lead group was significantly higher compared to the multi wire ECG lead group. It is indicated that less hidden cleft in an ECG lead will not reduce the risk of microbial contamination. In contrast with the snaps-on and clips-on type, the banana type ECG leads that is attached to a portable ECG device is intact, without hidden cleft, and thus easy to clean. Our finding, that these banana types of lead were significantly less contaminated, supported this logic. Nevertheless, lack of samples from portable ECG elicits the need to confirm this finding.
The close proximity of contaminated ECG leads with patient’s skin, open wound, intravenous lines, surgical dressing, and the duration of contact may poses patient at greater risk to infection, particularly those in critical conditions, immunocompromised or exposed to invasive device. A VRE outbreak in a burn ICU in Texas was directly related to a strain of VRE from previous infected patient that persisted for 13 weeks on an ECG lead wire [14]. This is in accordance with previous results that nosocomial pathogens may well survive in inanimate surfaces for weeks or months when regular disinfection practice is not performed [18]. In a hospital in Virginia, the surgical site infection (SSI) rate has been dropped by 40% after the use of disposable ECG lead wire without any changes being made to any other infection prevention practice [19]. Conversion to disposable ECG lead wires in three hospitals in South Florida and Southern California has also reduced the incidence of sternal wound infection by 90% [20]. We have not found any study on the role of ECG lead wire in cross infection in ICUs in Germany. However, our finding showed that variation of isolated pathogen from ECG lead wires in ICUs was similar with the pattern of the most common isolated pathogen accountable for device-associated infections in ICUs in Germany [21].
Conclusion
The study illustrates that ECG lead wires frequently are contaminated with pathogenic microorganisms and that they may serves as vector for transmission of nosocomial pathogens. The fact that almost all ECG devices has one or more contaminated lead wires implies that the required reprocessing quality at the investigated ICUs was not achieved by the established regular disinfecting methods and need to be improved. The safest option is to use a single-use ECG lead wires. Manual reprocessing of ECG lead wires by brushing the open ridges in a disinfectant solutions and disinfecting the lead with disinfectant wipes can be another potential alternatives. However efficacy of the brushing method to clean EECG lead wires needs to be demonstrated. If efficacy of the new reprocessing method has been confirmed, a reprocessing procedure needs to be standardized in a standard operating procedure (SOP). However, nothing replaces adequate hand hygiene and adherence to universal precautions, thus these prevention practices should always be performed.
Notes
Competing interests
The study is sponsored by Drägerwerk AG & Co. KGaA, Lübeck, Germany.
Acknowledgement
The author would like to thank the senior microbiologists (G. Lindstedt) of the Institut für Hygiene und Umweltmedizin, Universität Greifswald and the healthcare staff at studied ICUs for their kind assistance.
The study was conducted as part of the International Leadership Training for Hospital Management organized by Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH.
References
- 1.Pittet D, Allegranzi B, Storr J, Bagheri Nejad S, Dziekan G, Leotsakos A, Donaldson L. Infection control as a major World Health Organization priority for developing countries. J Hosp Infect. 2008 Apr;68(4):285–292. doi: 10.1016/j.jhin.2007.12.013. Available from: http://dx.doi.org/10.1016/j.jhin.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Collins AS. Preventing health care-associated infections. In: Hughes RG, editor. Patient safety and quality: an evidence-based handbook for nurses. Rockville (MD): Agency for Healthcare Research and Quality (US); Apr, 2008. p. Chapter 41. [PubMed] [Google Scholar]
- 3.Umscheid CA, Mitchell MD, Doshi JA, Agarwal R, Williams K, Brennan PJ. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol. 2011 Feb;32(2):101–114. doi: 10.1086/657912. Available from: http://dx.doi.org/10.1086/657912. [DOI] [PubMed] [Google Scholar]
- 4.Ranji SR, Shetty K, Posley KA, Lewis R, Sundaram V, Galvin CM, Winston LG. Prevention of healthcare-associated infections. Rockville, MD: Agency for Healthcare Research and Quality; 2007. (Closing the quality gap: a critical analysis of quality improvement strategies; 6). Available from: http://www.ncbi.nlm.nih.gov/books/NBK43982/ [PubMed] [Google Scholar]
- 5.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, Reinhart K EPIC II Group of Investigators. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009 Dec 2;302(21):2323–2329. doi: 10.1001/jama.2009.1754. Available from: http://dx.doi.org/10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 6.Rutala WA, Weber DJ. Disinfection and sterilization in health care facilities: what clinicians need to know. Clin Infect Dis. 2004 Sep 1;39(5):702–709. doi: 10.1086/423182. [DOI] [PubMed] [Google Scholar]
- 7.Mangi RJ, Andriole VT. Contaminated stethoscopes: a potential source of nosocomial infections. Yale J Biol Med. 1972 Dec;45(6):600–604. [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkins MC. Residual bacterial contamination on reusable pulse oximetry sensors. Respir Care. 1993 Nov;38(11):1155–1160. [PubMed] [Google Scholar]
- 9.Wright IM, Orr H, Porter C. Stethoscope contamination in the neonatal intensive care unit. J Hosp Infect. 1995 Jan;29(1):65–68. doi: 10.1016/0195-6701(95)90294-5. Available from: http://dx.doi.org/10.1016/0195-6701(95)90294-5. [DOI] [PubMed] [Google Scholar]
- 10.Brown DQ. Disposable vs reusable electrocardiography leads in development of and cross-contamination by resistant bacteria. Crit Care Nurse. 2011 Jun;31(3):62–68. doi: 10.4037/ccn2011874. Available from: http://dx.doi.org/10.4037/ccn2011874. [DOI] [PubMed] [Google Scholar]
- 11.de Gialluly C, Morange V, de Gialluly E, Loulergue J, van der Mee N, Quentin R. Blood pressure cuff as a potential vector of pathogenic microorganisms: a prospective study in a teaching hospital. Infect Control Hosp Epidemiol. 2006 Sep;27(9):940–943. doi: 10.1086/507284. Available from: http://dx.doi.org/10.1086/507284. [DOI] [PubMed] [Google Scholar]
- 12.Sykes A, Appleby M, Perry J, Gould K. An investigation of the microbiological contamination of ultrasound equipment. J Inf Prevent. 2006;7(4):16–20. [Google Scholar]
- 13.Jancin B. Antibiotic-resistant pathogens fund on 77% of ECG lead wires. Cardiol News. 2004 Mar;2(2):1. [Google Scholar]
- 14.Falk PS, Winnike J, Woodmansee C, Desai M, Mayhall CG. Outbreak of vancomycin-resistant enterococci in a burn unit. Infect Control Hosp Epidemiol. 2000 Sep;21(9):575–582. doi: 10.1086/501806. Available from: http://dx.doi.org/10.1086/501806. [DOI] [PubMed] [Google Scholar]
- 15.Albert NM, Hancock K, Murray T, Karafa M, Runner JC, Fowler SB, Nadeau CA, Rice KL, Krajewski S. Cleaned, ready-to-use, reusable electrocardiographic lead wires as a source of pathogenic microorganisms. Am J Crit Care. 2010 Nov;19(6):e73–e80. doi: 10.4037/ajcc2010304. Available from: http://dx.doi.org/10.4037/ajcc2010304. [DOI] [PubMed] [Google Scholar]
- 16.Yalaz M, Altun-Köroğlu O, Ulusoy B, Yildiz B, Akisu M, Vardar F, Ozinel MA, Kültürsay N. Evaluation of device-associated infections in a neonatal intensive care unit. Turk J Pediatr. 2012 Mar-Apr;54(2):128–135. [PubMed] [Google Scholar]
- 17.Dräger Monolead ECG lead-wire set. Lübeck, Germany;:Dräger Medical GmbH. [Google Scholar]
- 18.Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006 Aug 16;6:130. doi: 10.1186/1471-2334-6-130. Available from: http://dx.doi.org/10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnett TE. The not-so-hidden costs of surgical site infections. AORN J. 2007 Aug;86(2):249–258. doi: 10.1016/j.aorn.2007.03.012. Available from: http://dx.doi.org/10.1016/j.aorn.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Brown DQ. Electrocardiography wires: a potential source of infection. AACN NEWS. 2006;23(9):12–15. [Google Scholar]
- 21.Modul IT S-KISS. Referenzdaten Berechnungszeitraum: Januar 2007 bis Dezember 2011. Berlin: Nationales Referenzzentrum für Surveillance von nosokomialen Infektionen; 2012. [cited 2013 March 7]. Available from: http://www.nrz-hygiene.de/fileadmin/nrz/module/its/200701_201112_ITS_reference_ALLE.pdf. [Google Scholar]