Abstract
Introduction: In March 2010, more than 213 countries worldwide reported laboratory confirmed cases of influenza H1N1 infections with at least 16,813 deaths. In some countries, roughly 10 to 30% of the hospitalized patients were admitted to the ICU and up to 70% of those required mechanical ventilation. The question now arises whether breathing system filters can prevent virus particles from an infected patient from entering the breathing system and passing through the ventilator into the ambient air.
We tested the filters routinely used in our institution for their removal efficacy and efficiency for the influenza virus A H1N1 (A/PR/8/34).
Methods: Laboratory investigation of three filters (PALL Ultipor® 25, Ultipor® 100 and Pall BB50T Breathing Circuit Filter, manufactured by Pall Life Sciences) using a monodispersed aerosol of human influenza A (H1N1) virus in an air stream model with virus particles quantified as cytopathic effects in cultured canine kidney cells (MDCK).
Results: The initial viral load of 7.74±0.27 log10 was reduced to a viral load of ≤2.43 log10, behind the filter. This represents a viral filtration efficiency of ≥99.9995%.
Conclusion: The three tested filters retained the virus input, indicating that their use in the breathing systems of intubated and mechanically ventilated patients can reduce the risk of spreading the virus to the breathing system and the ambient air.
Keywords: influenza virus A (H1N1), pandemic, viral spread, heat and moisture exchanger
Abstract
Zielsetzung: Im März 2010 berichteten weltweit mehr als 213 Länder über laborchemisch bestätigte Influenza H1N1-Infektionen mit mindestens 16.813 Toten. In einigen Ländern wurden rund 10 bis 30% der hospitalisierten Patienten auf eine Intensivstation aufgenommen und bis zu 70% dieser Patienten benötigten eine mechanische Beatmung. Es stellt sich die Frage, ob Beatmungsfilter in der Lage sind Viruspartikel von einem infizierten Patienten daran zu hindern, das Beatmungsgerät sowie die Raumluft zu kontaminieren.
Wir untersuchten Filter, die routinemäßig in unserer Abteilung benutzt werden, auf ihre Wirksamkeit und Effizienz hinsichtlich des Influenza Virus H1N1 (A/PR/8/34)).
Methoden: Laboruntersuchungen von drei Filtern (PALL Ultipor® 25, Ultipor® 100 und Pall BB50T Beatmungsfilter, hergestellt von Pall Life Sciences) unter Verwendung eines monodispersen Aerosols von Influenza A (H1N1) Virus in einem Luftströmungsmodel mit Viruspartikeln quantifiziert als zytopathologische Effekte in kultivierten Kaninchen-Nierenzellen (MDCK).
Ergebnisse: Die initiale Viruslast von 7.74±0.27 log10 wurde hinter den Filtern auf eine Viruslast von ≤2.43 log10 reduziert. Dieses Ergebnis entspricht einer Virusfiltration von ≥99.9995%.
Schlussfolgerung: Die drei getesteten Filter verhinderten eine Ausbreitung des Virus, es deutet also daraufhin, dass die Verwendung dieser Filter das Risiko einer Virusübertragung von intubierten und beatmeten Patienten an das Beatmungsgerät und die Raumluft reduzieren kann.
Introduction
In the spring of 2009, an outbreak of influenza with the H1N1 virus strain was detected in Mexico and shortly thereafter in the USA and Europe [1], [2], [3]. This strain had a high lethality among previously healthy young people, who developed severe, rapidly progressing respiratory failure that required invasive ventilation and even extracorporeal membrane oxygenation [4], [5]. Early on, the question arose if the breathing system filters were able to prevent virus spread from an infected patient through the ventilator into the ambient air. If filters and filtering devices of this type were unable to contain the virus and protect health care workers, caring for infected patients would pose an unacceptably high risk. The primary concern of this investigation was therefore to test the efficiency of our routinely used breathing system filters, which are specifically designed to capture and retain bacteria and virus particles, in removing H1N1A virus from the airstream.
Materials and methods
Study design
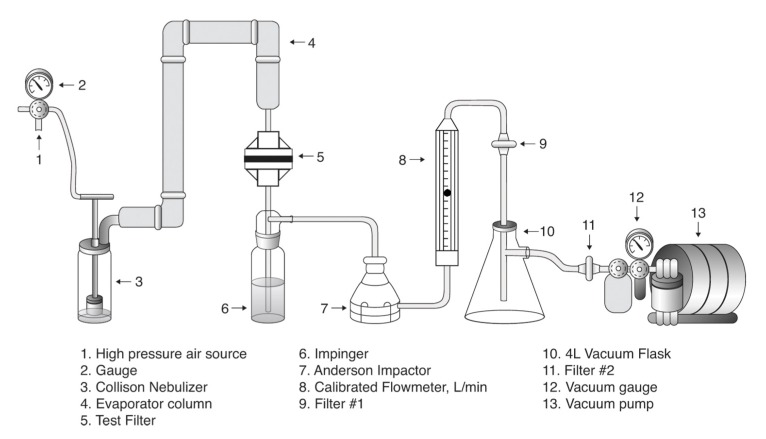
A diagram of the test system is shown in Figure 1 (Fig. 1). All tests were performed in a biohazard laminar flow cabinet. The nebulizer, connecting tubing, evaporation column, and impingers were autoclave sterilized prior to each test set. The test filters were provided pre-sterilized in sealed bags. The media used were autoclave sterilized or 0.2 µm aseptically filtered.
Figure 1. Filter efficacy test setup.
The test system consisted of a compressed air source set at 1380 mbar which was connected to a BGI Collision six jet nebulizer (P/N CN25). Under this pressurization the Collision nebulizer delivers about 12 L min–1 of aerosol (minute droplets in air) with a droplet size range of 0.78 to 9.0 µm.
Human influenza virus A (A/PR/8/34 – H1N1 – Charles River Laboratories) was used for the test challenges. It was prepared from frozen stocks and suspended in 0.1X Minimum Essential Medium (MEM) for nebulization. The virus particle aerosols for the control tests and the challenge tests were collected in impingers containing 40 mL of 0.1X MEM plus 0.02% gelatine.
All collected samples, the viral stock and the controls were assayed for infectious virus particles using monolayer cultures of Madin-Darby canine kidney (MDCK – ATCC CCL-34) cells grown in 1X MEM plus 10% fetal calf serum (FCS) in 24-well tissue culture plates. One ml aliquots of the blank, control, or samples, and/or of samples diluted in 1X MEM plus 1 µg mL–1 trypsin, were placed in each of four wells for each test run. The assay plates were incubated at 36ºC ±2ºC with 5 ±1% CO2 for 4 to 6 days. The host cells were then examined for virus-specific cytopathic effects (CPE). The 50% tissue culture infectious dose per mL (TCID50 mL–1) of the samples was calculated using the method of Spearman-Kärber [6]. If a sample contained no detectable virus CPE, the theoretical titer with 95% confidence limits was calculated using Poisson distribution.
All blanks, controls and filter tests were run for a period of 15 minutes (±15 seconds). Immediately after the challenge, the challenge nebulizer was replaced with a sterile nebulizer containing sterile aerosol media run for five minutes to move any remaining particles into the impinger. The nebulizer was then shut down and the vacuum run for two more minutes to clear remaining material from the system and to maximize capture by the impingers. The total viral challenge was calculated for each control and test from the volume nebulized multiplied by the virus concentration (infective particles mL–1) and by the system efficiency. The latter took into account the nebulized volume deposited in the system upstream of the filter.
Virus stock titer, cell viability and media sterility
At the beginning of each day on which tests were performed, the following quality tests were performed: a) The H1N1 virus stock was checked for titer; b) The MDCK cells evaluated for viability and absence of H1N1; c) The media was evaluated for sterility.
System blank
Prior to each daily set of filter tests, a system blank was performed by nebulizing sterile 0.1X MEM into the system, with the filter blank in place, to assure system cleanliness. The impinger was assayed for the presence of H1N1.
Controls
Prior to each daily test set, and after the system blank, two system control input tests were performed, using the 0.1X MEM in which the H1N1 stock was dispersed, to determine the system efficiency and to assure that the challenge level for each set of tests met the minimum of 107 infective units in a 15-minute test challenge.
Breathing circuit filter tests
Four units of each of three breathing system filter models: Pall Ultipor® 25; Pall Ultipor® 100; Pall BB50T were tested.
All three filters subjected to our tests are mechanical filters that contain a water repellent (hydrophobic) filter medium. The manufacturer claims a high airborne bacterial and viral retention efficiency (≥99.999%) for all three filter models and 100% retention efficiency for liquid-borne bacterial and viral contaminations. All test filters had been previously investigated for their ability to retain various pathogenic bacterial and viral species (e.g. Mycobacterium tuberculosis, Staphylococcus aureus, hepatitis C virus, human immunodeficiency virus) and pathogenic prion proteins, but not for H1N1.
The Ultipor 25 and 100 filters are both indicated for patient-side use in anesthesia. The Ultipor 100 filter is also indicated for use in ICU ventilation. The BB50T filter is indicated for use at the machine side of the ventilator.
Detailed description of the experimental setting
The nebulizer outlet was connected to a short, flexible one-inch diameter silicone tube attached to a two-foot total length of one-inch diameter stainless steel tubing that served both to facilitate evaporation to reduce the mean diameter of the droplets, and to remove larger droplets by sedimentation and by impingement on the angled tubing walls.
The volume lost in the tubing upstream of the filter affects system efficiency:
System efficiency (%) = [(impinger volume x impinger titer) / (nebulizer output volume x concentration of virus)] x 100.
The evaporation column outlet was connected to the inlet of the tested breathing system filters using silicone tubing. (For the system Blank and Control tests the test filter was replaced with an empty filter housing.)
The outlet of the test filter was connected with flexible tubing to the inlet of a glass liquid impinger designed to operate at 12 to 13 L min–1 under a vacuum of greater or equal to 508 mbar. A calibrated flow meter in the system downstream of the impinger assured that the intended airflow was maintained during the tests.
An Anderson two-stage impaction sampler, containing capture Petri plates, was placed between the impinger and flow meter to protect the flow meter from large droplets escaping from the impinger. Two 0.2 µm membrane filters were installed downstream of the flow meter to assure complete protection of the vacuum pump and to prevent any virus particles from being released into the test area.
The total virus challenge to the filter is calculated as follows:
Total Challenge to filter (log number of virus particles) = (nebulizer output volume x nebulizer titer) x (% system efficiency).
The filter efficiency is:
Filter efficiency (%) = [(impinger volume x impinger titer) / Total Challenge] x 100.
Titer reduction is the inverse of the above converted to a log10.
Results
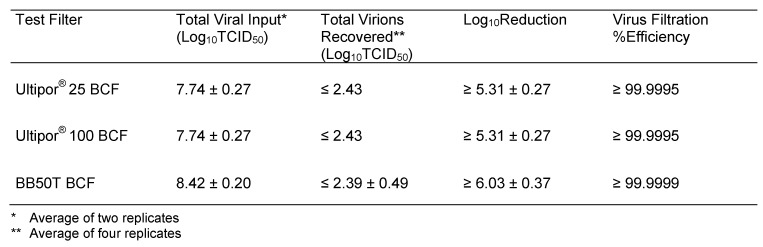
The results of the challenge tests for the three breathing circuit filters are shown in Table 1 (Tab. 1). A summary of the results with the average log efficiency (titer reduction) and average minimum percent efficiency for each filter model is shown in Table 2 (Tab. 2).
Table 1. H1N1 Challenge Data.
Table 2. Average titer reduction (% efficiency) for H1N1 removal.
The data in Table 1 (Tab. 1) show that all four tested units of Pall Ultipor® 25 and Ultipor® 100 completely retained the virus at the challenge level tested and at the detection limit of the tissue culture assay method. In addition, three of the four BB50T filters retained the entire input virus load while the fourth reduced the virus titer (log retention) by a factor of more than 105. The averaged data shown in Table 1 (Tab. 1) confirm that all three breathing circuit filter models (Ultipor® 25, Ultipor® 100 and BB50T) reduced the concentration of human influenza A virus H1N1 infective particles in an air stream by more than five orders of magnitude, with an efficiency of greater than 99.9995%. The slightly higher calculated efficiency of the BB50T BCF is due to the slightly higher (about 0.75 logs) input compared to the input for the Ultipor® 25 and Ultipor® 100 BCF units.
Discussion
The main finding of the investigation was that the PALL Ultipor® 25, Ultipor® 100 and BB50T breathing system filters effectively removed influenza H1N1A virus particles from the airstream.
Because of the frequency of severe cases caused by the 2009 swine flu virus, a prime endeavor in intensive care medicine was to keep the risk of infection of medical personnel and other patients as low as possible. The importance of using breathing filters is reflected in the new guidlines published by the German society for hospital hygiene and intensive care medicine for reducing possible bacterial or virus transmission [7].
The main transmission mode for the influenza virus is via aerosols that can remain airborne for more than 60 minutes because of their low settling velocity [8], [9]. This led to concerns of whether the routinely used breathing system filters could prevent airborne virus spread. This is particularly important during aerosol generating clinical procedures, such as all forms of mechanical ventilation, care of the artificial airway, specimen sampling from the respiratory tract, chest physiotherapy or administration of nebulized medication. Kola et al. showed in a meta-analysis of eight studies that the use of breathing system filters decreased the risk of ventilator-associated pneumonia. Conversely, this can be taken to indicate that the filters reduce the risk of releasing infectious particles into the ambient air [10]. The magnitude of this problem is illustrated by the SARS pandemic in 2003, during which e.g. in Hong Kong more than 20% of the patients with suspected or confirmed SARS were health care workers [11]. Arabi et al. reported in this context that health care workers performing or assisting endotracheal intubation had a 13-fold increased risk of being infected with SARS [12].
Marshall et al. showed that a significant risk factor for infection with H1N1 was working in an intensive care unit, where the majority of patients with confirmed H1N1 infections were intubated. It should be mentioned that even health care workers who had been vaccinated with the non-adjuvated split-virion 2009 H1N1 vaccine contracted swine flu [13].
In order to create a test situation simulating a highly infectious clinical situation, the test filters were subjected to a very high virus load of 7.74 ±0.27 log10 particles. In addition, the test rig was constructed to produce very small virus aerosol droplets. The short duration of the exposure period was considered adequate because earlier studies had shown that filtration efficiency does not diminish for at least 24 hours [14], [15], [16], [17]. The filtration efficiency of mechanical filters actually tends to increase over time as the substances retained on the filter form a layer (“filter cake”), which gradually increases particle retention.
The test system was designed to simulate the expiratory phase with the H1N1A droplets being carried in one direction, away from the “patient”. But under normal clinical conditions there is a bidirectional gas flow during the respiratory cycle with inspiratory and expiratory phases. While the exhaled air proximal to the filter is humid, the air distal to the filter is relatively dry, which can result in partial inactivation of the infectious particles. In addition, the gas flow during inspiration would carry escaped particles back into the filter and facilitate their capture.
The results of earlier investigations showed that the PALL Ultipor® 25 and 100 and the BB50T breathing system filters were able to prevent passage of hepatitis C and HIV virus [14], [15]. Previous studies had also shown that hydrophobic heat and moisture exchanger (HME) filters were impermeable to hepatitis C virus, and that the Ultipor® filters were effective at removing submicron particles using the salt crystal method [18]. But not all breathing system or HME filters are equally effective, as shown in the study of Ahmed et al. [19], who compared two filter models and found a significant colonization of the ventilator side of the filter with bacteria identical to the pulmonary aspirates in one model. Viruses are much smaller and would thus be more likely to pass through the filter.
The results of this investigation clearly show that the PALL Ultipor® 25 and 100 and the BB50T breathing system filters eliminated H1N1 influenza A virus from the air passing through the filters and are thus capable of preventing the spread of virus to the ventilator and the ambient air. It should be particularly noted that although the test setup was a worst case scenario, i.e. direct exposure of the filters to the “naked” virus and not a diluted virus aerosol, the removal effect of all filters was still efficient [15]. This study underlines the importance of assuring the filtration efficiency of HME and other types of breathing system filters, particularly for those used in ventilated patients with viral infections of the respiratory tract, in order to reduce the risk of transmission of viral infections to personnel and other patients.
Since we only tested the filters used in our institution we cannot comment on the suitability of other HME and breathing system filters for preventing virus spread from mechanically ventilated patients. This was a laboratory investigation and the results cannot be taken to indicate that the use of these filters can always prevent virus spread in the clinical situation, in which user errors can reduce the effectiveness of even the best filters.
Conclusion
With a filtration efficiency of more than 99.999%, the use of the PALL breathing system filters Ultipor® 25, Ultipor® 100, and BB50T renders the contamination of the respirator breathing system and the ambient air highly unlikely when properly employed under normal clinical conditions. One can conclude that they are suitable for use in ventilated patients with viral respiratory infections, including H1N1.
Notes
Competing interests
The authors declare that they have no competing interests.
Acknowledgements
The authors wish to express their appreciation to Dr. Steve Zhou, Microbiotest, a Division of Microbac Laboratories, and his staff for the excellent support given during the testing of the filters.
Authors' contributions
JFH, GH and MQ planned and designed the study. JFH and GH performed the measurements and analyzed the data. The tests were conducted by an independent laboratory (Microbiotest Inc., Sterling, VA, USA), with supervision and advice from GH. All authors (JFH, GH, TAC and MQ) participated in the analysis and interpretation of the results. The final manuscript was drafted by JFH, TAC and GH and was discussed and approved by all participating authors.
Funding
Pall Life Sciences, Port Washington, NY.
References
- 1.Westall GP, Paraskeva M. H1N1 Influenza: Critical Care Aspects. Semin Respir Crit Care Med. 2011;32(4):400–408. doi: 10.1055/s-0031-1283280. Available from: http://dx.doi.org/10.1055/s-0031-1283280. [DOI] [PubMed] [Google Scholar]
- 2.Rothberg MB, Haessler SD. Complications of seasonal and pandemic influenza. Crit Care Med. 2010;38(4 Suppl):e91–e97. doi: 10.1097/CCM.0b013e3181c92eeb. Available from: http://dx.doi.org/10.1097/CCM.0b013e3181c92eeb. [DOI] [PubMed] [Google Scholar]
- 3.Cohen J. Swine flu outbreak. Out of Mexico? Scientists ponder swine flu's origins. Science. 2009;324(5928):700–702. doi: 10.1126/science.324_700. Available from: http://dx.doi.org/10.1126/science.324_700. [DOI] [PubMed] [Google Scholar]
- 4.Roch A, Lepaul-Ercole R, Grisoli D, Bessereau J, Brissy O, Castanier M, Dizier S, Forel JM, Guervilly C, Gariboldi V, Collart F, Michelet P, Perrin G, Charrel R, Papazian L. Extracorporeal membrane oxygenation for severe influenza A (H1N1) acute respiratory distress syndrome: a prospective observational comparative study. Intensive Care Med. 2010;36(11):1899–1905. doi: 10.1007/s00134-010-2021-3. Available from: http://dx.doi.org/10.1007/s00134-010-2021-3. [DOI] [PubMed] [Google Scholar]
- 5.Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators; Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, Forrest P, Gattas D, Granger E, Herkes R, Jackson A, McGuinness S, Nair P, Pellegrino V, Pettilä V, Plunkett B, Pye R, Torzillo P, Webb S, Wilson M, Ziegenfuss M. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA. 2009;302(17):1888–1895. doi: 10.1001/jama.2009.1535. Available from: http://dx.doi.org/10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 6.Kärber G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Arch Exp Pathol Pharakol. 1931;162:480–483. doi: 10.1007/BF01863914. Available from: http://dx.doi.org/10.1007/BF01863914. [DOI] [Google Scholar]
- 7.Kramer A, Kranabetter R, Rathgeber J, Züchner K, Assadian O, Daeschlein G, Hübner NO, Dietlein E, Exner M, Gründling M, Lehmann C, Wendt M, Graf BM, Holst D, Jatzwauk L, Puhlmann B, Welte T, Wilkes AR. Infection prevention during anaesthesia ventilation by the use of breathing system filters (BSF): Joint recommendation by German Society of Hospital Hygiene (DGKH) and German Society for Anaesthesiology and Intensive Care (DGAI) GMS Krankenhaushyg Interdiszip. 2010;5(2):Doc13. doi: 10.3205/dgkh000156. Available from: http://dx.doi.org/10.3205/dgkh000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicas M, Nazaroff WW, Hubbard A. Toward understanding the risk of secondary airborne infection: emission of respirable pathogens. J Occup Environ Hyg. 2005;2(3):143–154. doi: 10.1080/15459620590918466. Available from: http://dx.doi.org/10.1080/15459620590918466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knight V. Viruses as agents of airborne contagion. Ann N Y Acad Sci. 1980;353:147–156. doi: 10.1111/j.1749-6632.1980.tb18917.x. Available from: http://dx.doi.org/10.1111/j.1749-6632.1980.tb18917.x. [DOI] [PubMed] [Google Scholar]
- 10.Kola A, Eckmanns T, Gastmeier P. Efficacy of heat and moisture exchangers in preventing ventilator-associated pneumonia: meta-analysis of randomized controlled trials. Intensive Care Med. 2005;31(1):5–11. doi: 10.1007/s00134-004-2431-1. Available from: http://dx.doi.org/10.1007/s00134-004-2431-1. [DOI] [PubMed] [Google Scholar]
- 11.Lau JT, Lau M, Kim JH, Tsui HY, Tsang T, Wong TW. Probable secondary infections in households of SARS patients in Hong Kong. Emerg Infect Dis. 2004;10(2):235–243. doi: 10.3201/eid1002.030626. Available from: http://dx.doi.org/10.3201/eid1002.030626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arabi Y, Gomersall CD, Ahmed QA, Boynton BR, Memish ZA. The critically ill avian influenza A (H5N1) patient. Crit Care Med. 2007;35(5):1397–1403. doi: 10.1097/01.CCM.0000262940.34596.4B. Available from: http://dx.doi.org/10.1097/01.CCM.0000262940.34596.4B. [DOI] [PubMed] [Google Scholar]
- 13.Ohara M, Tsubokura M, Naoto H, Kami M, Mochizuki T. H1N1 influenza A outbreak among young medical staff members who received single dose of non-adjuvanted split-virion 2009 H1N1 vaccine. Hum Vaccin. 2011;7(1):56–57. doi: 10.4161/hv.7.1.13459. Available from: http://dx.doi.org/10.4161/hv.7.1.13459. [DOI] [PubMed] [Google Scholar]
- 14.Lloyd G. Efficacy of a pleated hydrophobic filter as abarrier to human immunodeficiency virus transmission within breathing systems. Porton Down Salisbury Wiltshire, UK: Centre of Applied Microbiology and Research (CAMR); 1995. [Google Scholar]
- 15.Lloyd G, Howells J, Liddle C, Klineberg PL. Barriers to hepatitis C transmission within breathing systems: efficacy of a pleated hydrophobic filter. Anaesth Intensive Care. 1997;25(3):235–238. doi: 10.1177/0310057X9702500304. [DOI] [PubMed] [Google Scholar]
- 16.Preis I, Kobelt F, Joachim S, et al. Heat and moisture exchange filters for bacterial retention in intubated pigs. Intensive Care Medicine. 1995;21:38. [Google Scholar]
- 17.Vézina DP, Trépanier CA, Lessard MR, Gourdeau M, Tremblay C. Anesthesia breathing circuits protected by the DAR Barrierbac S breathing filter have a low bacterial contamination rate. Can J Anaesth. 2001;48(8):748–754. doi: 10.1007/BF03016689. Available from: http://dx.doi.org/10.1007/BF03016689. [DOI] [PubMed] [Google Scholar]
- 18.MHRA. 'Breathing system filters', a comparative assessment of 104 breathing system filters. London, UK: MHRA; 2004. (MHRA Device Evaluation Service publication; MHRA 04005). [Google Scholar]
- 19.Ahmed SM, Mahajan J, Nadeem A. Comparison of two different types of heat and moisture exchangers in ventilated patients. J Emerg Trauma Shock. 2009;2(3):164–169. doi: 10.4103/0974-2700.55327. Available from: http://dx.doi.org/10.4103/0974-2700.55327. [DOI] [PMC free article] [PubMed] [Google Scholar]