Abstract
Background: Antibiotic resistance of bacterial pathogens is an emerging problem worldwide. To combat multidrug resistant organisms (MRDOs) networks of care providers have been established in all states in Germany. The HICARE-network, a project to combat MRDOs, founded by the Federal Ministry of Education and Research, has published data from 2010 of a voluntary, German-wide, multicenter point-prevalence survey in 2011 conducted in collaboration with the German Society of Hospital Hygiene. The aim of the present survey was the re-evaluation of the situation in 2012.
Method: The survey was conducted as a voluntary, anonymous, point-prevalence in May 2012 using routine data of microbiological diagnostics of the hospitals. As in the former survey of 2010 it was differentiated between primary, secondary and tertiary care hospitals and only data from intensive care units, surgical and medical wards were collected. Based on the survey form used in 2010, an updated version was used including more pathogens and corrected issues observed in the former survey. Methicillin-resistant Staphylococcus aureus (MRSA) (total as well as separated in hospital-acquired (HA), community-acquired (CA) and lifestock-associated (LA) MRSA), vancomycin resistant Staphylococcus aureus (VRSA/GRSA), vancomycin resistant Enterococcus faecalis resp. Enterococcus faecium (VR-E. faecalis resp. VR-E. faecium), extended-spectrum-beta-lactamase-building (ESBL) E. coli (ESBL-EC) and Klebsiella pneumoniae (ESBL-KP), multiresistant Acinetobacter spp. (MAB), multiresistant Pseudomonas spp. (MRP), carbapenemase-producing Enterobacteriaceae (CRE) as well as Clostridium difficile (CD) infections and severe infections requiring ICU-treatment were included in the survey along with additional data on screening strategy, the equipment with infection control staff and possible confounders.
Results: Out of 1,550 hospitals asked to participate, 62 returned data (4%). Data from 56 hospitals including primary (26), secondary (20) and tertiary (10) care hospitals were analyzable (3.6%).
The most frequently reported organisms were MRSA 1.53% [CI95: 1.32–1.75], followed by CDAD 1.30% [CI95: 1.11–1.50], ESBL-EC 0.97% [CI95: 0.80–1.14], and ESBL-KP 0.27% [CI95: 0.18–0.36], regardless of the level of care. Prevalence of MRDOs depended on the level of care and on the type of ward, as expected. Overall prevalence was highest on intensive care wards, and prevalences were remarkably high on medical wards compared to surgical wards.
All tertiary care providers employed their own infection control nurse, while only ~70% of the secondary and primary care hospitals did. Surprisingly, in two of the ten participating tertiary care providers neither an internal nor an external infection control doctor was available.
Discussion: With more than 13,000 patients in 56 hospitals distributed all over Germany, the survey included more than three times as many patients as the first survey and therefore not only adds valuable information about the epidemiology of emerging nosocomial pathogens, but also helps to raise awareness of the problem of antibacterial resistance in Germany. The prevalences reported seem to be comparable to the results of the former survey and of other surveys published. Some hospitals reported to have no infection control personnel available at all. This statement is in line with another survey published in this issue.
Keywords: point prevalence, MRDOs, HICARE-network, MRSA, CDAD, ESBL, VRE, infection control staff, type of screening
Abstract
Hintergrund: Die Resistenzentwicklung gegen Antibiotika ist eine weltweit bedrohliche Situation. Zur Bekämpfung von multiresistenten Erregern (MRE) wurden in allen deutschen Bundesländern infektiologische Netzwerke der Leistungserbringer aufgebaut. Das HICARE-Netzwerk, ein vom Bundesministerium für Bildung und Forschung gefördertes Projekt, hat 2010 in Zusammenarbeit mit der Deutschen Gesellschaft für Krankenhaushygiene Ergebnisse einer auf freiwilliger Basis durchgeführten multizentrischen Punktprävalenzerhebung veröffentlicht. Mit der vorliegenden Studie sollten die Ergebnisse 2012 reevaluiert werden.
Methode: Die Erhebung wurde auf freiwilliger Basis anonymisiert als Punktprävalenz im Mai 2012 unter Zugrundelegung von Routinedaten der mikrobiologischen Diagnostik der Krankenhäuser durchgeführt. Wie in der vorangegangenen Erhebung von 2010 wurde zwischen Krankenhäusern der Maximal-, Schwerpunkt- und Regelversorgung unterschieden, und es wurden nur Daten von Intensivpflegeeinheiten sowie internistischen und chirurgischen Abteilungen erhoben. Die 2010 zugrunde gelegte Methode wurde in einigen Punkten korrigiert und um weitere MREs ergänzt. Eingeschlossen wurden Methicillin-resistente Staphylococcus aureus (MRSA) (insgesamt und unterschieden in Hospital-acquired (HA), Community-acquired (CA) and Lifestock-associated (LA) MRSA), Vancomycin resistente Staphylococcus aureus (VRSA/GRSA), Vancomycin resistente Enterococcus faecalis resp. Enterococcus faecium (VR-E. faecalis resp. VR-E. faecium), Extended-Spectrum-Beta-Lactamase bildende (ESBL) E. coli (ESBL-EC) und Klebsiella pneumoniae (ESBL-KP), multiresistente Acinetobacter spp. (MAB), multiresistente Pseudomonas spp. (MRP), Carbapenemase-bildende Enterobacteriaceae (CRE) und Clostridium difficile (CD) Infektionen einschließlich schwerer, intensivpflichtiger Verlaufsformen. Ergänzend wurden die Screeningstrategie, die Ausstattung mit Hygienefachpersonal und mögliche Confounder erfasst.
Ergebnisse: Von 1.550 angefragten Krankenhäusern beteiligten sich 62 (4%). Die Daten von 56 Krankenhäusern (3,6%) waren auswertbar, davon 26 der Regelversorgung, 20 der Schwerpunktversorgung und 10 der Maximalversorgung.
MRSA stand unabhängig vom Versorgungs- und Stationstyp in der Häufigkeit an erster Stelle mit 1,53% [CI95: 1,32–1,75], gefolgt von CDAD 1,30% [CI95: 1,11–1,50], ESBL-EC 0,97% [CI95: 0,80–1,14] und ESBL-KP 0,27% [CI95: 0,18–0,36]. Wie erwartet war die Prävalenz aller MRE am höchsten in Intensivtherapieeinheiten und verglichen mit chirurgischen Stationen relativ hoch in internistischen Stationen.
Während die Krankenhäuser der Maximalversorgung ausnahmslos eine hauptamtliche Hygienefachkraft beschäftigten, war das bei Schwerpunkt- und Regelversorgern nur in etwa 70% der Fall. Überraschenderweise verfügten zwei der zehn Krankenhäuser der Maximalversorgung weder über einen hauptamtlichen Krankenhaushygieniker, noch über einen nebenamtlich beschäftigten.
Diskussion: Mit mehr als 13.000 Patienten von 56 bundesweit verteilten Krankenhäusern schloss die Umfrage im Vergleich zur ersten Erhebung 2010 mehr als dreimal so viel Patienten ein. Damit werden nicht nur wertvolle Hinweise zur Epidemiologie gefährlicher nosokomialer Pathogene erhalten, sondern die Studie trägt auch dazu bei, dass die Prävalenzen mit den Ergebnissen früherer Erhebungen verglichen werden können. Einige Krankenhäuser verfügten über keinerlei Hygienefachpersonal. Dies steht in Übereinstimmung mit einer anderen in dieser Ausgabe veröffentlichten Analyse.
Introduction
Antibiotic resistance of bacterial pathogens is an emerging problem worldwide. While no longer limited to hospitals, inpatient care is still a focal point for problems associated with bacterial resistance. Besides MRSA, prevalence of other emerging nosocomial pathogens like multiresistant Enterobacteriacae and C. difficile have remarkably increased recently [1].
As multidrug resistant organisms (MDRO) fail to respond to antimicrobial therapy, infections due to these pathogens are prolonged, more severe and cause more complications. They also lead to higher tangible as well as intangible costs [2], [3], [4], [5], [6], [7], [8], [9], [10]. Outbreaks with these organisms do not only affect and harm numerous patients but can also lead to closure or severe impairment of the function of medical facilities, causing enormous costs [11].
To combat MRDOs networks of care providers have been established in all states in Germany. These networks are coordinated by the local, regional or state health authorities supported by the Robert Koch-Institute [12]. For a start, the unified management of MRSA was the main objective in most of these networks. To increase awareness of as well as knowledge on the regional epidemiology of MRDOs, some networks have conducted prevalence surveys, and some of these have been published [12].
In addition, the HICARE-network, established 2010 as part of a project, founded by the Federal Ministry of Education and Research of Germany to combat MRDOs, has published data from a voluntary, German-wide, multicenter point-prevalence survey in 2011 conducted in collaboration with the German Society of Hospital Hygiene. Besides MRSA, the prevalence of other MRDOs was assessed. The study, including 3,411 patients of five tertiary and four secondary care hospitals across Germany, showed a prevalence of 1.8% of MRSA, 0.45% of ESBL-E. coli, 0.41% of ESBL-Klebsiella spp., 0.53% of multiresistant Pseudomonas spp., 0.15% of multiresistant Acinetobacter spp., 0.49% of VRE and 1.01% of CDAD, with great local differences [13].
To reevaluate the epidemiology and support awareness of MRDO in Germany, a succession survey was initiated by the HICARE-network [14] and conducted in May 2012.
Method
The survey was conducted as a voluntary, anonymous, point-prevalence in May 2012 using routine data of microbiological diagnostics that have to be present in hospitals in Germany by law [15]. To allow comparison to the former survey as well as to the former distinction between primary, secondary and tertiary care hospitals, only data from intensive care units, surgical and medical wards were collected.
Based on the survey form used in 2010 [13], an updated version including more pathogens and corrected issues observed in the former survey was generated and converted into an active PDF-form (Adobe Acrobat X). The form was sent by E-mail to 1550 hospitals by the last week of April in 2012. Returned surveys were collected and consolidated using build-in functions of Adobe Acrobat and Microsoft Excel.
The following emerging bacterial pathogens were included in the survey: Methicillin-resistant Staphylococcus aureus (MRSA) (total as well as separated in hospital-acquired (HA), community-acquired (CA) and, lifestock-associated (LA) MRSA), vancomycin resistant S. aureus (VRSA/GRSA), vancomycin resistant (VR) Enteroococcus (E.) faecalis/E. faecium, extended-spectrum-beta-lactamase-building (ESBL) E. coli (ESBL-EC) and Klebsiella pneumoniae (ESBL-KP), multiresistant Acinetobacter spp. (MAB), multiresistant Pseudomonas spp. (MRP), carbapenemase-producing Enterobacteriaceae (CRE) as well as Clostridium difficile (CDAD) infections including severe infections requiring ICU-treatment.
To exclude outbreaks as possible confounder, hospitals were asked whether an outbreak with these pathogens was ongoing at the day of the survey.
Additionally, structure data on the level of care, number of beds, staffing with infection control personnel and the presence of admission screening for the pathogens were assessed. Finally, we assessed by whom and by which method the epidemiological data were provided.
Results
Response rate and data on the structure of hospitals and infection control
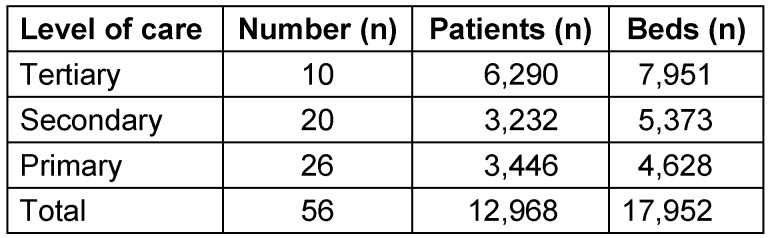
From the 1,550 hospitals asked to participate, 62 returned data (4%). Data from 56 hospitals (3.6%) were analyzable and included in the study. Out of the 56 hospitals ten (18%) were tertiary care providers, 20 (36%) were secondary and 26 (46%) primary care hospitals (Table 1 (Tab. 1)).
Table 1. Number, level of care, beds and patients treated in the included hospitals.
Data collection
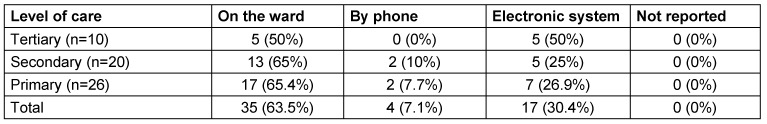
In most hospitals, data were collected at the wards. Some, especially tertiary care hospitals used data stored in electronic systems (Table 2 (Tab. 2)).
Table 2. Methods of data collection.
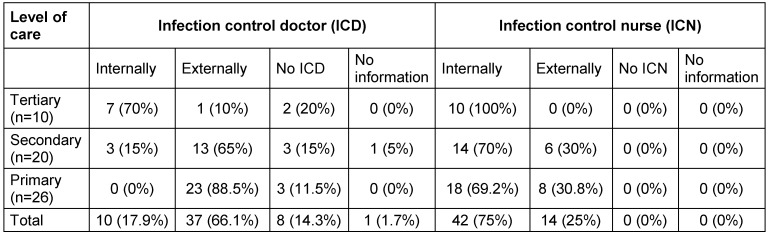
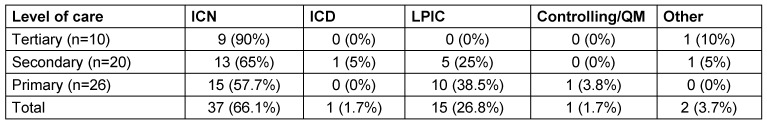
In most hospitals, data were collected by infection control personnel, mostly infection control nurses (Table 3 (Tab. 3)).
Table 3. Responsible personnel for collection of data (ICN = infection control nurse, ICD= infection control doctor, LPIC = link physician for infection control).
Prevalence data
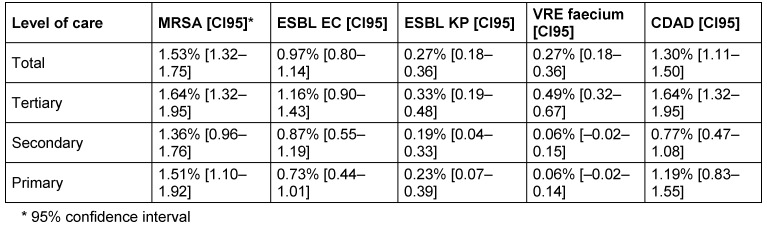
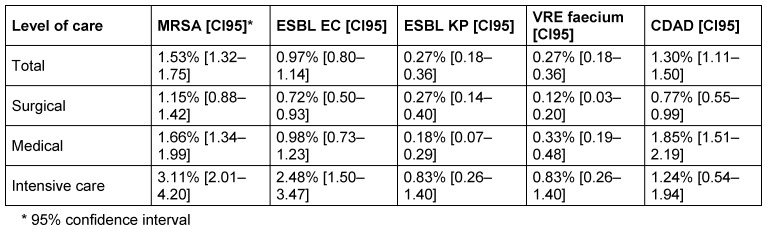
MRSA was the most frequently reported organism with 1.53% [CI95: 1.32–1.75], followed by CDAD 1.30% [CI95: 1.11–1.50], ESBL-EC 0.97% [CI95: 0.80–1.14], ESBL-KP 0.27% [CI95: 0.18–0.36] and VR-E. faecium 0.27% [CI95: 0.18–0.36], regardless of the level of care or ward. MRP were less frequent (depending on level of care 0.14–0.17% and depending on ward 0.03–0.72%). MAB was rarely reported (overall 0.02–0.1%).
CA-MRSA, LA-MRSA, VRSA, VR-E. faecalis and Carbapenemase-producing Enterobacteriaceae were not reported. Only a small percentage of CDAD infections required ICU-treatment (overall 0.02–0.6%).
As expected, the prevalence of MRDOs depended on the level of care (Table 4 (Tab. 4)) and on the type of ward (Table 5 (Tab. 5)). While confidence intervals of prevalence overlapped for MRSA and ESBL-EC and ESBL-KP between levels of care, VR-E. feacalis was rarely reported in secondary and primary care hospitals compared to tertiary care hospitals (Table 4 (Tab. 4)).
Table 4. Prevalences of the most frequently reported pathogens, divided into levels of care.
Table 5. Prevalences of the most frequently reported pathogens, divided into types of wards.
Overall prevalence was highest on intensive care wards (Table 5 (Tab. 5)) without overlapping confidence intervals for MRSA and ESBL-EC. The prevalences were remarkably high on medical wards compared to surgical wards (Table 5 (Tab. 5)).
Staffing with infection control personnel
As expected, the survey revealed differences between different levels of care. While all tertiary care providers employed their own infection control nurse, secondary and primary care hospitals employed infection control nurse only in about 70%. Surprisingly not in all tertiary hospitals an internal infection control doctor existed. In two tertiary care providers neither an internal nor an external infection control doctor was available (Table 6 (Tab. 6)).
Table 6. Percentage of infection control doctors and nurses, divided into levels of care.
Screening regime
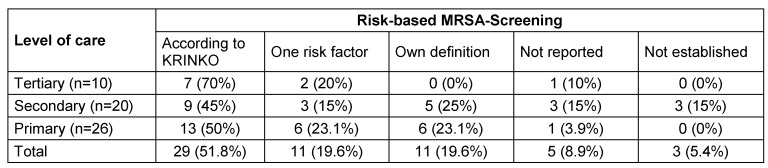
Most hospitals reported to have a MRSA-admission screening established, but using different methods. The definitions of the KRINKO guideline [16] are most often used to identify patients eligible for screening. This includes the screening of patients with two defined risk factors. 11 of the 56 hospitals screen patients with only one risk factor and 11 hospitals used their own definition (Table 7 (Tab. 7)). In one tertiary medical centre beyond to the screening of patients with one risk factor each newly admitted patient is screened on medical and surgical ICUs, stroke unit, weaning unit, dermatology, neonatology as well as all surgical patients with planned perioperative antibiotic prophylaxis. Also, in this centre a screening for VRE and 4 MRGN is established. Two other hospitals screen each admitted in-patient. It was noticeable that the screening in all hospitals was performed with internal infection control staff. Up to now no screening is established for other MDROs.
Table 7. MRSA-admission screening.
Discussion
This survey is an update to a survey conducted two years earlier using the same approach to collect data. However, a direct comparison between both surveys is not possible, because the samples are not identical and both surveys were anonymous, thus it is unclear if the same hospitals had participated. Finally the questionnaire used has been improved, controlling for possible confounders and assessing more information on the structure and organization of infection control measures in the participating hospitals.
With almost 13,000 patients in 56 hospitals distributed all over Germany, the second survey includes more than three times more patients as the first survey and therefore not only adds valuable information on the epidemiology of emerging nosocomial pathogens, but also helps to raise awareness of the problem of antibacterial resistance in Germany. The prevalences reported here are lower compared to our former survey [13] but tend to be higher than those reported in 2009 [17]. The explanation for the slightly lower prevalence compared to our first survey is the dominance of secondary and primary levels of care in the new prevalence study and furthermore the larger sample size.
Most remarkably, despite the frequency of MRDOs and the problems typically associated with these pathogens, staffing with infection control personnel seems to be inadequate in some hospitals, especially in some secondary and primary care hospitals. Some hospitals reported to have no infection control personnel available at all. These results are in line with another survey reported in this issue [18].
Conclusion
Point-prevalence studies, using existing routine data, can help to raise and maintain awareness as well as knowledge of the epidemiology of MRDOs and can therefore contribute to successful prevention strategies. While prevalences of individual MRDOs vary, antimicrobial resistance is an issue in all hospitals and wards regardless of the level of care or type of ward. Awareness, knowledge and responsibility are needed in order to not only control but primarily to prevent transmission as well as infection [19].
Notes
Competing interests
The authors declare that they have no competing interests.
Acknowledgement
We like to thank Sabine Gorynia, Oncotest GmbH, Freiburg, Germany, for the linguistic proof, and Ipse communication GmbH for providing an extensive list with e-mail contacts of hospitals.
This work was supported by the Federal Ministry of Education and Research and the Ministry of Education, Science and Culture of the state Mecklenburg Western-Pomerania.
References
- 1.European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2011. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) Stockholm: ECDC; 2012. Available from: http://ecdc.europa.eu/en/publications/Publications/antimicrobial-resistance-surveillance-europe-2011.pdf. [Google Scholar]
- 2.Gould I. Costs of hospital-acquired methicillin-resistant Staphylococcus aureus (MRSA) and its control. International J Antimicrob Ag. 2006;28(5):379–384. doi: 10.1016/j.ijantimicag.2006.09.001. Available from: http://dx.doi.org/10.1016/j.ijantimicag.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Haecker A. MRSA im Krankenhaus, Konsequenzen und Lösungen für das Klinikmanagement. KU Gesundheitsmanagement. 2009;8:61–62. [Google Scholar]
- 4.Hübner C, Hübner NO, Kramer A, Fleßa S. Cost-analysis of PCR-guided pre-emptive antibiotic treatment of Staphylococcus aureus infections: an analytic decision model. Eur J Clin Microbiol Infect Dis. 2012;31(11):3065–3072. doi: 10.1007/s10096-012-1666-y. Available from: http://dx.doi.org/10.1007/s10096-012-1666-y. [DOI] [PubMed] [Google Scholar]
- 5.Hübner NO, Hübner C, Kramer A. Ökonomische Aspekte des Hygienemanagements von MRSA. [Economic aspects of the management and control of MRSA]. Gesundheitswesen. 2009;71(11):771–776. doi: 10.1055/s-0029-1241891. (Ger). Available from: http://dx.doi.org/10.1055/s-0029-1241891. [DOI] [PubMed] [Google Scholar]
- 6.Lee NY, Lee HC, Ko NY, Chang CM, Shih HI, Wu CJ, Ko WC. Clinical and economic impact of multidrug resistance in nosocomial Acinetobacter baumannii bacteremia. Infect Control Hosp Epidemiol. 2007;28(6):713–719. doi: 10.1086/517954. Available from: http://dx.doi.org/10.1086/517954. [DOI] [PubMed] [Google Scholar]
- 7.Morales E, Cots F, Sala M, Comas M, Belvis F, Riu M, Salvado M, Grau S, Horcajada JP, Montero MM, Castells X. Hospital costs of nosocomial multi-drug resistant Pseudomonas aeruginosa acquisition. BMC Health Serv Res. 2012;12:122. doi: 10.1186/1472-6963-12-122. Available from: http://dx.doi.org/10.1186/1472-6963-12-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nulens E, Broex E, Ament A, Deurenberg RH, Smeets E, Scheres J, van Tiel FH, Gordts B, Stobberingh EE. Cost of the meticillin-resistant Staphylococcus aureus search and destroy policy in a Dutch university hospital. J Hosp Inf. 2008;68(4):301–307. doi: 10.1016/j.jhin.2008.01.018. Available from: http://dx.doi.org/10.1016/j.jhin.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Nathwani D. Health economic issues in the treatment of drug-resistant serious Gram-positive infections. J Infect. 2009;59 Suppl 1:S40–S50. doi: 10.1016/S0163-4453(09)60007-4. Available from: http://dx.doi.org/10.1016/S0163-4453(09)60007-4. [DOI] [PubMed] [Google Scholar]
- 10.Maragakis LL, Perencevich EN, Cosgrove SE. Clinical and economic burden of antimicrobial resistance. Expert Rev Anti Infect Ther. 2008;6(5):751–763. doi: 10.1586/14787210.6.5.751. Available from: http://dx.doi.org/10.1586/14787210.6.5.751. [DOI] [PubMed] [Google Scholar]
- 11.Piednoir E, Thibon P, Borderan GC, Godde F, Borgey F, Le Coutour X, Parienti JJ. Long-term clinical and economic benefits associated with the management of a nosocomial outbreak resulting from extended-spectrum beta-lactamase-producing Klebsiella pneumoniae. Crit Care Med. 2011;39(12):2672–2677. doi: 10.1097/CCM.0b013e31822827e0. [DOI] [PubMed] [Google Scholar]
- 12.Mielke M. Bericht über das 3. Treffen der Moderatoren der Regionalen MRE-Netzwerke am 15. und 16. Dezember 2011 am Robert Koch-Institut. [Report of the third meeting of the coordinators of the regional MRP networks in Germany on 15 and 16 December 2011 at the Robert Koch Institute]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2012 Nov;55(11-12):1474–1482. doi: 10.1007/s00103-012-1553-9. (Ger). Available from: http://dx.doi.org/10.1007/s00103-012-1553-9. [DOI] [PubMed] [Google Scholar]
- 13.Kramer A, Ryll S, Wegner C, Jatzwauk L, Popp W, Hübner NO. One-day point prevalence of emerging bacterial pathogens in four secondary and five tertiary care German hospitals – results from a pilot study of the German Society for Hospital Hygiene (Deutsche Gesellschaft für Krankenhaushygiene, DGKH) GMS Krankenhaushyg Interdiszip. 2011;6(1):Doc20. doi: 10.3205/dgkh000177. Available from: http://dx.doi.org/10.3205/dgkh000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linder R, Thoms I, Pfenning I, Schadowski R, Möws V. The project HICARE: cross-sectoral action alliance against multi-resistant pathogens. GMS Krankenhaushyg Interdiszip. 2011;6(1:Doc25 DOI):10. doi: 10.3205/dgkh000182. Available from: http://dx.doi.org/10.3205/dgkh000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gesetz zur Änderung des Infektionsschutzgesetzes und weiterer Gesetze (IfSGuaÄndG), Geltung ab 04.08.2011. Bgbl I. 2011;41:1622. [Google Scholar]
- 16.Empfehlung zur Prävention und Kontrolle von Methicillinresistenten Staphylococcus aureus-Stämmen (MRSA) in Krankenhäusern und anderen medizinischen Einrichtungen. Mitteilung der Kommission für Krankenhaushygiene und Infektionsprävention am RKI. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 1999;42(12):954–958. [Google Scholar]
- 17.Geffers C, Gastmeier P. Nosokomiale Infektionen und multiresistente Erreger in Deutschland: Epidemiologische Daten aus dem Krankenhaus-Infektions-Surveillance-System. Dt Ärztebl Int. 2011;108(6):87–93. doi: 10.3238/arztebl.2011.0087. Available from: http://dx.doi.org/10.3238/arztebl.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer A, Assadian O, Helfrich J, Krüger C, Pfenning I, Ryll S, Perner A, Loczenski B. Questionnaire-based survey on structural quality of hospitals and nursing homes for the elderly, their staffing with infection control personal, and implementation of infection control measures in Germany. GMS Hyg Infect Control. 2013;8(1):Doc11. doi: 10.3205/dgkh0002111. Available from: http://dx.doi.org/10.3205/dgkh0002111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mielke M, Schaade L. Aufmerksamkeit, Wissen und Verantwortung. [Awareness, knowledge and responsibility]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2012;55(11-12):1361–2. DOI 10. doi: 10.1007/s00103-012-1561-9. (Ger). Available from: http://dx.doi.org/10.1007/s00103-012-1561-9. [DOI] [PubMed] [Google Scholar]