Abstract
Neuroprotective agents are becoming significant tools in the repair of central nervous system injuries. In this study, we determined whether ginkgolides (Gin, extract of GinkgoBiloba) and Acanthopanax senticosus saponins (ASS, flavonoids extracted from Acanthopanax herbal preparations) have protective effects on rat spinal cords exposed to anoxia and we explored the mechanisms that underlie the protective effects. Spinal motor neurons (SMNs) from rat spinal cords were obtained and divided into five groups with 10 wells in each group. In control group, SMNs suffered no injury under normal oxygen; in hypoxia- inducible (HI) group, SMNs suffered injury from hypoxia; in Gin group, 37.5µg/ml Gin were used before 24 hrs of hypoxia; in ASS group, 50µg/ml ASS were used before 24 hrs of hypoxia;in glial cell-lined derived neurotrophic factor (GDNF) group, 0.1µg/ml GDNF were used before 24 hrs of hypoxia. Changes in morphology, neuron viability, and lactate dehydrogenase (LDH) release were observed. In addition, the expression of HIF-1α induced by hypoxia was measured. The neuronal viability in the Gin, ASS, and GDNF pretreated groups was higher than that in the HI group (P<0.05). The viability in the Gin group was better than that in the ASS group (P<0.05), but there was no significant difference between the ASS and GDNF groups (P>0.05). The quantity of LDH released in the three pretreated groups was lower than that in the HI group (P<0.05). The expression of HIF-1α in the HI group was greater than that in the control group (P<0.05), and the expression in the three pretreated groups was greater than that in the HI and the control groups (P<0.05). Our results indicate that Gin and ASS which was not as effective as Gin, but its effects were similar to those of GNDF could all enhance the viability of SMNs and have protective effects on hypoxic neurons.
Keywords: ginkgolides, Acanthopanax senticosus saponins, apoptosis, hypoxia, motor neurons, hypoxia-inducible factor-1, rats
Introduction
Acute spinal cord injury remains a hard-to-cure disease. Apoptosis of neurons has been reported to occur after spinal cord injury. Thus, the main objective of neuroprotection is to delay or block apoptosis of neurons (Johnson et al., 1995; Beattie et al., 1997). Screening for neuroprotective agents and studies of their pharmacological mechanisms is becoming a research hot spot in the field of central nervous system injury repair.
Ginkgolides (Gin) consists of the diterpene trilactones of Ginkgo biloba, including ginkgolides a, b, c, j and m, which are powerful platelet-activating factor antagonists. Gin reportedly has neuroprotective effects in vivo and in vitro (Wu and Zhou, 1999). Acanthopanax senticosus saponins (ASS), which is a flavonoid preparation extracted from the Chinese medicinal herb Acanthopanax senticosus Harms, was reported to be protective to ischemic brain tissue (Wu and Zhou, 1999). Gin and ASS also have been shown to protect the ischemic cerebral cortex neurons of embryonic rats by increasing SOD, decreasing MDA, and antagonizing the toxicity of excitatory amino acids (Jin et al., 2006). Accordingly, Gin and ASS are presumed to be effective in curing acute spinal cord injury. Ischemia-hypoxia injury, which is caused by secondary injury after spinal cord injury in spinal tissue, has been shown to induce the expression of hypoxia-inducible factor 1 (HIF-1) in spinal tissue. This eukaryotic transcription factor is one of the key regulators of oxygen homeostasis, and it could affect the gene expression responsible for cell survival, growth, differentiation, and apoptosis. The activation of HIF-1 was considered to be the key component in cellular responses to hypoxia (Huang and Bunn, 2003).
The objective of this in vitro study was to survey the protective effects of Gin and ASS on spinal motor neurons (SMNs) from rat embryos with ischemia-hypoxia injury and to outline the possible mechanisms -including the activation of HIF-1—for their observed pharmacological effects.
Materials and Methods
Animals and reagents
This study was conducted at the Key Laboratory of Neural Regeneration of Jiangsu Province, Medical College of Nantong University, from March 2004 to May 2005. Gin was provided by China Pharmaceutical University, and ASS was provided by the Department of Organic Chemistry of Jilin University. Polylysine, trypsinase, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), and dimethyl sulphoxide were purchased from Sigma (St. Louis, MO, USA). Rabbit anti-mouse neuronal specific enolase (NSE) antibody and biotinylated goat anti-rabbit IgG were purchased from Beijing Zhongyuan Company (Beijing, P.R. China). Dulbecco's modified eagle medium (DMEM), fetal bovine serum (FBS), and glia cell-derived neurotrophic factor (GDNF) were purchased from Gibco BRL (Grand Island, NY, USA). Sprague-Dawley (SD) rats were provided by the Experimental Animal Centre of Nantong University (Nantong, Jiangsu Province, P.R. China).
Culturing of SMNs from rat embryos in vitro and staining characterization (Kuhn, 2003; Guigoni and Coulon, 2002)
Pregnant SD rats at 15 days of gestation were placed under ether anesthesia, and five embryos were removed under sterile conditions. The spinal cords of embryos were isolated, and the spinal cord anterior horn tissue from the ventral part of the spinal cord was dissected and digested in 0.25% trypsin (Sigma) and 1% collagenase (Sigma) at 37 °C. Spinal cord anterior horn cells were suspended in DMEM with 10% FBS and purified using the differential velocity adherent technique. Cells were centrifuged and resuspended in neurobasal medium, and the cell density was adjusted to 1 × 106 cells/mL. The cell suspension was incubated at 37 °C in a 5% CO2 incubator, and the medium was changed twice per week.
Immunohistochemical staining for NSE was conducted as follows: Cells at 10 days of culture were fixed in 4% paraformaldehyde at room temperature, and 0.6% H2O2 in methanol was added to quench endogenous peroxidase activity. The cells were incubated in 10% (v/v) normal sheep serum for 20 min and then incubated overnight at 4°C with rabbit anti-mouse NSE antibody (1:200). Subsequently, the sections were incubated with biotinylated goat anti-rabbit IgG (1:200) followed by incubation with avidin-biotin peroxidase complex for 60 min. The enzyme reaction was developed using diaminobenzidine. The sections were dehydrated and cover slipped with Cytoseal, and cells were observed under a light microscope.
Establishing the model of ischemia-hypoxia injury (Romijn et al., 1988)
Ischemia-hypoxia injury was induced in SMNs that had been cultured for 7 days. The medium was changed to L-15 medium without serum in 24-well cell culture plates, and the wells were sealed off with paraffin for 3 days to generate hypoxic conditions. The experimental groups were as follows (10 wells per group):
Control group: SMNs were cultured in normal conditions for 10 days.
Hypoxia injury (HI) group: SMNs were incubated with 800 µl of 0.01 mol/L PBS (pH 7.2) for 24 hr before hypoxia injury was induced.
Gin group: SMNs were preincubated with 800 µl of 37.5 µg/ml Gin 24 hr after being cultured in plates and 24 hr before hypoxia injury was induced.
ASS group: SMNs were preincubated with 800 µl of 50 µg/ml ASS 24 h after being cultured in plates and 24 hr before hypoxia injury was induced.
GDNF group (positive control group): SMNs were preincubated with 800 µl of 0.1 µg/ml GDNF 24 hr after being cultured in plates and 24 hr before hypoxia injury was induced.
Morphological observations
The status and morphological changes that occurred in SMNs cultured under different conditions were observed directly under an inverted microscope. For scanning electron microscope (SEM) analysis, SMNs were inoculated onto the cover glass slides for 10 days and then were fixed with 4% glutaraldehyde and washed in buffer, refixed in 1% buffer osmic acid, washed again in buffer, dehydrated in 100% acetonitrile, dried by vacuum, and sputter coated before observation under a JSM-T300 SEM (JEOL, Jap). For transmission electron microscope (TEM) analysis, adherent SMNs were obtained and fixed with 4% glutaraldehyde, refixed in 1% buffer osmic acid, dehydrated in a graded ethanol series, embedded in Epson 812 resin, sliced with a LKB-V ultramicrotome (LKB, Swe), and double-stained with uranyl acetate and lead citrate before examination under a JEM 100S TEM (JEOL).
Determination of the neuron survival rate using the MTT method
MTT stock solution was added to each well in 96-well plates so that the final concentration of MTT was 1 mg/ml, and the cells then were incubated for 4 hr at 37 °C. The reaction was stopped by adding 100 µl of solution containing 50% N,N-dimethylformamide and 20% SDS to each well. After incubation for 20–24 hr at 37 °C, the absorbance (D570) was measured with a microplate reader at a test wavelength of 570 nm. The survival rate of the cells was calculated as follow:
Survival rate = (D570 treated/D570 control) × 100%
Determination of the release of LDH (Liu, et al., 2000)
To measure the release of LDH, 1.3 ml of 0.194 mg/ml pyruvate solution and 1.3 ml of 0.154 mg/ml nicotinamide adenine dinucleotide-reduced (NADH) solution were added to K2HPO4/KH2PO4 buffer (pH 7.5). Next, 0.4 ml of culture solution was mixed in to the pyruvate/NADH solution quickly at 37 °C. The absorbance at 340 nm (D340) was measured with a microplate reader 30 s later and every 10 s from then on for 5 min. One unit of LDH activity was defined as a decrease in absorbance at 340 nm of 0.001 per minute per ml of culture solution.
Detection of HIF-1α protein expression of SMNs using SDS-PAGE and Western blotting
The proteins were extracted from each group and loaded onto an SDS-PAGE gel. Vertical slab gel electrophoresis was run, followed by transmembranal transport. The rabbit anti-mouse NSE antibody and biotinylated goat anti-rabbit antibody were added to the antigen-antibody reaction. SX-300 four Stars image analyzing equipment (Shanghai, P.R. China) was used to analyze the image after the reaction was developed, fixed, and scanned.
Statistical analysis
Data were expressed as mean ± SD, and the results were analyzed with SPSS 13.0 software. One-way ANOVA followed by a Scheffe test was used for intragroup comparison. P < 0.05 was considered to be significant.
Results
The growth state of SMNs and staining characterization
The connections between neurons, neurons, and non-neuronal cells (horizontal cells mainly) were observable when SMNs had been cultured for 10 days. Fibroblasts and neuroglia cells grew on the underlayer and neurons grew on the upper layer. After NSE immunohistochemical staining of SMNs cultured for 10 days, neurons were stained brown and the morphology could be observed clearly (Figure 1). Thus, SMNs were cultured successfully using this method.
Figure 1.
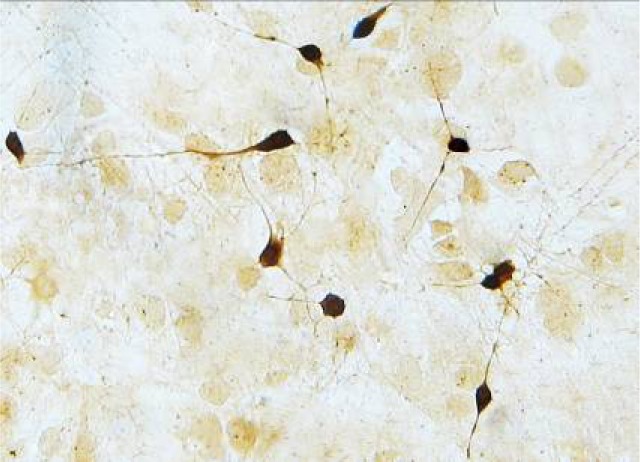
Neurons were stained brown after the immunohistochemical staining (inverted microscope ×400).
Morphological observations of SMNs
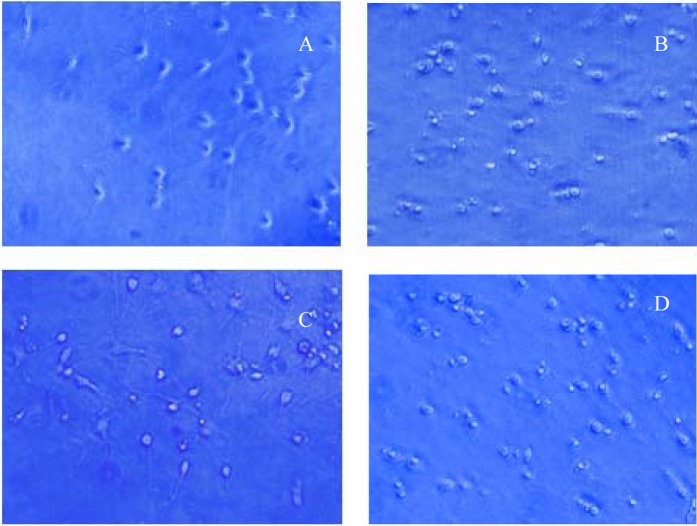
Inverted microscope: The cultured neurons in the control group grew well and halos formed around them. The neurites were slender and neurite networks were developed (Figure 2A). In the HI group, the transmittance of the neurons decreased, the cells were crimped, neurites were broken, and the networks disappeared. Some cracked neurons and suspended dead cells were observed (Figure 2B). In the Gin (Figure 2C) and ASS groups, the morphology of the neurons was better than that in the HI group, and more neurites remained. However, some cellular necrosis and swelling were observed. The cellular morphology in the GDNF group (Figure 2D) was similar to that in the Gin and ASS groups.
Figure 2.
(A) Neurons cultured for 10 days grew well, formed halos, and showed increased neurites (inverted microscope ×400). (B) Neurons cultured for 3 days under hypoxia: The transmittance decreased, cells crimpled, and many dead cells were present (inverted microscope ×400). (C) Neurons cultured for 3 days pretreated with Gin under hypoxia: The morphology of neurons was better than that in the hypoxia injury group, although some cellular necrosis and swelling was visible (inverted microscope ×400). (D). Neurons cultured for 3 days pretreated with GDNF under hypoxia: The morphology of neurons was better than that in the hypoxia injury group and most neurite lies (inverted microscope ×400).
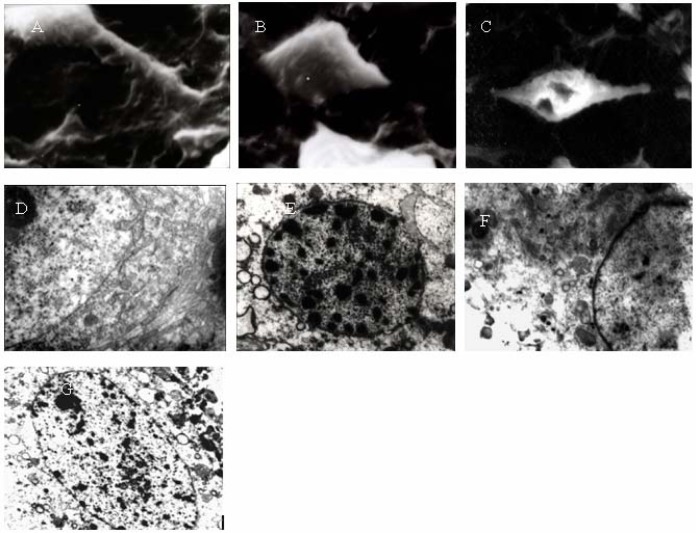
SEM: Under the SEM, the cell bodies of SMNs cultured for 10 days in the control group were well stacked, the cellular membranes were smooth, and the neurites were thick (Figure 3A). In contrast, the neurons in the HI group were crimped or pierced and the neurites were broken or had disappeared (Figure 3B). In the Gin (Figure 3C) and ASS groups, the cell bodies of the neurons were smaller and the neurites were thinner and even broken in places compared to the control group.
Figure 3.
(A) The normal morphology of neurons: The cell bodies were well stacked, the cellular membranes were smooth, and the neurites were thick (SEM ×3500). (B) The morphology of neurons under hypoxia: The cell bodies were smaller, the cellular membranes were crimpled, and the neurites were broken or had disappeared (SEM ×3500). (C). The morphology of neurons pretreated with Gin under hypoxia: The neurites were thinner and broken or had disappeared (SEM ×3500). (D) The normal morphology of neurons: Nuclear membranes were complete and smooth; chromatin was well distributed, and the structure of the endoplasmic reticulum was clear (TEM ×6000). (E) The morphology of neurons under hypoxia: The color of the cell nucleus was deepened, the nuclear membranes were not complete, the kytoplasm was condensed, and the rough endoplasmic reticulum was expanded (TEM ×6000). (F) The morphology of neurons pretreated with Gin under hypoxia: The nuclear membranes were complete, the kytoplasm was condensed slightly, and the rough endoplasmic reticulum was expanded slightly (TEM ×6000). (G) The morphology of neurons pretreated with GDNF under hypoxia: The nuclear membranes were complete; the kytoplasm was condensed slightly, and the rough endoplasmic reticulum was expanded slightly (TEM ×6000).
TEM: TEM analysis revealed that the morphology of neurons in the control group was normal. Smooth nuclear membranes and a round or oval nucleus were apparent, chromatin and perinuclear cisterna were well distributed, and the nucleolus was easily visible. Moreover, the structures of the intracytoplasmic mitochondria and rough endoplasmic reticulum were clear and ribosomes were numerous (Figure 3D). In contrast, the cell bodies of neurons in the HI group were smaller and rounder. Some small introcessions in the nuclear membranes were present, and the neurites were broken or had disappeared. The cytoplasm was condensed, the mitochondria were swollen and vacuolated, the rough endoplasmic reticulum had expanded, and the chromatin was condensed (Figure 3E). In the Gin (Figure 3F), ASS, and GNDP groups (Fig. 3G), the morphology of the neurons was much more normal than that in the HI group, and the cellular damage was lighter. Most of the nuclear chromatin was well distributed, and the ultramicrostructures of the cells were similar to those in the control group. The structures of mitochondria were clear and ribosomes were numerous.
The effects of Gin and ASS on survival rate, LDH release, and HIF-1α expression of SMNs
Table 1 shows the survival rate, LDH release, and HIF-1α expression of the SMNs used in this study. The D570 of SMNs decreased significantly after hypoxia injury was induced, indicating that the survival rate of the cells in the HI group decreased notably compared to that of control group (P < 0.01). The D570s of the Gin, ASS, and GDNF groups were significantly higher than that in the HI group (P < 0.05), which indicated that Gin, ASS, and GDNF could improve the activities of SMNs significantly. Moreover, the effect of Gin was better than that of ASS (P < 0.05), and the effects of GDNF and ASS were much more similar (P > 0.05). The LDH release in the HI group was much higher than that in the control group (P < 0.01). The release of LDH in the Gin, ASS, and GDNF groups was lower than that in the HI group (P < 0.01). The effect of Gin was better than that of ASS (P < 0.05), and the effects of GDNF and ASS were not statistically different (P > 0.05).
Table 1.
The effects of ASS and Gin on the survival rate, LDH release, and HIF-1α expression of SMNs under ischemia-hypoxia injury.
| Groups | Survival rate (D570) |
LDH release (D340) |
Gray value rate of HIF-1α/β-actin |
| Control | 0.26 ± 0.035 | 14.15 ± 0.416 | 0.16 ± 0.003 |
| Hypoxia injury | 0.15 ± 0.012a | 40.7 ± 1.885a | 0.72 ± 0.027a |
| ASS | 0.21 ± 0.028b | 28.6 ± 1.309b | 1.15 ± 0.016a,b |
| Gin | 0.25 ± 0.059b,c | 22.8 ± 1.645 b,c | 1.28 ± 0.019a,b,c |
| GDNF | 0.20 ± 0.026b,d | 26.5 ± 0.885b,d | 1.12 ± 0.016a,b,d |
Note:One-way ANOVA revealed significant effects in these groups (P < 0.001). Values represent mean ± SD (n = 10).
P < 0.05 compared with the control group,
P < 0.05 compared with the hypoxia injury group,
P < 0.05 compared with the ASS group,
P < 0.05 compared with the Gin group.
Western blot analysis was conducted after the proteins of SMNs were extracted. There was no HIF-1α expression in the control group. The injury treatment induced HIF-1α expression of neurons in the injury group, and the levels of expression were enhanced in the Gin, ASS, and GDNF pretreatment groups.
Discussion
With a better understanding of the molecular mechanism responsible for ischemia-hypoxia spinal cord injury, research of neuroprotective agents became significant and spectacular. Gin was reported to have a protective effect on neurons cultured in vitro as well as an effect on auxoaction of peripheral nerve regeneration and reduction of neuron apoptosis (Chandrasekaran et al., 2003). ASS was shown to have the pharmacological activities of anti-hypoxia, anti-edema, removal of free radicals, raising of intracellular SOD, blocking of the calcium channel, inhibiting endothelin secretion and NO release, and reducing platelet aggregation. ASS was reported to cure experimental brain injury and clinical cerebrovascular insufficiency (Chen et al., 2006). Many reports exist about the protective effects of Gin and ASS on cortical neurons with ischemia-hypoxia injury and their mechanisms of action (Ahlemeyer et al., 1999; Chen et al., 2007; Saleem et al., 2008). However, this is the first report about the effects of Gin or ASS on rat SMNs with ischemia-hypoxia injury cultured in vitro.
In this study, SMNs, which are sensitive to ischemia-hypoxia, were cultured in vitro. The pathological processes of neuronal injury in our ischemia-hypoxia injury model were similar with those reported for injury of spinal tissue under ischemia-hypoxia after clinical spinal cord injury. This model also could be used to study the pathogenesis of secondary injury in spinal tissue under early ischemia-hypoxia conditions and early drug intervention. GDNF, the cholinergic neurotrophic factor with the most specificity, could improve the activities, promote the differentiation, and enhance the metabolism of the SMNs (Chen et al., 2006). For this reason, GDNF was chosen as the positive control in this study. HIF-1, which is one of the transcription factors and one of the key regulators of oxygen homeostasis in hypoxia injury for mammals and humans, could activate the expressions of various hypoxia-response genes (Oppenheim et al., 1995). Therefore, the expression of HIP-1 in spinal tissue under ischemia-hypoxia injury was used to evaluate the degree of spinal cord injury and it's treatment.
In our study, the survival rate of SMNs with ischemia-hypoxia injury decreased significantly relative to that of the control, whereas the survival rate of cells in the Gin and ASS groups was significantly higher than that of the injury group. In the neurons treated with ischemia-hypoxia, the typical ultramicrostructure characteristic of apoptosis were visible under SEM and TEM: The cell bodies of impaired neurons became smaller and rounder, the neurites were broken or had disappeared, microvilli were missing, the cytoplasm was condensed, the rough endoplasmic reticulum expanded, the cell membrane was vacuolated and pierced, the nucleus shrank, the nuclear membranes broke, and the chromatin condensed. However, the ultramicrostructures of the neurons treated with Gin, ASS, and GDNF were similar to those of the control group. These results revealed that Gin, ASS, and GDNF could protect neurons against apoptosis caused by ischemia-hypoxia injury and the protective effect of Gin was better than those of ASS and GDNF. The results of the LDH release experiment and Western blot analysis revealed that ischemia-hypoxia injury could induce HIF-1α expression of neurons, and this expression was enhanced when the neurons were pretreated with Gin, ASS, and GDNF. This result suggests that the mechanism of protection against ischemia-hypoxia injury for Gin and ASS might be related to enhancing stabilization of the neuron cellular membrane under ischemia-hypoxia injury and to the HIF-1α expression of neurons. Previous studies revealed that bonding of the antisense oligonucleotides of HIF-1α with the initiator codon upstream on the mRNA could interrupt the translation of proteins and thereby block HIF-1α expression (Bergeron et al., 2000; Qian et al., 2004). Our results about protection of Gin and ASS might also up-regulate HIF-1α expression in the initial stage of ischemia-hypoxia injury of neurons, start the expression of downstream genes, promote the removal of oxygen free radicals, and enhance the stabilization of the cellular membrane and intra-cellular environment, thereby working against ischemia-hypoxia injury. However, how Gin and ASS up-regulate HIF-1α expression and whether the same signal transduction pathway is at work for Gin and ASS remains unknown and requires further research.
Abbreviations
- GIN
ginkgolides
- ASS
Acanthopannx Senticosus Saponins
- SMNs
spinal motor neurons
- HI
hypoxia-inducible
- GDNF
glial cell-lined derived neurotrophic factor
- LDH
lactate dehydrogenase
References
- 1.Ahlemeyer B, Mowes A, Krieglstein J. Inhibition of serum deprivation and staurosporine-induced neuronal apoptosis by Ginkgo biloba extract and some of its constituents. Eur J Pharmacol. 1999;367:423–430. doi: 10.1016/s0014-2999(98)00903-0. [DOI] [PubMed] [Google Scholar]
- 2.Beattie MS, Bresnahan JC, Komon J, Tovar CA, Van Meter M, Anderson DK, Faden AI, Hsu CY, Noble LJ, Salzman S, Young W. Endogenous repair after spinal cord contusion injuries in the rat. Exp Neurol. 1997;148:453–463. doi: 10.1006/exnr.1997.6695. [DOI] [PubMed] [Google Scholar]
- 3.Bergeron M, Gidday JM, Yu AY, Semenza GL, Ferriero DM, Sharp FR. Role of hypoxia-inducible factor-1 in hypoxia-induced ischemic tolerance in neonatal rat brain. Ann Neurol. 2000;48:285–296. [PubMed] [Google Scholar]
- 4.Chandrasekaran K, Mehrabian Z, Spinnewyn B, Chinopoulos C, Drieu K, Fiskum G. Neuroprotective effects of bilobalide, a component of Ginkgo biloba extract (EGb 761) in global brain ischemia and in excitotoxicity-induced neuronal death. Pharmacopsychiatry. 2003;36(Suppl 1):89–94. doi: 10.1055/s-2003-40447. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Zhu L, Pan YJ. Acanthopannx senticosus saponins induced tolerance to ischemia and its possible molecular mechanism in PC12 cells. Zhonghua Erke Za Zhi. 2007;451:38–142. [PubMed] [Google Scholar]
- 6.Chen Z, Gu YJ, Bao SY. Protective effects of Acanthopanax senticosus saponins on cortical neuronal ischemia-hypoxia injury. J Clin Neurol. 2006;19:127–129. [Google Scholar]
- 7.Guigoni C, Coulon P. Rabies virus is not cytolytic for rat spinal motoneurons in vitro. J Neurovirol. 2002;8:306–317. doi: 10.1080/13550280290100761. [DOI] [PubMed] [Google Scholar]
- 8.Huang LE, Bunn HF. Hypoxia-inducible factor and its biomedical relevance. J Biol Chem. 2003;278:19575–19578. doi: 10.1074/jbc.R200030200. [DOI] [PubMed] [Google Scholar]
- 9.Jin GH, Huang Z, Tan XF, Tian ML, Zhang XH, Qin JB, Xu HJ, Yew DT, Mak YT. Effects of Ginkgolide on the development of NOS and AchE positive neurons in embryonic basal forebrain. Cell Biol In. 2006;30:500–504. doi: 10.1016/j.cellbi.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Johnson EM, Greenlund LJ, Akins PT, Shu CY. Neuronal apoptosis: current understanding of molecular mechanisms and potential role in ischemic brain injury. J Neurotrauma. 1995;12:843–852. doi: 10.1089/neu.1995.12.843. [DOI] [PubMed] [Google Scholar]
- 11.Kuhn B. Growing and working with spinal motor neurons. Methods Cell Biol. 2003;71:67–87. doi: 10.1016/s0091-679x(03)01005-7. [DOI] [PubMed] [Google Scholar]
- 12.Liu B, Du L, Hong JS. Naloxone protects rat dopaminergic neurons against inflammatory damage through inhibition of microglia activation and superoxide generaten. J Pharmacol Exp Ther. 2000;293:607–617. [PubMed] [Google Scholar]
- 13.Oppenheim RW, Houenou LJ, Johnson JE, Lin LF, Li L, Lo AC, Newsome AL, Prevette DM, Wang S. Developing motor neurons rescued from programmed and axotomy-induced cell death by GDNF. Nature. 1995;373:344–346. doi: 10.1038/373344a0. [DOI] [PubMed] [Google Scholar]
- 14.Qian D, Lin HY, Wang HM, Zhang X, Liu DL, Li QL, Zhu C. Normoxic induction of the hypoxic-inducible factor-1 alpha by interleukin-1 beta involves the extracellular signal-regulated kinase 1/2 pathway in normal human cytotrophoblast cells. Biol Reprod. 2004;70:1822–1827. doi: 10.1095/biolreprod.103.025031. [DOI] [PubMed] [Google Scholar]
- 15.Romijn HJ, Ruijter JM, Wolters PS. Hypoxia preferentially destroys GABA-ergic neurons in developing rat neocortex explants in culture. Exp Neurol. 1988;100:332–340. doi: 10.1016/0014-4886(88)90112-4. [DOI] [PubMed] [Google Scholar]
- 16.Saleem S, Zhuang H, Biswal S, Christen Y, Doré S. Ginkgo biloba extract neuroprotective action is dependent on heme oxygenase in ischemic reperfusion brain injury. Stroke. 2008;393:389–3396. doi: 10.1161/STROKEAHA.108.523480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu WR, Zhou XZ. Involvement of monoamine oxidase inhibition in neuroprotective and neurorestorative effects of Ginkgo biloba extract against MPTP-induced nigrostriatal dopaminergic toxicity in C57 mice. Life Sci. 1999;65:157–164. doi: 10.1016/s0024-3205(99)00232-5. [DOI] [PubMed] [Google Scholar]