Abstract
This study evaluated the antioxidant and anti-fatigue activities of flavonoids from Puerariae radix (FPR). In vitro antioxidant activities of FPR were investigated through hydroxyl and superoxide radical scavenging activities. In vivo anti-fatigue activity of FPR was investigated through loaded swimming exercise of mice. Results showed that FPR had not only in vitro antioxidant activities, but also an in vivo anti-fatigue activity in mice. FPR possessed superoxide and hydroxyl radical scavenging activity in in vitro experimental studies. In vivo experimental studies, FPR could evidently extend exhaustive swimming time of mice, inhibit the increase of blood lactic acid (BLA), decrease serum urea nitrogen (BUN) and malondialdehyde (MDA) contents, promote increases in the activities of superoxide dismutase (SOD) and glutathione peroxidase (GPX) of mice after swimming. The results provided an important basis for developing the FPR as a novel antioxidant and anti-fatigue compound.
Keywords: Puerariae radix, flavonoids, antioxidant, anti-fatigue
Introduction
Fatigue can be defined as the reversible decline in skeletal muscle contractile performance due to intense muscle activity (Allen et al., 2008). Fatigue is associated with many physiological factors including reduced neural input (central and peripheral) and disruptive metabolic changes in skeletal muscle such as lactic acidosis and the production of oxidative free radicals (Mach et al., 2010) Free radicals are intermediate metabolites of many vital biochemical events in the body, and they are in a dynamic balance between their production and clearance. Research evidence has accumulated in the past decade that intense exercise can produce an imbalance between the body's oxidation system and its anti-oxidation system. The accumulation of reactive free radicals will put the body in a state of oxidative stress and bring injury to the body by attacking large molecules and cell organs. Enzymatic and nonenzymatic antioxidants play a vital role in protecting tissues from excessive oxidative damage during exercise. Recent research suggests that supplementation of exogenous dietary antioxidants can also decrease the contribution of exercise-induced oxidative stress and improve the animal's physiological condition (Wang et al., 2006; Trapp et al., 2010). The reason may be that exogenous antioxidants promote interact with endogenous antioxidants to form a cooperative network of cellular antioxidants (Mizuno et al., 2008; You et al., 2011).
Flavonoids, which are naturally spread in food and plants, might delay, inhibit, or even prevent oxidation reaction by terminating the oxidation chain transmission or by chelating metal ions, etc. (Fukumoto and Mazza, 2000). Therefore, a number of studies have been focused on biological activities of flavonoids as a potential antioxidant and free radical scavengers. Puerariae radix (PR), the root of Pueraria labata (Willd.) Ohwi, a wild creeper leguminous plant, is one of the earliest and most important crude herbs used in Chinese medicine for various medicinal purposes (Wang et al., 2003). It is used to treat cardiovascular diseases, myocardial infarction, hypertension, and potential diabetes (Xu et al. 2005; Yan et al. 2006). Pharmacological studies reveal that the active ingredients in Puerariae radix are flavonoids. Xu and He (2007) have indicated that puerarin (daidzein 8-C-glucoside), daidzein and rutin are the major flavonoids in PR. In vitro antioxidant activities and in vivo anti-fatigue activity of flavonoids from PR (FPR) have not been previously published. Hence, the present work investigated the possible antioxidant and anti-fatigue activities. In this study, we examined the antioxidant activities of FPR, employing various in vitro assay systems, such as the hydroxyl and superoxide radical scavenging. In vivo anti-fatigue activity of FPR was investigated through loaded swimming exercise of mice.
Materials and Methods
Plant material
Puerariae radix was bought from the Chengdu Chinese herb factory, and authenticated by Dr. Li (College of Life Science, Sichuan Normal University, Chengdu, China). A voucher specimen (Rm-17-217) was deposited in a herbarium of Sichuan Normal University. Samples were ground into powder.
Chemicals
Trolox (6-hydroxy-2,5,7,8-tetramethylchromancarboxylic acid) was obtained from Sigma Chemical Co. (St. Louis, MO, USA). Commercial kits used for determination of Blood lactic acid (BLA), serum urea nitrogen (BUN), malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GPX) were all purchased from Jiancheng Institute of Biotechnology (Nanjing, China). All other chemicals and solvents used in this study were of analytical grade and obtained from Sichuan Reagent Company (Chengdu, China).
Preparation of flavonoids from Puerariae radix
Flavonoids from Puerariae radix (FPR) were prepared by the method of Li et al. (2010). Samples of 100g were weighted and mixed with 80% aqueous ethanol (solvent: sample= 10:1, v/w) for extraction. The process was refluxed in haven for three times and 40 min for each. The extracted solutions were filtered and evaporated to 100 mL under reduced pressure at 60 °C. Then, the filtrate was added into a glass column (4.0 cm×60 cm, containing 200.0 g D101 macroporous resin purchased from the Chemical Plant of Nankai University, Tianjin, China). The column was first washed with water (600 mL) to remove the un-desired compounds, and then the resin was eluted with 40% aqueous ethanol (800 mL) to elute the targets. The 40% aqueous ethanol elution was collected and evaporated under reduced pressure at 60°C to dryness, and 4.75 g powder was produced, which was stored in a refrigerator for subsequent experiments.
Hydroxyl radical scavenging activity
The hydroxyl radical scavenging activity was carried out by the modified method of Yang et al (2010). Briefly, the reaction mixture contained 2.06×10−3 mM methyl violet (MV), 0.2 mM Fe2+, 0.35 M H2O2, and 0.1 M Tris-HCl (pH 4.7) and various concentrations of FPR samples in a final volume of 5 mL. After incubation for 5 min at 20 °C, the absorbance of the methyl violet complex was measured at 582 nm. Trolox was used as control.
The hydroxyl radical scavenging rate (HSR) was calculated with the equation:
Where A0 was the absorbance of the negative control (without Fe2+ and H2O2), A1 was the absorbance of the positive control (without extract), A2 was the absorbance in the presence of the extract.
Superoxide radical scavenging activity
The superoxide radical scavenging activity was carried out by the modified method of Xiang and Ning (2008). Briefly, the reaction mixture contained 70 uL 10 mM pyrogallol, 4.5 mL 50 mM Tris-HCl (pH 8.2) and 0.5 mL various concentrations of FPR samples. The absorbance at 325 nm was recorded immediately at 30 s and then recorded once every minute. Trolox was used as control.
The superoxide scavenging rate (SSR) was calculated with the equation:
Where A0 was the absorbance of the control (without extract), A1 was the absorbance in the presence of the extract, A2 was the absorbance without pyrogallol.
Experimental animals
Male ICR mice (at the age of 6 weeks) were obtained from the Experiment Animal Center of Sichuan, and the research was conducted in accordance with the internationally accepted principles for laboratory animal use and care as found in the European Community guidelines. The mice were housed at 25°C with a 12-hr light/dark cycle with free access to standard food pellets and tap water throughout the study. After one week adaptation to the laboratory conditions, the healthy animals were used. A total of 64 mice were equally randomly divided into four groups, each consisting of 16 mice. The first group designated as control group (control) was administered with distilled water by gavage every day for 5 weeks. The second, third and fourth group designated as FPR treatment groups were administered with FPR of 25, 50 and 75 mg/kg body weight, respectively, by gavage every day for 5 weeks. The doses of FPR and 5 weeks treatment time used in this study were confirmed to be suitable and effective in tested mice, according to preliminary experiments.
Anti-fatigue activity of FPR
The anti-fatigue activity of FPR was evaluated by the weight loaded swimming exercise of mice. After the final treatment with FPR or distilled water, the mice were allowed to rest for 30 min. Then, eight mice were taken out from each group for weight loaded swimming exercise. The animals were placed in the swimming tank (50 cm × 50 cm ×40 cm) 30 cm deep with water maintained at 25 ± 2 °C. Each mouse's tail was loaded with a bundle of lead pieces, which was 10 % of its body weight. Exhaustion was determined by observing loss of coordinated movements and failure to return to the surface within 10 s (Wu et al., 2007), and the exhaustive swimming time was immediately recorded.
Measuring biochemical parameters related to fatigue
After the final treatment with FPR or distilled water, the mice were allowed to rest for 30 min. Then, the remaining eight mice were taken out from each group for biochemical parameters analyses. Mice were forced to swim for 30 min after weight loading (2% body weight), and blood was collected from the orbital sinus to determine BLA and BUN contents (Jung et al., 2007). The mice were killed by cervical dislocation, and the skeletal muscles were removed to determine MDA contents, SOD and GPX activities.
Statistical analysis of the data
The results were presented as mean ± S.D. Statistical analysis was performed using ANOVA following Mann-Whitney U-test. A value of P < 0.05 was considered to indicate a significant difference between groups.
Results and discussion
Antioxidant activities of FPR assay
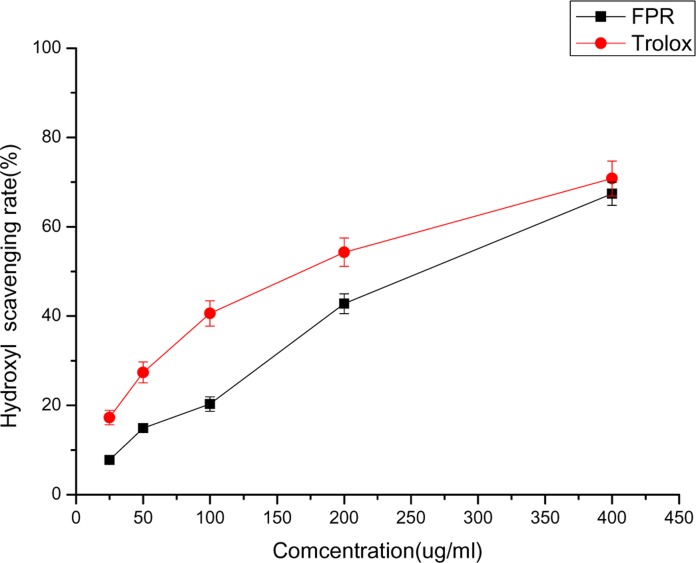
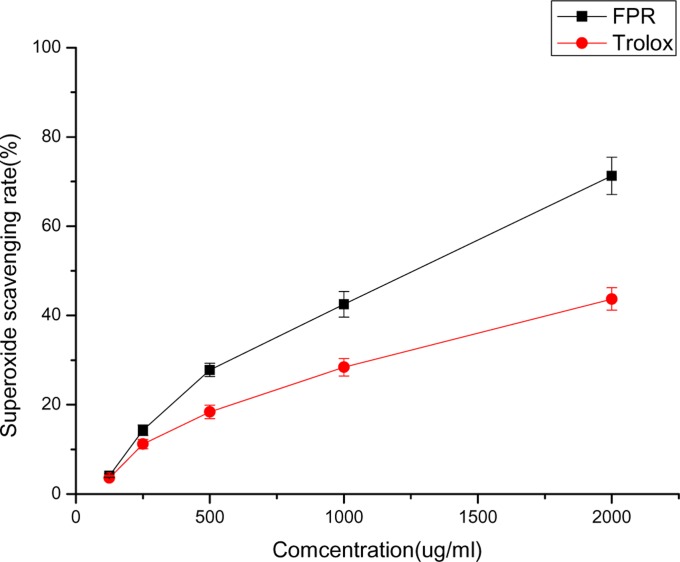
Figure 1 depicts the hydroxyl radical scavenging activity of FPR. When the concentration was from 25 to 400 ug/mL, the percentage scavenging of FPR ranged from 7.8 % to 67.4 %, and Trolox ranged from 17.3 % to 70.8 %, demonstrating enhanced hydroxyl radical scavenging ability relative to controls. Figure 2 depicts the superoxide radical scavenging activity of FPR. FPR showed strong superoxide radical scavenging activity. At 2000 ug/mL, FPR and Trolox have 71.3 % and 43.7 % scavenging effect, and FPR was more efficient than Trolox.
Figure 1.
The hydroxyl radical scavenging activity of FPR
Figure 2.
The superoxide radical scavenging activity of FPR
Free radicals such as hydroxyl radical and superoxide radical are generated from sequential reduction of oxygen during the normal course of aerobic metabolism. Over abundant radicals cause oxidative stress, which can lead to cell injury and tissue damage (Yu et al., 2008). Moreover, the generation of oxidative stress may play an important role in the etiology of many pathological conditions (Karuna et al., 2009). Antioxidant can be used as the indication of prevention from increased oxidative stress and recovery from fatigue (Morihara et al., 2006; Powers and Jackson, 2008). In this study, the data indicated that FPR has in vitro antioxidant activities, which possessed superoxide and hydroxyl radical scavenging activity. Therefore, FPR may have the potential to reduce the oxidative stress in vivo and to fight fatigue.
Anti-fatigue activity of FPR assay
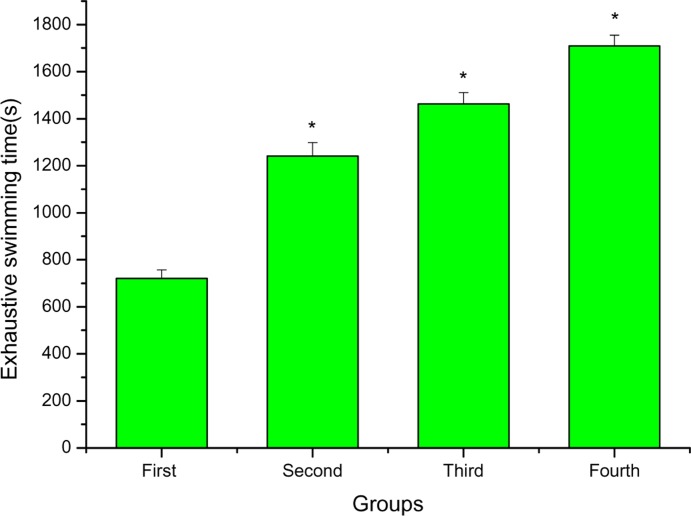
Loaded swimming exercise of mice was selected for evaluation of Anti-fatigue activity. The length of the exhaustive swimming time indicates the degree of fatigue. As shown in Figure 3, the exhaustive swimming time of the second, third and fourth groups were longer than that of the first group (P < 0.05), and increased by 72.12%, 102.77% and 137.03%, respectively.
Figure 3.
The effects of FPR on exhaustive swimming time of mice. *P < 0.05 when compared to the control group (first group).
Loaded swimming exercise of mice has been used as an endurance test and also to examine whether certain agents have anti-fatigue effects (An et al., 2006). Dawson and Horvath (1970) pointed out that swimming has advantages over other forms of exercise, including the treadmill. Training is not required because rodents have a natural swimming ability and they are assumed to be highly motivated to avoid drowning when fatigue is imminent, assuring a high level of performance. Weights have been attached to the tail to standardize the workload and reduce the swimming time in the tank (Matsumoto et al., 1996). In this study, the data indicated that FPR could evidently extend exhaustive swimming time of mice, which suggested that FPR had anti-fatigue activity.
Biochemical parameters related to fatigue assay
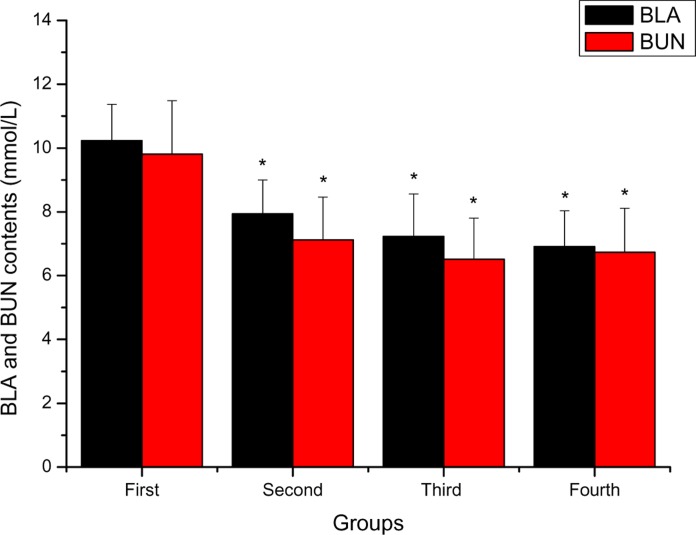
Figure 4 depicts the effects of FPR on BLA and BUN of mice after swimming. BLA contents of the second, third and fourth groups were lower than that of the first group (P < 0.05), and decreased by 28.84 %, 41.91 % and 48.05 %, respectively. BUN contents of the second, third and fourth groups were lower than that of the first group (P < 0.05), and decreased by 37.78 %, 50.69 % and 45.77 %, respectively.
Figure 4.
The effects of FPR on BLA and BUN of mice after swimming *P < 0.05 when compared to the control group (first group).
BLA and BUN are important blood biochemical parameters related to fatigue. BLA is the glycolysis product of carbohydrate under an anaerobic condition, and glycolysis is the main energy source for intense exercise in a short time. Therefore, the blood lactate is one of the important indicators for judging the degree of fatigue (Yu et al., 2008). In this study, the data indicated that FPR could inhibit the increase of BLA and of mice after swimming, which suggested that FPR could postpone the appearance of fatigue. BUN is a sensitive index to evaluate the bearing capability when human bodies suffer from a physical load and caused by catabolism of proteins and amino acids. Protein and amino acids have a stronger katabolic metabolism when the body cannot obtain enough energy by sugar and fat catabolic metabolism. Therefore, correlation between the urea nitrogen in vivo and the exercise tolerance (Wei et al., 2010). In this study, the data indicated that FPR could decrease BUN contents of mice after swimming, which suggested that FPR could reduce catabolic decomposition of protein for energy and ameliorates fatigue.
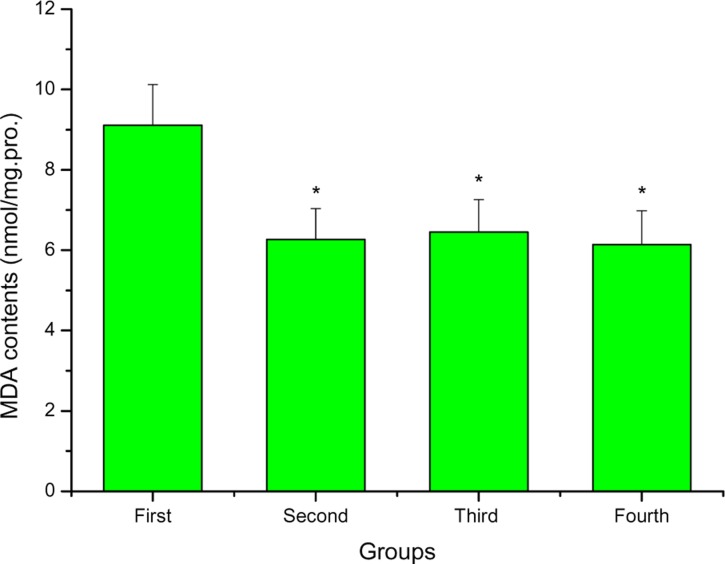
Figure 5 depicts the effects of FPR on MDA of mice after swimming. MDA contents of the second, third and fourth groups were lower than that of the first group (P < 0.05), and decreased by 45.29%, 41.24 % and 48.37 %, respectively. MDA is a physiologic ketoaldehyde produced mainly by peroxidative decomposition of unsaturated lipids as a byproduct of arachidonic acid metabolism (Griffiths et al., 2002). The product generated from MDA due to tissue injury can combine with free amino groups of proteins yielding modified protein adducts. The modification of proteins could potentially alter biological properties. Previous studies indicated that MDA had been frequently measured as an indicator of lipid peroxidation and oxidative stress in vivo (Javier Ordonez and Rosety-Rodriguez, 2007). In this study, the data indicated that FPR could decrease MDA contents of mice after swimming, which suggested that FPR could effectively reduce lipid peroxidation.
Figure 3.
The effects of FPR on MDA of mice after swimming *P < 0.05 when compared to the control group (first group).
Table 1 depicts the effects of FPR on SOD and GPX of mice after swimming. SOD activities of the second, third and fourth groups were higher than that of the first group (P < 0.05), and increased by 49.98 %, 73.03 % and 80.26 %, respectively. GPX activities of the second, third and fourth groups were higher than that of the first group (P < 0.05), and increased by 50.73 %, 97.87 % and 101.46 %, respectively. Some previous reports have shown that reactive oxygen species are responsible for exercise-induced protein oxidation, and contribute strongly to muscle fatigue (Powers et al., 2010). Muscle cells contain two major classes (non-enzymic and enzymic) of endogenous cellular defense mechanisms to elimcontrolinate reactive oxygen species. The primary antioxidant enzymes include SOD and GPX. SOD dismutates superoxide radicals to form hydrogen peroxide. GPX is the major hydrogen peroxide scavenger enzyme. Together with its substrate glutathione (GSH) they form a formidable defense against hydrogen peroxides and lipid peroxides (Sun et al., 2010). These antioxidant defense mechanisms become weaker during chronic fatigue and other disease conditions. So, the improvement in the activities of these defense mechanisms can help to fight against fatigue (You et al., 2011). In this study, the data indicated that FPR could promote increases in the activities of SOD and GPX after swimming, which suggested that FPR could reduce exercise-induced oxidative stress, again supporting that FPR had an anti-fatigue activity.
Table 1.
The effects of FPR on SOD and GPX of mice after swimming
| Groups | SOD (U/mg.pro.) | GPx (U/mg. pro.) |
| First | 102.26 ± 11.12 | 7.51 ± 0.94 |
| Second | 153.37 ± 13.26* | 11.32 ± 1.06 |
| Third | 176.94 ± 14.37* | 14.86 ± 1.14* |
| Fourth | 184.33 ± 11.89* | 15.13 ± 1.03* |
P < 0.05 when compared to the control group (first group).
Conclusion
In conclusion, our results indicated that FPR had in vitro antioxidant activities, with superoxide and hydroxyl radical scavenging activity. In vivo experimental studies, the data indicated that FPR had anti-fatigue activity, which could evidently extend exhaustive swimming time of mice, inhibit the increase of BLA, decrease BUN and MDA contents, promote increases in the activities of SOD and GPX of mice after swimming. One of the putative mechanisms of anti-fatigue activity is, FPR has higher antioxidant activities, which as exogenous antioxidants, can promote or interact with endogenous antioxidants to form a cooperative network of cellular antioxidants. The results provide an important basis for developing the FPR as a novel antioxidant and anti-fatigue compound. However, further research needs to be carried out to evaluate its anti-fatigue activity on humans and its anti-fatigue mechanism at the cellular and molecular levels.
Acknowledgments
We thank Dr. Zhang Guoman for providing technical assistance and advice. This work was supported by the Development of Science and Technology of the Sichuan Normal University.
References
- 1.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 2.An HJ, Choi HM, Park HS, Han JG, Lee EH, Park YS, Um JY, Hong SH, Kim HM. Oral administration of hot water extracts of Chlorella vulgaris increases physical stamina in mice. Ann Nutr Metab. 2006;50:380–386. doi: 10.1159/000094303. [DOI] [PubMed] [Google Scholar]
- 3.Dawson CA, Horvath SM. Swimming in small laboratory animals. Med Sci Sports. 1970;2:51–78. [PubMed] [Google Scholar]
- 4.Griffiths HR, Møller L, Bartosz G, Bast A, Bertoni-Freddari C, Collins A, Cooke M, Coolen S, Haenen G, Hoberg AM, Loft S, Lunec J, Olinski R, Parry J, Pompella A, Poulsen H, Verhagen H, Astley SB. Biomarkers. Mol Aspects Med. 2002;23:101–208. doi: 10.1016/s0098-2997(02)00017-1. [DOI] [PubMed] [Google Scholar]
- 5.Fukumoto LR, Mazza G. Assessing antioxidant and prooxidant activities of phenolic compounds. J Agric Food Chem. 2000;48:3597–3604. doi: 10.1021/jf000220w. [DOI] [PubMed] [Google Scholar]
- 6.Javier Ordonez F, Rosety-Rodriguez M. Regular exercise attenuated lipid peroxidation in adolescents with Down's syndrome. Clin Biochem. 2007;40:141–142. doi: 10.1016/j.clinbiochem.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Jung K, Kim IH, Han D. Effect of medicinal plant extracts on forced swimming capacity in mice. J Ethnopharmacol. 2004;93:75–81. doi: 10.1016/j.jep.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Karuna R, Reddy SS, Baskar R, Saralakumari D. Antioxidant potential of aqueous extract of Phyllanthus amarus in rats. Indian J Pharmacol. 2009;41:64–67. doi: 10.4103/0253-7613.51342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li T, Zheng CM, Zhang T, Tao JS. Study on Determination of Pueraria Flavonoids in Extracts of Pueraria labta (Willd.) Ohwi. Lishizhen Med Maert Med Res. 2010;21:56–58. [Google Scholar]
- 10.Mach J, Midgley AW, Dank S, Grant RS, Bentle DJ. The Effect of Antioxidant Supplementation on Fatigue during Exercise: Potential Role for NAD+(H) Nutrients. 2010;2:319–329. doi: 10.3390/nu2030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumoto K, Ishihara K, Tanaka K, Inoue K, Fushiki T. An adjustable-current swimming pool for the evaluation of endurance capacity of mice. J Appl Physiol. 1996;81:1843–1849. doi: 10.1152/jappl.1996.81.4.1843. [DOI] [PubMed] [Google Scholar]
- 12.Mizuno K, Tanaka M, Nozaki S, Mizuma H, Ataka S, Tahara T. Antifatigue effects of coenzyme Q10 during physical fatigue. Nutrition. 2008;24:293–299. doi: 10.1016/j.nut.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Morihara N, Ushijima M, Kashimoto N, Sumioka I, Nishihama T, Hayama M, Takeda H. Aged garlic extract ameliorates physical fatigue. Biol Pharm Bull. 2006;29:962–966. doi: 10.1248/bpb.29.962. [DOI] [PubMed] [Google Scholar]
- 14.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powers SK, Smuder AJ, Kavazis AN, Hudson MB. Experimental guidelines for studies designed to investigate the impact of antioxidant supplementation onexercise performance. Int J Sport Nutr Exerc Metab. 2010;20:2–14. doi: 10.1123/ijsnem.20.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun L, Shen W, Liu Z, Guan S, Liu J, Ding S. Endurance exercise causes mitochondrial and oxidative stress in rat liver: Effects of a combination of mitochondrial targeting nutrients. Life Sci. 2010;86:39–44. doi: 10.1016/j.lfs.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Trapp D, Knez W, Sinclair W. Could a vegetarian diet reduce exercise-induced oxidative stress? A review of the literature. J Sports Sci. 2010;28:1261–1268. doi: 10.1080/02640414.2010.507676. [DOI] [PubMed] [Google Scholar]
- 18.Wang JJ, Shieh MJ, Kuo SL, Lee CL, Pan TM. Effect of red mold rice on antifatigue and exercise-related changes in lipid peroxidation in endurance exercise. Appl Microbiol Biotechnol. 2006;70:247–253. doi: 10.1007/s00253-005-0051-5. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Wu J, Chiba H, Umegaki K, Yamada K, Ishimi Y. Puerariae radix prevents bone loss in ovariectomized mice. J Bone Miner Metab. 2003;21:268–275. doi: 10.1007/s00774-003-0420-z. [DOI] [PubMed] [Google Scholar]
- 20.Wei W, Zheng LY, Yu MY, Jiang N, Yang ZR, Luo X. Anti-fatigue activity of extract form the submerged fermentation of Ganoderma Lucidum using Radix astragali as substrate. J Anim Plant Sci. 2010;6:677–684. [Google Scholar]
- 21.Xiang ZN, Ning ZX. Scavenging and antioxidant properties of compound derived from chlorogenic acid in South-China honeysuckle. LWT-Food SciTech. 2008;41:1189–1203. [Google Scholar]
- 22.Xu ME, Xiao SZ, Sun YH, Zheng XX, Ou-yang Y, Chen G. The study of anti-metabolic syndrome effect of puerarin in vitro. Life Sci. 2005;77:3183–3196. doi: 10.1016/j.lfs.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 23.Yan LP, Chan SW, Chan ASC, Chen SL, Ma XJ, Xu HX. Puerarin decreases serum total cholesterol and enhances thoracic aorta endothelial nitric oxide synthase expression in diet-induced hypercholesterolemic rats. Life Sci. 2006;79:324–330. doi: 10.1016/j.lfs.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Yang QM, Pan XH, Kong WB, Yang H, Su YD, Zhang L, Zhang YN, Yang YL, Ding L, Liu GA. Antioxidant activities of malt extract from barley (Hordeum vulgare L.) toward various oxidative stress in vitro and in vivo. Food Chem. 2010;118:84–89. [Google Scholar]
- 25.You LJ, Zhao MM, Regenstein JM, Ren TY. In vitro antioxidant activity and in vivo anti-fatigue effect of loach (Misgurnus anguillicaudatus) peptides prepared by papain digestion. Food Chem. 2011;124:188–194. doi: 10.1021/jf2016368. [DOI] [PubMed] [Google Scholar]
- 26.Yu B, Lu ZX, Bie XM, Lu FX, Huang XQ. Scavenging and antifatigue activity of fermented defatted soybean peptides. Eur Food Res. 2008;226:415–421. [Google Scholar]