Abstract
Compound Kushen Injection (CKI) is Sophora Flavescens and Heterosmilacis Japonicae extract. Meta-analysis confirmed that CKI plus transcatheter arterial chemoembolization (TACE) is more superior to TACE alone for unresectable hepatocellular carcinoma (UHCC) patients.
Keywords: Compound Kushen Injection, Transcatheter arterial chemoembolization, TACE, hepatocellular carcinoma
Introduction
Hepatocellular carcinoma is one of the commonest forms of malignancies. Its global morbidity ranks the seventh, while mortality is at fourth position in the world. Statistics from the World Health Organization (WHO, 2008) show that 749,744 cases of hepatocellular carcinoma were diagnosed, of these, 695,726 cases died in 2008. Southern Africa, Southeast Asia and the Mediterranean coast is the high incidence area of hepatocellular carcinoma. Hepatocellular carcinoma has been the second leading cause of death by cancer in China, there were 372,079 deaths in 2008 (Ferlay et al., 2008). However, due to the spread of hepatitis B, the incidence and mortality of hepatocellular carcinoma also show a rising trend (Liaw et al., 1986).
For unresectable hepatocellular carcinoma (UHCC) patients, most of them are at an advanced stage, few meaningful therapeutic options are available (Bruix et al., 2002). TACE is considered as a primary and complementary measure for the treatment of UHCC (Roche et al., 2003; Stuart, 2003; Venook et al., 1990). However, the adverse events of TACE, such as post embolization syndrome, hepatic insufficiency and myelosuppression, are frequent leads to interruption of TACE treatment (Chung et al., 1996). As a result, searching new drugs which can combine with TACE to enhance the therapeutic effects and reduce its adverse events has become a hotspot.
Many clinical trials showed that CKI plus TACE can reduce the adverse reactions and improve quality of life for UHCC (Chen, 2009; Tong, 2010; Zhang et al., 2006; Zhu and Li, 2006). CKI was extracted from the mixture of Sophora flavescens and Heterosmilacis Japonicae with the mass ratio of 7 than 3. Experimental studies confirmed that CKI has significant killing effect on the tumor cells, such as Hep, H22, LAC and Lewis in vitro (Lin et al., 2009). Meanwhile, animal experiment showed that CKI can depress the growth of hepatocellular carcinoma and its angiogenesis in SD rat (Sprague Dawley Rat) (Li et al., 2007; Zhu et al., 2011). These results revealed that CKI may enhance the therapeutic effects of TACE. Until now, however, rigorously designed, randomized, large, multi-center, double-blind, controlled trials for UHCC have not been reported.
The purpose of this meta-analysis is to evaluate whether CKI enhances therapeutic effects for UHCC after TACE. It is anticipated that this systematic review will provide evidence-based information for clinical practice.
Materials and Methods
Criteria for Inclusion and Exclusion
The articles were read by two reviewers (Qizhe Sun and Yuan Gao) and studies were selected systematically according to the following criteria: (1) hepatocellular carcinoma patients were confirmed cytologically or pathologically, or diagnosed by CT; (2) trials were described as randomized clinical trials (RCTs); (3) published trials included a treatment group receiving CKI plus TACE and a control group receiving TACE; and (4) the published data of primary interest were tumor response and quality of life for calculation of the odds ratio (OR) at a 95% confidence interval (CI). Trials were excluded if they did not meet the criteria above and included the following: (1) involved animal studies or in vitro studies; (2) did not represent primary research (review articles, letters to the editor, etc); or (3) represented duplicate publications of other studies previously identified in our systematic evaluation.
Literature Search Strategy
Retrieval of trials was performed through the Cochrane Database of Systematic Reviews (The Cochrane Library, Issue 5, 2011), CENTRAL (the Cochrane Central Register of Controlled Trials, January 1966 to May 2011), MEDLINE (January 1966 to May 2011), EMBASE (January 1966 to May 2011), CNKI (Chinese National Knowledge Infrastructure, January 1979 to May 2011) and CBMdisk (Chinese Biomedical Database, January 1978 to May 2011). All searches were performed without language limitations to identify all relevant trials. The main search terms were: hepatocellular carcinoma? hepatic tumor, hepatic cancer, liver cancer, or liver tumor and transcatheter arterial chemoembolization, TACE transcatheter arterial embolization or TAE and Compound Kushen Injection, Yan Shu, Kushen, Sophora flavescens or Sophorae. The search results were downloaded to a reference database and screened further.
Outcome Measurements
The main outcome measurements were as follows: (1) Tumor response was evaluated according to the WHO standard for evaluating therapeutic efficacy on solid tumors (Therasse, 2002). Based on the degree of tumor regression, efficacy was evaluated as following: CR (complete response, CT and/or MRI revealed complete clearance of the lesion); PR (partial response, lesion decreased more than 50%); SD (lesion decreased less than 50% or increased less than 25%); PD (size of lesion increased more than 25% after treatment). Tumor responses were defined as CR+PR. (2) Quality of life was evaluated according to the Karnofsky performance score (KPS) (Yates et al., 1980), which was classified as: Improvement (KPS improved ⩾10 points after treatment); Stabilization (KPS improved <10 points or decreased <10 points); Deterioration (KPS decreased ⩾10 points after treatment). (3) One-year survival. (4) Adverse events were evaluated, based on the WHO criteria for evaluation of acute and subacute toxic and adverse reactions (Miller et al., 1981).
Review Methods
Data extraction
The trials selection and the data extraction were performed independently by two investigators. For conflicts, an agreement was reached by discussion among reviewers. The following information was collected from each study: (1) the information about patients (the number of patients allocated, clinical stage, and KPS); (2) the characteristics of methods (the randomization procedure, concealment of allocation, blinding procedure, withdrawal and reasons, and protection against contamination); (3) The characteristics of interventions (dosage and duration of therapy, TACE course, and any co-interventions; (4) the outcomes (tumor response, quality of life, one-year survival and adverse events).
Quality assessment
Methodological quality was evaluated according to the Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0) (Higgins et al., 2011). The evaluation was performed in the following aspects: (1) selection bias (random sequence generation and allocation concealment); (2) performance bias (blinding of participants and personnel); (3) detection bias (blinding of outcome assessment); (4) attrition bias (incomplete outcome data); (5) reporting bias (selective reporting); (6) other bias (other sources of bias). The judgement of each term was assigned as: ‘Low risk’ of bias (the description of methods are adequate and the procedures are correct), ‘High risk’ of bias (the methods or procedures are improper), or ‘Unclear risk’ of bias (without description of methods and procedures). The methodological quality evaluation was performed independently by two reviewers, and discrepancies were resolved through discussion.
Statistical method
Meta-analysis was performed according to recommendations from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins et al., 2011) using the statistical software RevMan5.1.2 (Update Software Ltd, Oxford, England) provided by the Cochrane Collaboration. Statistical heterogeneity was explored using the x2 test (P<0.10 was considered representative of significant statistical heterogeneity). The random-effects model was used when there was significant statistical heterogeneity (P<0.10); otherwise the fixed-effects model was used. For dichotomous variables, OR (Odds Ratio) with 95%CI was calculated. Patients with incomplete or missing data were counted as treatment failures. The sensitivity analysis was performed by comparing the results of two different statistical models (random-effects model and fixed-effects model). Potential publication bias was examined by funnel plot.
Results
Common characteristics
The initial search identified 795 trials for possible inclusion in the review, but all of these trials were reported in Chinese journals. According to the inclusion criteria, 785 trials were excluded as obvious error, non-clinical studies, duplicates, or study objectives different from the aim of this review. Ultimately, 10 trials (Cao et al., 2009; Chen et al., 2006; Chen et al., 2007; Deng et al., 2009; Guan et al., 2006; Huang et al., 2006; Wang and Cheng, 2009; Xu et al., 2010; Yang, 2006; Yu and Kang, 2009) were identified for further study (726 patients), but without a multi-center study. Characteristics of the included trials are listed in Tables 1 and 2.
Table 1.
Characteristics of Included Trials
| Study Author (year) |
No. Enrolled Patients | Stage | KPSa | Treatment vs Control | Outcomes | ||
| Treatment | Control | Treatment | Control | ||||
| Cao et al., 2009 | 30 | 30 | II, III | ≥60 | ≥60 | TACE-Kushen vs TACE | Tumor response, KPS, and adverse events |
| Chen et al., 2006 | 16 | 14 | II, III | >50 | >50 | TACE-Kushen vs TACE | Tumor response, KPS, and adverse events |
| Chen et al., 2007 | 46 | 40 | II, III | ≥70 | ≥70 | TACE-Kushen vs TACE | Tumor response, KPS, survival, and adverse events |
| Deng et al., 2009 | 20 | 20 | II, III | ≥70 | ≥70 | TACE-Kushen vs TACE | Tumor response, KPS, and adverse events |
| Guan et al., 2006 | 58 | 59 | II, III | >50 | >50 | TACE-Kushen vs TACE | Tumor response, KPS, survival, and adverse events |
| Huang et al., 2006 | 35 | 33 | II, III | ≥50 | ≥50 | TACE-Kushen vs TACE | Tumor response, KPS, and adverse events |
| Wang et al., 2009 | 27 | 30 | II, III | ≥70 | ≥70 | TACEb-Kushen vs TACE | Tumor response, KPS, and survival |
| Xu et al., 2010 | 53 | 53 | II, III | — | — | TACE-Kushen vs TACE | Tumor response, KPS, survival, and adverse events |
| Yang, 2006 | 33 | 33 | II, III | ≥60 | ≥60 | TACE-Kushen vs TACE | Tumor response, KPS, and survival |
| Yu et al., 2010 | 48 | 48 | II, III | >60 | >60 | TACE-Kushen vs TACE | Tumor response, KPS, and adverse events |
KPS, Karnofsky Performance Scale;
TACE, transcatheter arterial chemoembolization;
Table 2.
Compound Kushen Injection and Transcatheter Arterial Chemoembolization Drugs Used in Included Studies
| Study Author (year) |
Anticancer Drug | Embolizing | Duration Days |
Compound Kushen Injection Dosage |
Injection Method |
||
| LP | Agents | Adjuvant Drug | |||||
| Cao et al., 2009 | 5-Fu, DDP, MMC | Yes | GS particles | MMC | 56<D<224 | given 20ml per time, once daily over 14 days; began in the TACE operation day; | I.v.drip |
| Chen et al., 2006 | 5-Fu, DDP, EPI, | Na | Na | Na | 63 | given 20ml per time, once daily over 21 days; begin in the TACE operation day; | I.v.drip |
| Chen et al., 2007 | 5-Fu, MMC, EPI, | Yes | Na | Na | 84<D<126 | given 20ml per time, once daily over the whole duration; | I.v.drip |
| Deng et al., 2009 | THP-ADM | Yes | Na | Na | 61 | given 20ml per time, once daily over 14 days; begin in the 2nd day after TACE operation; | I.v.c. infusion |
| Guan et al., 2006 | (5-Fu, DDP, ADM) or (THP-ADM,5-Fu, CBP) | Na | Na | Na | 45<D<60 | given 20ml per time, once daily over the whole duration; | I.v.drip |
| Huang et al., 2006 | 5-Fu, ADM, HCPT | Yes | Na | Na | 56<D<224 | given 15∼30ml per time, once daily over 10∼20 days; begin in the TACE operation day; | I.v.drip |
| Wang et al., 2009 | 5-Fu, DDP, ADM | Yes | GS particles | Na | 140<D<168 | given 20ml per time, once daily over 10 days; begin in the TACE operation day; | I.v.drip |
| Xu et al., 2010 | 5-Fu, DDP,CF | Yes | Na | THP-ADM or HCPT | 60<D<120 | given 20ml per time, once daily over 15 days; begin in the 3rd day before TACE operation; | I.v.drip |
| Yang, 2006 | DDP, MMC, ADM | Yes | GS particles | Na | 40<D<240 | given 20ml per time, once daily over 20 days; began in the TACE operation day; | I.v.drip |
| Yu et al., 2010 | 5-Fu, ADM, HCPT | Yes | GS particles | Na | 84 | given 20ml per time, once daily over 15 days; begin in the TACE operation day; | I.v.drip |
5-Fu=fluorouracil, DDP=cisplatin, ADM=Adriamycin, CF=Calcium Folinate, EPI=Epirubicin, MMC=Mitomycin, THP-ADM=pirarubicin hydrochloride, CBP=carboplatin, HCPT=Hydroxycamptothecin, LP=Lipiodol, GS=Gelatin sponge, Na=not described, D=days, I.v.drip=intravenous drip, I.v.c.infusion=intravenous continuous infusion.
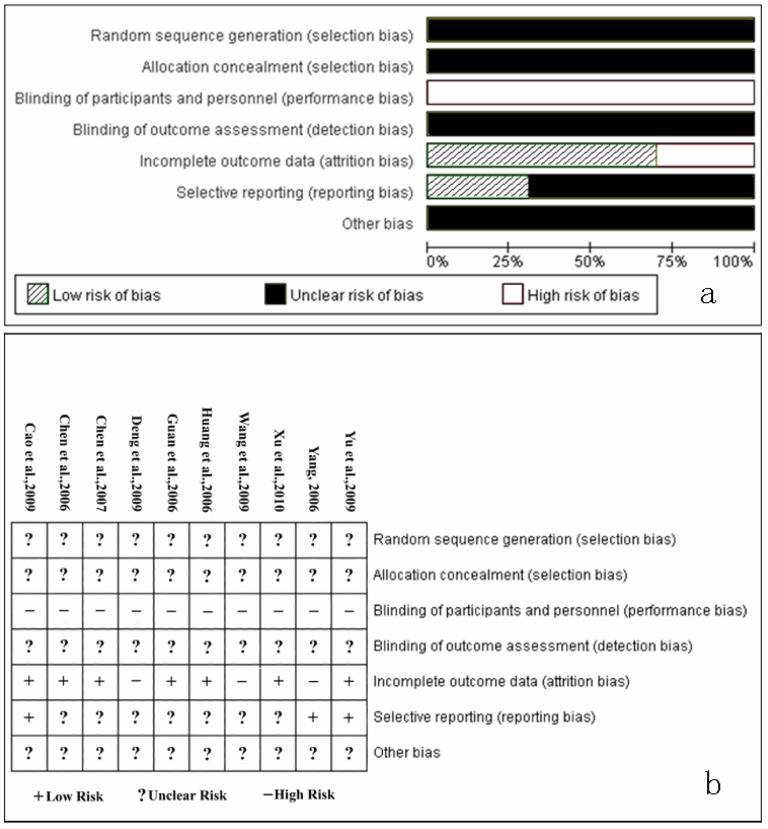
In terms of quality, all trials mentioned “randomization,” but none stated the generation of a random allocation sequence. No trials described information on allocation concealment and blinding. No trials reported the withdrawals and dropouts. (Figure 1)
Figure 1.
Quality of all included trials
a. Risk of bias percentage graph of all included studies
b. Risk of bias summary of all included studies
Meta-analysis Outcomes
Tumor response
Ten trials (726 patients) (Cao et al., 2009; Chen et al., 2006; Chen et al., 2007; Deng et al., 2009; Guan et al., 2006; Huang et al., 2006; Wang et al., 2009; Xu et al., 2010; Yang, 2006; Yu et al., 2009) were identified with a CR+PR outcome measurement of tumor response. The fixed-effects model was used because of heterogeneity of the results of trials (Heterogeneity: Chi2=4.74, df=9 (P=0.86); I2=0%). The pooled analysis showed that compared with TACE alone, CKI plus TACE significantly improved the tumor response (OR=2.01; 95% CI [1.47, 2.74]; P < 0.0001) (Figure 2).
Figure 2.
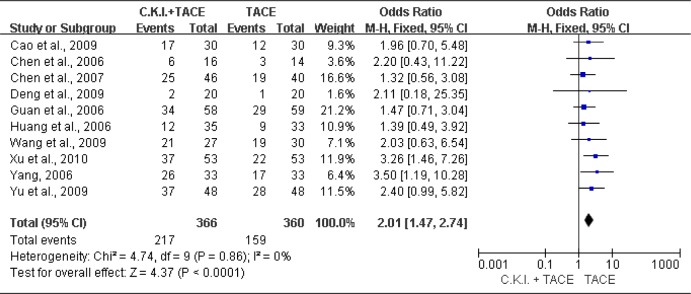
Meta-analysis of tumor response.
Compound Kushen Injection (C.K.I.) plus transcatheter arterial chemoembolization (TACE) versus TACE alone for tumor response (CR+PR) of patients with unresectable hepatocellular carcinoma.
Quality of life
There were 9 trials (Cao et al., 2009; Chen et al., 2006; Chen et al., 2007; Guan et al., 2006; Huang et al., 2006; Wang et al., 2009; Xu et al., 2010; Yang, 2006; Yu et al., 2009) contained a KPS improvement of >10 points. The fixed-effects model was used (Heterogeneity: Chi2=5.51, df=8 (P=0.70); I2=0%). The results showed that CKI plus TACE significantly improved KPS (OR=2.47; 95% CI [1.77, 3.44]; P < 0.00001) (Figure 3).
Figure 3.
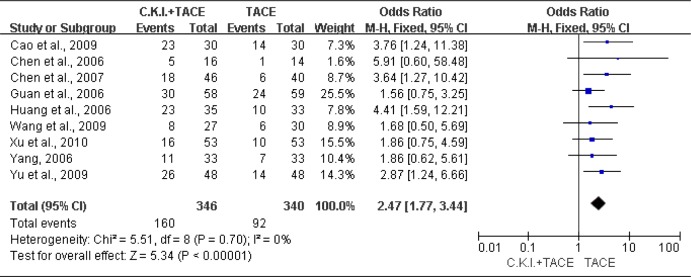
Meta-analysis of quality of life improvement.
Compound Kushen Injection (C.K.I.) plus transcatheter arterial chemoembolization (TACE) versus TACE alone for KPS (>10 points) of patients with unresectable hepatocellular carcinoma.
One-year survival
Five trials (432 patients) (Chen et al., 2007; Guan et al., 2006; Wang et al., 2009; Xu et al., 2010; Yang, 2006;) were identified with the outcome measurements of one-year survival. The fixed-effects model was used (Heterogeneity: Chi2=1.92, df=4 (P=0.75); I2=0%). The meta-analysis showed that CKI plus TACE significantly improved one-year survival (OR= 2.16; 95% CI [1.45, 3.23]; P=0.0002) (Figure 4).
Figure 4.
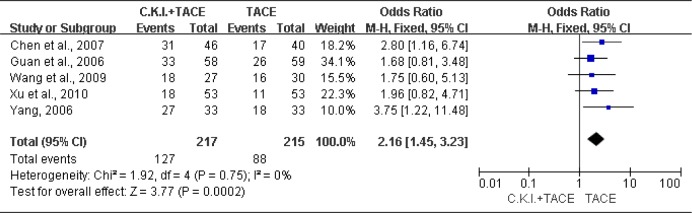
Meta-analysis of one-year survival.
Compound Kushen Injection (C.K.I.) plus transcatheter arterial chemoembolization (TACE) versus TACE alone for one-year survival of patients with unresectable hepatocellular carcinoma.
Adverse Effects
We found 8 trials (Cao et al., 2009; Chen et al., 2006; Chen et al., 2007; Deng et al., 2009; Guan et al., 2006; Huang et al., 2006; Xu et al., 2010; Yu et al., 2009) mentioned adverse events. All of the eight trials reported that the common symptoms such as abdominal pain, abdominal distension, diarrhea, hepatic insufficiency, and bone marrow depression were found in both groups. These might be due to the complications of TACE. There were six trials (Cao et al., 2009; Chen et al., 2006; Chen et al., 2007; Huang et al., 2006; Xu et al., 2010; Yu et al., 2009) showed that compared with TACE alone, CKI plus TACE significantly reduced the complications of TACE. In addition, two trials (Deng et al., 2009; Guan et al., 2006) reported that no adverse events attributable to CKI were observed. Therefore, we concluded that the use of CKI has no significant adverse events.
Sensitivity Analysis
Sensitivity analysis was performed by comparing fixed-effects versus random- effects. In the primary analysis, outcome on quality of life was applied to the fixed-effects model (OR=2.47; 95% CI [1.77, 3.44]; P < 0.00001). Therefore, a random-effects model was used to re-analyzed it (OR= 2.45; 95% CI [1.75, 3.43]; P < 0.00001). The results were virtually identical.
Publication Bias
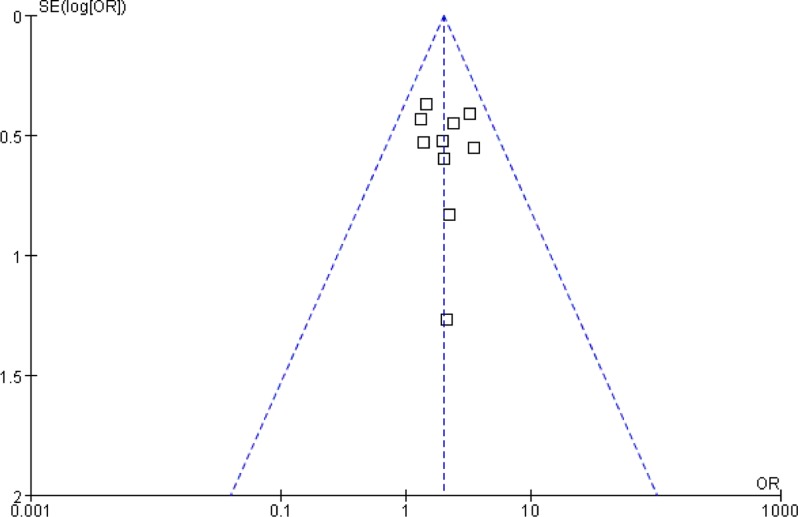
We used the funnel plot to access the publication bias of literatures (Figure 5). The shape of the funnel plots seemed symmetric, and suggesting there was no obvious publication bias.
Figure 5.
Funnel plot of tumor response (CR+PR).
A graphical display of the logarithm of odds ratio (OR) plotted against OR. The vertical line indicates that there was no publication bias.
Discussion
Experimental studies Show CKI can significantly enhance the apoptosis rates of HepG2 cells and inhibit its transition from G2 phase to M phase in vitro. Therefore, the proliferation of tumor cells, such as HepG2 cells, BEL-7402 cells and SGC-7901 cells could be restrained by CKI. Further Studies show that the expression of tumor metastasis suppressor gene — nm23 could remarkably be promoted by CKI in BEL-7402 cells, meanwhile in contrast, the expression of CD44v6 in BEL-7402 cells could be depressed (Li et al., 2006). Based on these data, CKI could be a complementary drug with TACE to inhibit the growth of liver cancer, suppress the tumor metastasis, and improve quality of patients' life for UHCC.
The results show that TACE plus CKI seemed superior to TACE alone for UHCC in respect to patients' tumor response, quality of life and one-year survival. Although quality of included literature are low, the results of this study present credible evidence that the administration of CKI plus TACE is worthy of additional study. Hence, larger, longer-term, rigorously designed, multi-center, randomized, double-blind, controlled trials are required to fully assess whether CKI plus TACE is more outstanding than TACE alone.
There are some limitations in this meta-analysis. Due to all literatures we found were of poor methodological quality, the definite conclusions could not be made base on our data. All included trials mentioned “randomized,” but all of them did not describe the information of randomization sequence generation, allocation concealment, and blinding. All included literatures did not report the withdrawals and dropouts. We found that the number of patients in experimental group and control group has no change before and after treatment. So it is possible that there are no withdrawals and dropouts happened. However, the evaluation of one-year survival should be interpreted with caution.
Acknowledgments
We thank the anonymous referee for his/her helpful comments, which remarkably improved the quality of this paper.
The list of abbreviations
- CKI
Compound Kushen Injection
- TACE
transcatheter arterial chemoembolization
- UHCC
unresectable hepatocellular carcinoma
- WHO
World Health Organization
- SD rat
Sprague Dawley Rat
- OR
Odds Ratio
- CI
Confidence Interval
- KPS
Karnofsky Performance Score
References
- 1.Bruix J, Llovet J M. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35(3):519–524. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- 2.Cao J, Wang Z L, Fang J, Liu H Q, He Y. Clinical Study of Compound Kushen Injection Combined with TACE for Treatment of Advanced Liver Cancer. Shandong Medicine. 2009;49(4):74–76. [Google Scholar]
- 3.Chen G H, Li SR, Yang L. Therapeutic effects of complex kushen injection combined with double interventional therapy on treating primary hepatocarcinoma. Chin J Int egr T rad West Med Dig. 2007;15(4):239–241. [Google Scholar]
- 4.Chen Y H. Efficacy of transcatheter arterial chemoembolization combined with complex prescription kushen injection in the treatment of primary advanced liver cancer. China Modern Medicine. 2009;16(17):5–6. [Google Scholar]
- 5.Chen Y L, Jia Y J, Zhang Y. Clincial effect Observation of Complex Sophorae Injection Combining with Chemotherapy on Treating Liver Cancer. Journal of Tianjin University of Traditional Chinese Medicine. 2006;25(3):166–167. [Google Scholar]
- 6.Chung J W, Park J H, Han J K, Choi B I, Han M C, Lee H S, Kim C Y. Hepatic tumors: predisposing factors for complications of transcatheter oily chemoembolization. Radiology. 1996;198(1):33–40. doi: 10.1148/radiology.198.1.8539401. [DOI] [PubMed] [Google Scholar]
- 7.Deng L, Li Z W, Wu H T, Lu Q H, Chen L Z, Wu J Y, Wu X Y, Chen Q Q. herapeutic Effect of Continuous Infusion of Compound Kushen Injection Combined with Hepatic Arterial Chemoembolization for Advanced Liver Cancer: An Observation of 20 Cases. Journal of New Chinese Medicine. 2009;41(4):28–29. [Google Scholar]
- 8.Ferlay J, Shin HR, Bray F. GLOBOCAN 2008, Cancer incidence and mortality worldwide. IARC CancerBase [DB/OL]; 2008. http://globocan.iarc.fr. [Google Scholar]
- 9.Guan C N, Cai LZ, Yue L Q, Zhang Y. Clinicel study on treatment of advanced primary liver cancer by Yanshu injection combining with chemotherapy. China Journal of Chinese Materia Medica. 2006;31(6):510–512. [PubMed] [Google Scholar]
- 10.Higgins JPT, Green S, editors. The Cochrane Collaboration. 2011. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] Available from http://www.cochrane-handbook.org. [Google Scholar]
- 11.Huang R W, Chen S X, Huang X P. Clinical Observation On Advanced Primary Hepatic Carcinoma Treated by Yanshu Injection Combined with Transcatheter Arterial Chemoembolization. Hebei Medicine. 2006;12(5):443–445. [Google Scholar]
- 12.Li M, Qian XP, Liu B R. Experimental Study of Composite Radix Sophora Flavescentis Injection with Thermotherapy Inhibiting Angiogenesis. Journal of Clinical Medicine in Practice. 2007;11(3):57–61. [Google Scholar]
- 13.Li R, Du JP, Hou Y S, Chen Z T. Experimental study of Yanshu injection acted on SGC-7901, HepG2 and BEL-7402 tumor cell. Cancer Research and Clinic. 2006;18(1):8–10. [Google Scholar]
- 14.Lin L, Zhou D H, Chen Y, Liu Q H, Chen X X. Experimental Study of Anti - tumor Effect of Compound Radix Sophorae Flavescentis Injection on Lung Cancer Cells and Hepatic Carcinoma Cells. Traditional Chinese Drug Research & Clinical Pharmacology. 2009;20(1):21–23. [Google Scholar]
- 15.Miller A B, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47(1):207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Roche A, Girish B V, de Baere T, Baudin E, Boige V, Elias D, Lasser P, Schlumberger M, Ducreux M. Trans-catheter arterial chemoembolization as first-line treatment for hepatic metastases from endocrine tumors. Eur Radiol. 2003;13(1):136–140. doi: 10.1007/s00330-002-1558-0. [DOI] [PubMed] [Google Scholar]
- 17.Stuart K. Chemoembolization in the management of liver tumors. Oncologist. 2003;8(5):425–437. doi: 10.1634/theoncologist.8-5-425. [DOI] [PubMed] [Google Scholar]
- 18.Therasse P. Evaluation of response: new and standard criteria. Ann Oncol. 2002;13(Suppl 4):127–129. doi: 10.1093/annonc/mdf649. [DOI] [PubMed] [Google Scholar]
- 19.Tong H Z. The observation of the clinical effects of transcatheter arterial chemoembolization and complex prescrption kuseng injection on the advanced period of liver cancer. Jiangxi Medical Journal. 2010;45(7):637–639. [Google Scholar]
- 20.Venook A P, Stagg R J, Lewis B J, Chase J L, Ring E J, Maroney T P, Hohn D C. Chemoembolization for hepatocellular carcinoma. J Clin Oncol. 1990;8(6):1108–1114. doi: 10.1200/JCO.1990.8.6.1108. [DOI] [PubMed] [Google Scholar]
- 21.Wang H M, Cheng X M. Composite Kushen injection combined with hepatic artery embolism on unresectable primary liver cancer. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2009;18(12):1334–1335. [Google Scholar]
- 22.Xu P, Wang WD, Lu J, Fan C. Efficacy of Compound Kushen Injection in the Prevention and Treatment of the Adverse Reactions Following Transcatheter Hepatic Arterial Chemoembolization in Patient with Liver Cancer. Evaluation and analysis of drug-use in hospitals of China. 2010;10(5):457–458. [Google Scholar]
- 23.Yang S M. Yan Shu Injecdtion Combined with Chemotherapy on Treating advanced primary liver cancer. Medicine Industry Information. 2006;3(17):209–210. [Google Scholar]
- 24.Yates J W, Chalmer B, McKegney F P. Evaluation of patients with advanced cancer using the Karnofsky performance status. Cancer. 1980;45(8):2220–2224. doi: 10.1002/1097-0142(19800415)45:8<2220::aid-cncr2820450835>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 25.Yu M L, Kang XH. Clinical Study of Compound Kushen Injection Combined with Hepatic Arterial Chemoembolization for Primary Liver Cancer. Guide of China Medicine. 2009;8(7):123–125. [Google Scholar]
- 26.Zhang A M, Wang W M, Wu J J. Effect of Yanshu on Primary Hepatic Carcinoma Treated with Interventional Therapy. Evaluation and analysis of drug-use in hospitals of China. 2006;6(6):374–375. [Google Scholar]
- 27.Zhu X F, Li G A. Study of Yanshu injection combined with chemoembolization on middle and advanced stage liver cancer. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2006;15(4):431–432. [Google Scholar]
- 28.Zhu Y Z, Kong XB, Yan L. Effect of compound radix sophorae flavescentis injection on Hedgehog signaling pathway activation in rat hepatocarcinoma models. Journal of Clinical Rehabilitative Tissue Engineering Research. 2011;15(2):257–260. [Google Scholar]