Abstract
Vitex doniana is traditionally used in Togo to treat various diseases including wounds. The aim of this work was to evaluate the efficiency of Vitex doniana on cutaneous wound healing. Wounds were induced in ICR mice divided into four groups as following: Group I received carbopol 974P NF empty gel, Groups II and III were treated topically with carbopol gel containing 2.5% and 5% of Vitex doniana extract. Group IV received Betadine® 10% as standard drug. The efficacy of treatment was evaluated by planimetry and histological analysis. We secondary used the gel containing Vitex doniana at 2.5% and the pure extract at 10 mg/ml on the model of ear edema induced by xylene. Skin toxicity test was performed with the gel containing Vitex doniana at 5% and the pure extract at 30 mg/ml. Vitex doniana at 5% and 2.5% provided better wound contraction (91.14% and 86.38%) at day 12 post-excision when compared to control (51.15%). The results of histological evaluation supported the outcome of excision wound model. Moreover Vitex doniana inhibited significantly edema induced by xylene when compared to control (p< 0.05). In skin toxicity test, no abnormal symptoms were developed over 14 day-time period. Vitex doniana inhibits the topical inflammation and accelerate cutaneous wound repair.
Keywords: Vitex doniana, wound healing, inflammation, histology
Introduction
Wounds have tremendous impact on the healthcare economy. Chronic wound represents a major resources burden and drain on healthcare resources in developed countries. According to World Alliance for Wound and Lymphedema Care (Macdonald and Asiedu 2010) WAWLC report, chronic leg wounds in the USA account for the estimated loss of two million work days per year. The impact of loss of self-esteem, continued pain, and possible depression is difficult to quantify but is certainly real. In the United Kingdom, the attributable cost of wound care in 2006–2007 was 9.89 million Pounds. In developing countries, chronics infected wounds are most common such as Sub-Saharan African and South Asian countries, than in developed countries (Sasidharan et al. 2010). Current estimates indicate that nearly 6 million people suffer from chronic wounds worldwide. The prevalence of chronic wounds in the community was reported as 4.5 per 1000 population, whereas that of acute wounds was nearly double, at 10.5 per 1000 population (WAWLC). The poor hygienic condition in some third world countries is the main cause of this problem. Besides that, most people in developing countries who suffer from chronic wound cannot afford to purchase modern drugs, which are very expensive and might have side effects. Interestingly, in developing countries, it's estimated that 70–80% of patients is treated by traditional healers and herbal practitioners (Agyare et al., 2009; Nyika, 2007).
There are several reports stating that the extracts of many plants used in Africa have wound healing properties (Reddy et al., 2002; Raina et al., 2008; Mahé et al., 2006). V. doniana is one of these plants commonly used in Togo to treat chronic cutaneous wounds, infection, diarrhea and diabetes. The genus Vitex belonging to the family of Verbenaceae approximately includes 270 known species of trees and shrubs within tropical and sub-tropical regions, although few species may be found in temperate zones. Vitex species (V. piramidata, V. pubescens, V. agnus-castus, V. doniana, V. gaumeri, V. trifolia, V. cienkowskii, V. rehmannii) has been reported to be used in traditional medicine to treat a wide range of ailments, such as depression, venereal diseases, malaria, asthma, allergy, wounds, skin diseases, snake bite, inflammation and body pains (Hernandez et al 1999, Montiel-Herrera et al. 2004, Dongmo et al. 2011, Nyiligira et al. 2008, Agunu et al. 2005). Vitex doniana (plack plum) is the commonest species in West Africa (Ladedji and Okoye, 1993). Any work in ours acknowledges has being evaluated V. doniana effect on skin wound healing.
Wound healing process involves several steps including coagulation, inflammation, formation of granulation tissue, matrix formation and acquisition of wound strength (Scaffer and Nannyl, 1996). Villagers traditionally use the poultice prepared from different crude extract to treat a variety of skin ailments including wound. The aim of this study was to evaluate cutaneous wound healing effect of V. doniana in mice.
Materials and methods
Plant material
V. doniana stem bark (voucher number Togo09270-09283) was collected locally in the savannah of Tsevie (35 km north of Lomé) and taken to the herbarium of laboratory of Botany of Universite de Lome where the plant was authenticated. The stem bark of the plant was washed in water, cut into pieces, freeze-dried and reduced to powder. To this powder (325 g) was added 3 l of hydroalcohol solution (v/v). The filtrate was evaporated on rotavapor (Buchi R-210) and the extract obtained was mixed to the gel for the tests.
Preparation of gels
The gel was prepared as following: 0,3 g of Carbopol 974P NF (Goodrich, USA) was dispersed in 27 g of distilled water and mixed by stirring continuously in a magnetic stirrer (IKA-Combimag RCT) at 800 rpm for 1 h. The mixture under continuous stirring was neutralized by drop-wise addition of NaOH 1 mol/l. Mixing was continued until a transparent gel was formed (Trombetta et al., 2006). Three types of gel formulations were prepared viz. empty gel, gel containing 2.5% and 5% of V. doniana extract. For standard treatment we used dermal Betadine® 10% (povidone ioded) as positive control.
Animal and grouping
ICR mice (30–35 g weight) purchased from Nogoschi Memorial Medical Research Institute of The University of Legon (Accra, Ghana) were used for this study. They were housed for 10 days at Laboratoire de Physiologie Animale of Université de Lomé (Togo) for acclimatization. They were maintained on standard experimental conditions of temperature, 12h light/dark cycle and fed on normal pallet diet and water ad libitum throught the experiment (Trombetta et al., 2006). Except the agents under study, no topical or systematic therapy was given to animals. The mice were divided into four groups consisting of eight in each group, where group-I was keep as control receiving Carbopol empty gel. Goups-II and III animals were locally applied with Carbopol gel containing 2.5% and 5% of V. doniana extract respectively. Group-IV received the standard treatment with Betadine® 10%.
Wound healing model
Wound healing activity was studied using excision wound model. Mice were anesthetized by the open mask method with diethyl ether and their dorsal surface was shaved by using a shaving machine (Gurung and Skalko-Basnet, 2009). Circular wound of 6 mm diameter was created on the dorsal region shaved area of mice (Tramontina et al., 2002). The wounds were left undressed to the open environment and observed daily. The treatments were applied topically once a day, starting from the wound induction until 12 day post-excision.
Measurement of wound area
The progressive changes in wound area were monitored by camera (Pentax, Optio E75, China) every other day. Later on, wound area was evaluated by using Image J program. Wound contraction (WC) was calculated as percentage of the reduction in wound area by using the mathematic expression: WCd= (1-WAd/WA0) × 100 where WAd is area of the wound on the day d and WA0 the wound area on the zero day (Sadaf et al., 2006).
Histological studies
Skin specimens from different wounds of each group were collected on days 8 and 12 immediately after the wound area measurement was performed. The specimens were fixed in 10% neutral buffered formalin, processed and blocked with paraffin. Five-micrometer skin sections were cut and stained with hematoxylin-eosin (McManus and Mowry, 1965). The tissues were qualitatively assessed under the light microscope (Olympus BX 51) at 200x or 400x magnification and graded as absent (−), mild (+), moderate (++) and severe (+++) for epidermal or dermal remodeling. Epithelialization, ulceration and necrosis in epidermis, congestion edema, fibroblast proliferation, mononuclear and polynuclear cells and neovascularization in dermis were analyzed (Akkol et al., 2009).
Topical anti-inflammatory activity
The anti-inflammatory activity was evaluated by using model of xylene-induced ear edema in mice according to Kou (Kou et al., 2005) with some modifications. The animals were randomly allocated to groups GI - IV, each group consisting of five mice, as follows: GI received only distilled water, (positive control); GII received Carbopol empty gel (negative control); GIII received gel containing V. doniana at 2.5% and GIV the pure extract of V. doniana at 10mg/ml, applied topically immediately after inducing edema. The edema was induced by topical application of xylene (50 µl) on the inner and outer surfaces of the right ear lobe. The left ear was considered as control. One hour after induction of inflammation, the mice were euthanized by overdose of ether anesthesia and both ears were removed and weighed.
The edematous response was measured as the weight difference between the right and left ears. The anti-inflammatory activity was expressed as a percentage of the inhibition of edema in treated mice in comparison to positive control mice.
Acute toxicity studies
Twelve mice were used for skin irritation study. The animals were shaved on the back of the body and were left under close observation for 24 h in order to ascertain no abnormal skin responses including irritation, post-positive swelling, redness, itching, and inflammation or any other symptoms present in the shaved area. The tested mice were then equally divided into 3 groups. The group-I and -II were respectively treated topically with single dose of the gel containing V. doniana at 5% and the pure extract of V. doniana at 30mg/ml according to OECD guidelines 404. Group-III received distilled water in the identical manner to those in group-I/II. The systematic responses, in particular, the local skin reactions, to the topical treatment were monitored on the daily basis for successive 14 days.
Statistical analysis
Results were expressed as mean ± SEM. Data were analyzed by a one-way ANOVA, followed by Bonforroni test for comparison between two groups. P < 0.05 was considered to be significant.
Results
Wound healing activity
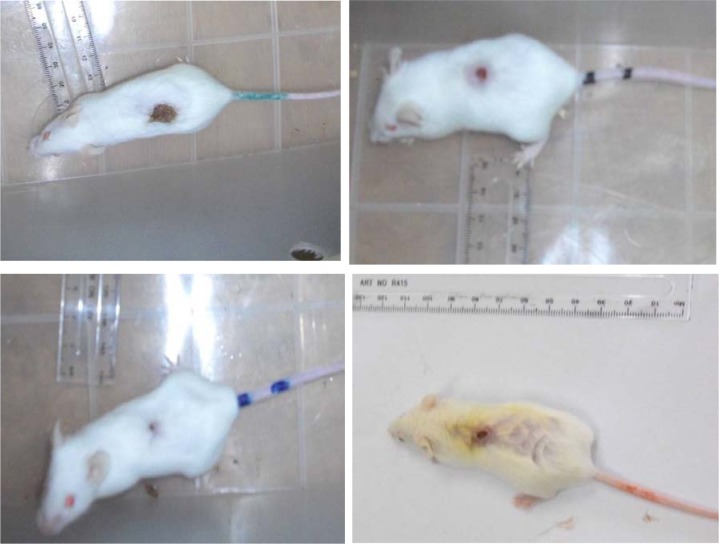
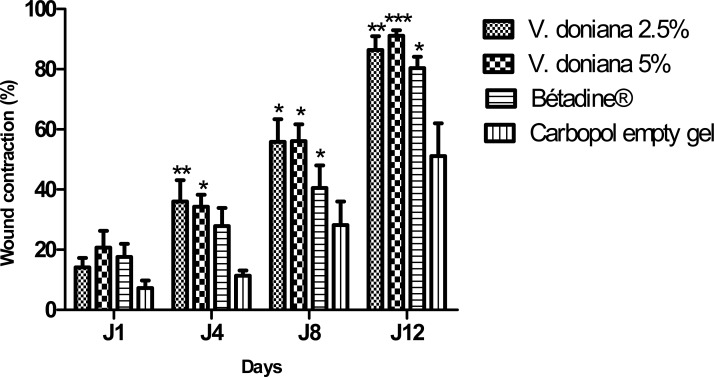
Topical application of Vitex doniana extract at 5% and 2.5% enhances cutaneous wound healing by stimulating wound contraction. Macroscopically we observed better contraction of the wound in the mice treated by V. doniana extract (Figure 1). Wound contraction is one parameter used to assess wound healing. Wound contracting ability of V. doniana extract topical application in different concentrations was significantly greater than that of the control. For example significant (p< 0.05) wound contraction was initiated from day 4 in treated groups (II and III). Animals treated with Vitex doniana 5% and 2.5% showed respectively 91.14% and 86.38% contraction versus 51.15% for control on day 12 (Figure 2).
Figure 1.
Effect of topical application of V. doniana on skin excision wound on day 12 post-excision, showing better wound contraction in groups II–III and IV
Figure 2.
Effect of topical application of V. doniana on skin excision wound.
Wound contraction was expressed on percentage of wound closure using wound area on day d compared with wound area on the first day. Results are expressed on means ± SEM of n=8 mice per group. Difference is significant which *p< 0.05 ; **p< 0.01 ; ***p<0.001 control vs treated
Histological studies
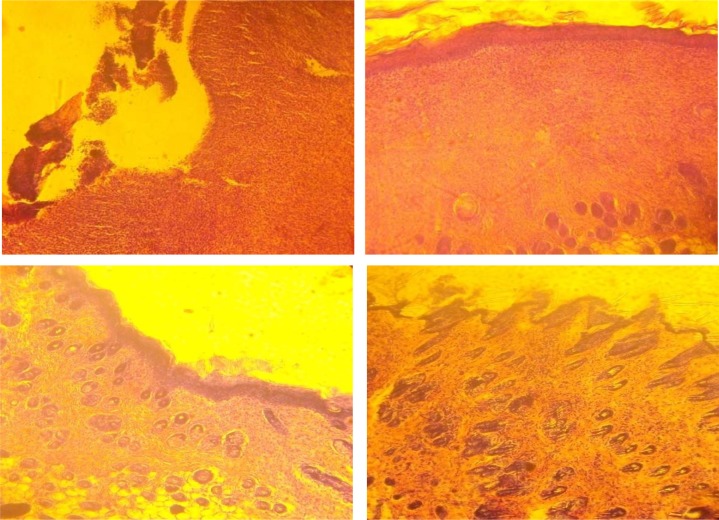
On the histopathological examination, our findings showed that the granulation tissue is much greater in skin wounds treated with t V. doniana extract than in control wound. In fact, on day 8, the main activity observed was the proliferation of inflammation cells, neovascularisation and the epithelialization in the mice treated with Vitex doniana and Betadine®. In those treated with the carbopol empty gel it were mainly ulceration and necrosis (table 1). On the day 12, histological sections showed better epithelialization in all groups but when compared to the vehicle and Betadine®, epithelialization capacity of V. doniana extracts was the highest (Fig. 3).
Table 1.
Histological evaluation of wound healing process of the vehicle, extracts of Vitex doniana and Betadine® administrated mice on day 8.
| Treatment groups | Wound healing process | ||||||||
| C | Ed | PMN | MNC | N | FP | NV | Ep | U | |
| Vehicle | +++ | +++ | +++ | ++ | ++ | + | + | − | ++ |
| Vitex doniana 2.5% | + | + | + | ++ | + | ++ | + | + | + |
| Vitex doniana 5% | ++ | + | ++ | ++ | + | ++ | ++ | ++ | + |
| Bétadine® | + | ++ | + | + | + | ++ | + | + | + |
Hematoxilin-eosin stained sections were scored as mild (+), moderate (++) and severe (+++) for epidemal and/or dermal remodeling. Epithelialization and ulceration were scored as present (+) and absent (−). C : congestion ; Ed : edema ; PMN : polymorphonuclear cells ; MNC : mononuclear cells ; N : necrosis ; FP : fibroblast proliferation ; NV : neovascularization ; Ep : epithelialization ; U : ulceration.
Figure 3.
Histological appearance of the 12 days old wounded skin treated topically with the vehicle alone (Carbopol empty gel), Vitex doniana extracts and Betadine®. U : ulceration ; N : necrosis ; Ed : edema ; Inf : :mixed type inflammatory cells ; C : congestion ; Ep : epithelialization ; HF : hair follicle ; Fp : fibroblast proliferation. (Hematoxilin-eosin stain, ×200
Topical anti-inflammatory activity
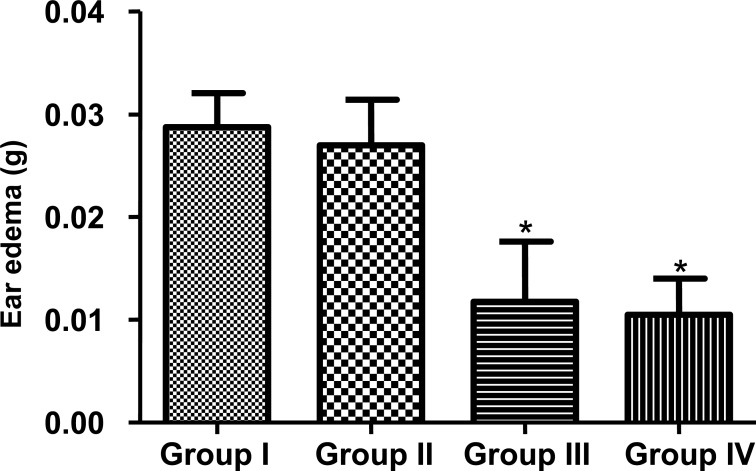
Results of acute model of inflammation presented in figure 4 showed that V. doniana extract attenuated the xylene-induced ear edema. Pure extract of V. doniana and the extract at 2.5% showed respectively 65.52% and 58.62% inhibition of edema at time of 1 h after induction of edema (Figure 4).
Figure 4.
Effect of Vitex doniana extracts on the xylene-induced ear edema.
Ear edema was induced by topical application of xylene on the inner and outer surfaces of the right ear lobe. Edema was evaluated by calculate difference between right and left portion weigh. Each group contained n=5 rats, results are expressed on means ± SEM. Difference is significant which *p< 0.05 control vs treated.
Acute toxicity study
The evaluation scores of irritation response to one-dosage showed that neither the erythema formation nor skin swelling were developed during a 14-day time period. Before the fourteenth day, the growing hair in shaved area was observed in mice. V. doniana may be used in topical application for wound healing without side effect on skin.
Discussion
Wound healing is an extreme complex phenomenon involving regeneration of parenchymal cells, migration and proliferation of both parenchymal and connective tissue cells, synthesis of extracellular matrix protein, remodeling of connective tissue parenchymal components, collagenization and acquisition of wound strength (Schaffer and Nanney, 1996). These phenomenon may be categorized into three phases viz inflammation, proliferation and remodeling phases. In the inflammation phase, various growth factors such as tumor necrosis factor (TNF), interleukins (IL) are released to initiate the proliferation phase. The latter is characterized by angiogenesis, collagen deposition, granular tissue formation, epithelialization and wound contraction (Chang et al., 2004; Pierce et al., 1992). In this paper we studied wound healing potential of V. doniana extracts. We observed that the topical application of V. doniana extracts demonstrated very significant wound healing properties. Wound contraction, a part of the proliferative phase of wound healing, occurs through the centripetal movement of the tissues surrounding the wound, which is mediated by myofibroblasts (Adams and Richarada, 1999). The increased wound contraction in the treated group may be due to the enhanced activity of fibroblasts. Others hand ours histological studies results showed that V. doniana extract enhanced nevascularization in treated group compared to control at day 8. Angiogenesis plays an important role in wound healing and newly formed blood vessels comprise 60% of the repair tissue. Neovascularization helps hypoxic wounds to attain noroxic condition (Ehrlich et al., 1972). V. doniana may promote angiogenesis as showed histological studies. This histological observation provided additional evidence for the wound healing effect of V. doniana based on the contraction value of wound area.
Control of inflammation is an important feature of a wound-healing process, since excessive inflammation delayed wound healing. Such prolonged, inflammation and its consequent effect is a major challenge in chronic wounds (Vinaya et al 2009). The xylene is a flogistic agent, promoter neurogenic inflammation that act on target cells in the periphery such as mast cells and vascular smooth muscle producing inflammation, which is characterized by redness and warmth, swelling and hypersensitivity. The inflammatory symptoms result from the release of substances from primary sensory nerve terminals (Richardson and Vasko, 2002). Antiinflammatory effect of V. doniana may be benefict for wound repair.
The dermal toxicity was one of the major problems associated with the topical application of certain drugs in wound healing. Topical application of V. doniana showed no sign of toxicity of the skin. V. doniana extract application promoted skin wound healing effect without skin toxicity.
Conclusion
V. doniana promote experimental cutaneous wound healing by enhance wound contration and this finding support their utilization in togolese folk medicine.
References
- 1.Adam JS, Richarada FC. Cutaneous wound healing. New Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 2.Agyare C, Asase A, Lechtenberg M, Niehues M, Deters A, Hensel A. An ethnopharmacological survey and in vitro confirmation of ethnopharmacological use of medicinal plants used for wound healing in Bosomtwi-Atwima-Kwanwoma area, Ghana. J Ethnopharmacol. 2009;125:393–403. doi: 10.1016/j.jep.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Agunu A, Yusuf S, Andrew GO, Zezi AU, Abdurahman EM. Evaluation of five medicinal plants used in diarrhea treatment in Nigeria. J Ethnopharmacol. 2005;101:27–30. doi: 10.1016/j.jep.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 4.Akkol EK, Koca U, Pesin I, Yilmazer D, Toker G, Yesilada E. Exploring the wound healing activity of Arnebia densiflora (Nordm.) Ledeb. By in vivo models. J Ethnopharmacol. 2009;124:137–141. doi: 10.1016/j.jep.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Chang HY, Senedon JB, Alizadeh AA, Sood R, West RB, Montgomery K, Chi JT, van de Rijn M, Botstein D, Brown PO. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2004;2:E7. doi: 10.1371/journal.pbio.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dongmo AB, Azebaze AGB, Donfack FM, Dimo T, Nkeng-Efouet PA, Devkota K P, Sontia B, Wagnerg H, Sewald N, Vierling W. Pentacyclic triterpenoids and ceramide mediate the vasorelaxant activity of Vitex cienkowskii via involvement of NO/cGMP pathway in isolated rat aortic rings. J Ethnopharmacol. 2011;133:204–212. doi: 10.1016/j.jep.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 7.Ehrlich HP, Grislis G, Hunt TK. Metabolic and circulatory contribution to oxygen gradient in wounds. Surgery. 1972;72:576–583. [PubMed] [Google Scholar]
- 8.Gurung S, Skalko-Basnet N. Wound healing properties of Carica papaya latex: In vivo evaluation in mice burn model. J Ethnopharmacol. 2009;121:338–341. doi: 10.1016/j.jep.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez MM, Heraso C, Villarreal ML, Vargas-Arispuro I, Aranda E. Biological activities of crude plant extracts from Vitex trifolia L. (Verbenaceae. J Ethnopharmacol. 1999;67:37–44. doi: 10.1016/s0378-8741(99)00041-0. [DOI] [PubMed] [Google Scholar]
- 10.Kou J, Sun Y, Lin Y, Cheng Z, Zheng W, Yu B, Xu Q. Anti-inflammatory activities of aqueous extract from Radix Ophiopogon japonicus and its two constituents. Biol Pharm Bull. 2005;28:1234–1238. doi: 10.1248/bpb.28.1234. [DOI] [PubMed] [Google Scholar]
- 11.Ladeji O, Okoye ZSC. Chemical analysis of the fruit of Vitex doniana (Verbanaceae) J Sci Food Agric. 1993;63:483–484. [Google Scholar]
- 12.Macdonald J, Asiedu K. WAWLC: World Alliance for Wound and Lymphedema Care. Wounds. 2010;22:55–59. [PubMed] [Google Scholar]
- 13.Mahé A, Faye O, N'Diaye HT, Konare H, Keita S, Traore AK, Hay R. Definition of an algorithm for the management of common skin disease at primary care level in sub-Suharan Africa. Trans R Soc Trop Med Hyg. 2006;99:39–47. doi: 10.1016/j.trstmh.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 14.McManus JFA, Mowry RW. Stainning methods, histologic and histochemical. New York, Evanston, London: Harper Raw; 1965. [Google Scholar]
- 15.Montiel-Herrera M, Camacho-Hernandez IL, Rios-Morgan A, Delgado-Vargas F. Partial physicochemical and nutritional characterization of the fruit of Vitex mollis (Verbenaceae) J Food Compost Anal. 2004;17:205–215. [Google Scholar]
- 16.Nyika A. Ethical and regulatory issues surrounding African traditional medicine in the context of HIV/AIDS. Dev World Bioeth. 2007;7:225–234. doi: 10.1111/j.1471-8847.2006.00157.x. [DOI] [PubMed] [Google Scholar]
- 17.Nyiligira E, Viljoen AM, Van Heerden FR, Van Zyl RL, Van Vuuren SF, Steenkamp PA. Phytochemistry and in vitro pharmacological activities of South African Vitex (Verbenaceae) species. J Ethnopharmacol. 2008;119:680–685. doi: 10.1016/j.jep.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Pierce GF, Tarpley JE, Yanaghira D, Mustoe TA, Fox GM, Thomason A. Platelet-derived growth factor (BB homodimer), transforming growth factor-bl, and basic growth factor in dermal wound healing. Am J Pathol. 1992;140:1375–1388. [PMC free article] [PubMed] [Google Scholar]
- 19.Raina R, Prawez S, Verma PK, Pankaj NK. Medecinal plants and their role in wound healing. Vet Scan. 2008;3:1–8. [Google Scholar]
- 20.Reddy JS, Rao PR, Reddy MS. Wound healing effects of Heliotropium indicum, Plumbago zeylanicum and Acalypha indica in rats. J Ethnopharmacol. 2002;79:249–251. doi: 10.1016/s0378-8741(01)00388-9. [DOI] [PubMed] [Google Scholar]
- 21.Richardson JD, Vasko MR. Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther. 2002;302:839–845. doi: 10.1124/jpet.102.032797. [DOI] [PubMed] [Google Scholar]
- 22.Sadaf F, Saleem R, Ahmed M, Ahmad SI, Navaid-ul Z. Healing potential of cream containing extract of Sphaeranthus indicius on dermal wounds in Guinea pigs. J Ethnopharmacol. 2006;107:161–163. doi: 10.1016/j.jep.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 23.Sasidharan S, Nilawatyi R, Xavier R, Latha LY, Amala R. Wound Healing Potential of Elaeis guineensis Jacq Leaves in an Infected Albino Rat Model. Molecules. 2010;15:3186–3199. doi: 10.3390/molecules15053186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaffer CJ, Nanney LB. Cell biology of wound healing. Int Rev Cytol. 1996;169:151–181. doi: 10.1016/s0074-7696(08)61986-5. [DOI] [PubMed] [Google Scholar]
- 25.Tramontina VA, Machado MA, Nogueira Filho Gda R, Kim SH, Vizzioli MR, Toledo S. Effet of bismuth subgallate (local hemostatic agent) on wound healing in rats. Histological and histometric findings. Brazil Dental J. 2002;13:11–16. [PubMed] [Google Scholar]
- 26.Trombetta D, Puglia C, Perri D, Licata A, Pergolizzi S, De Pasquale A, Saija A, Bonina FP. Effect of polysaccharides from Opuntia ficus-indica (L.) clatodes on the healing of dermal wounds in the rat. Phytomedicine. 2002;13:352–358. doi: 10.1016/j.phymed.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Vinaya K, Raja Naika H, Ananda Kumar CS, Benaka Prasad SB, Chandrappa S, Ranganatha SR, Krishna V, Rangappa KS. Evaluation of in vivo wound-healing potential of 2-[4-(2,4-dimethoxy-benzoyl)-phenoxy]-1-[4-(3-piperidin-4-yl-propyl)-piperidin-1-yl]-ethanone derivatives. Eur J Med Chem. 2009;44:3158–3165. doi: 10.1016/j.ejmech.2009.03.012. [DOI] [PubMed] [Google Scholar]