Abstract
This study explores the anti-inflammatory and anti-nociceptive activities of Patrinia villosa, a Chinese medicinal plant, and to explore its effects on the proinflammatory cytokines of the rats with pelvic inflammation model. The animals were randomly divided into Patrinia villosa group (PV group), dexamethasone group (DEX group), and model-control group (CON group) to perform an ear edema test, a carrageenin-induced paw edema test, a cotton pellet-induced granuloma formation test, and an acetic acid-induced writhing test. The model rats with pelvic inflammation were established, and the serum levels of interleukin-6 (IL-6), interleukin-8 (IL-8) and tumor necrosis factor-alpha (TNF-α) in each group was detected with the Enzyme-Linked ImmunoSorbent Assay (ELISA). The results of the ear edema test, carrageenin-induced paw edema test, cotton pellet-induced granuloma formation test, and acetic acid-induced writhing test all showed that Patrinia villosa had strong anti-inflammatory and anti-nociceptive effects. In the experiment using model rats with pelvic inflammation, we found that the serum levels of IL-6, IL-8 and TNF-α in PV and DEX group were all significantly lower than those of the CON group, and the serum levels of IL-6 and IL-8 in PV group were significantly lower than those of the DEX group. Patrinia villosa, with its strong anti-inflammatory and anti-nociceptive activities, can be used to treat pelvic inflammation and to relieve the associated pain.
Keywords: Patrinia villosa, anti-inflammatory, anti-nociceptive, pelvic inflammation
Introduction
Patrinia villosa (PV) is a commonly used Chinese medicinal plant. It grows in eastern, central, southern and southwest areas of China(Hu et al., 1992; Li et al., 2004; Li et al., 2006; Wei et al., 1999). It has been widely used in the Chinese medicinal formula when treating carbuncles, acute appendicitis, hepatitis, amygdalitis, angina parotidea, anthracia, stasis, intestinal abscess, postpartum pain, dysmenorrhoea and endometriosis(But and Chang, 1986). The inhibitory effects of a Chinese medicinal formula were prominently decreased when PV was eliminated from the formula in treating severe acute respiratory syndrome (SARS) (Li et al., 2006). The methanol extract of PV (EPV) has been used in the treatment of cutaneous diseases for the antipruritic activity, and the methanol extracts have inhibitory activities against Substance P-induced itching(Kuraishi et al., 2000).
Interleukin-6 (IL-6), interleukin-8 (IL-8) and tumor necrosis factor-alpha (TNF-α) are inflammatory cytokines. The chemokine IL-8 could represent a direct link between chronic inflammation and autocrine/paracrine stromal cell proliferation, in agreement with its marked secretion by a combination of Th1 and Th17 cell-derived inflammatory cytokines(Maggi et al., 2010). Some of these cytokines may directly trigger infiltration by peritoneal leukocytes (Gazvani et al., 2002; Harada et al., 2001). The concentrations of IL-1β, IL-6, and IL-8 were significantly greater in biopsy specimens from pouchitis compared to those from pouches without pouchitis or normal ileal mucosa (Gionchetti et al., 1994). To explore the roles of proinflammatory cytokines including IL-1β, IL-6, IL-8 and TNF-α in pelvic inflammatory disease (PID), Lee et al (2008) used multiplex bead array analysis to measure the plasma levels of these proinflammatory cytokines in 50 healthy controls as well as in 41 PID patients before and after routine protocol treatment. They finally found IL-1β, IL-6, IL-8 and TNF-α were significantly elevated in PID patients before antibiotic treatment than after treatment. However, IL-8 was not significantly different between healthy controls and PID patients. The relative increase in ratio of IL-6 was significantly correlated with white blood cell count, neutrophil count and C-reactive protein level (Lee et al., 2008). IL-1β, IL-6, IL-8 and TNF-α may play an important role in the pathogenesis of PID (Lee et al., 2008). These biomarkers, particularly IL-6, could be useful adjuncts for the clinical diagnosis of PID(Lee et al., 2008).
Although PV has been used for many years clinically, few studies have been done on the involved mechanism underlying the effects of PV on pelvic inflammation. In this study, the anti-inflammatory, anti-nociceptive activities of PV and its effects on the proinflammatory cytokines of the model rats with pelvic inflammation were investigated.
Materials and Methods
Animals
Female Imprinting-Control-Region (ICR) mice weighing 40–60g were used in the ear edema test and acetic acid-induced writhing test. Female Sprague-Dawley (SD) rats were used in the carrageenan-induced rat paw edema test, cotton pellet-induced granuloma formation test, and in the experiment exploring the effects of EPV on the model rats with pelvic inflammation. All the animals were provided by the Laboratory Animal Center of Zhejiang Chinese Medicine University (Hangzhou, China). The animals were kept in a room under environmentally controlled conditions of 22±2°C and a 12h light–12h dark cycle. Before starting the study, all the animals were acclimatized at least a week. The research was carried out according to the National Research Council's protocol for the care and use of laboratory animals.
The preparation of plant materials
PV was purchased from Huqing Yutang Pharmaceutical Co., Ltd (Hangzhou, China) and was identified by Zhejiang Chinese Medicine University (Hangzhou, China). EPV was prepared with 30g of PV extracted by refluxing it with 300ml of 70% ethanol for 60 min followed by filtration. The same extraction procedures were repeated once. The obtained solution was combined and condensed to a concentration of 1g/ml, which was then dried into EPV. The dosage of PV administered was within the standard dosage levels according to the guideline from State Administration of Traditional Chinese Medicine of the People's Republic of China.
Anti-inflammatory and anti-nociceptive activity
Ear edema test in mice
The test was conducted following the methods of Brattsand et al.(1983) and Young et al.(1981). 18 female ICR mice (body weight 40–60g) were randomly divided into Patrinia villosa group (PV group), dexamethasone group (DEX group), and model-control group (CON group) with N=6 in each group. Ear edema was induced by topical application of arachidonic acid (AA) dissolved in acetone to the inner and outer surfaces of both ears with an automatic microliter pipette. Before the edema induction and at 0.25h, 0.5h, 1h and 2h after the edema induction, the thickness of each ear was measured by vernier calipers. Percent inhibition was calculated: inhibition (%)= (Vc−Vt)/Vc×100%, where Vc and Vt respectively represented the average ear edema volume of the model-control rat and the rat under drug treatment. PV group was orally administrated EPV at 0.08g/kg. The DEX group was orally administrated dexamethasone at 0.02mg/kg. The CON group was orally administrated saline at 8ml/kg.
Carrageenan-induced rat paw edema test
The test was performed as described by Winter et al(1962). 18 female SD rats (with a body weight of 100–120g) were randomly divided into PV group, DEX group and CON group (N=6 in each group). The PV group was orally administrated EPV at 0.55g/kg and DEX group was orally administrated dexamethasone at 9.97 mg/kg. The CON group was orally administrated saline at 8ml/kg. 0.1ml of 1% freshly prepared suspension of carrageenin was administered into the sub-planter region of the right hind paws to lead to the formation of edema in situ due to localized inflammation. Percent inhibition was calculated: inhibition (%)=(Vc−Vt)/Vc×100%, where Vc and Vt respectively represented the average paw volume of the model-control rat and the rat under drug treatment. Test drugs were administrated orally 1h prior to the carrageenin injection.
Cotton pellet induced granuloma test
The method of Trnavsky et al.(1965) was used in the test. The SD rats were divided into three groups (PV group, DEX group and CON group), each group consisting of six rats. After shaving off the fur, the animals were ether anaesthetized. Sterile pre-weighed cotton pellets (50±1 mg) were implanted in the lumbar region of each rat through an incision. The PV group was orally administrated EPV at 0.08g/kg and DEX group was orally administrated dexamethasone at 1.44mg/kg for 7 consecutive days. The CON group was orally administrated saline at 8ml/kg for 7 consecutive days. Test drugs were administered once daily during the whole experimental period. 24 hours after the treatment ended, the animals were sacrificed and the cotton pellets were excised, which were then dried until the weight remained constant. The increase of the pellet weight was considered as granuloma tissue deposit.
Acetic acid induced writhing test
This test was performed using the method described by Collier et al. (Collier, 2009) 18 female ICR mice (body weight 40–60g) were randomly divided into three groups (PV group, DEX group and CON group) with 6 in each group. Muscle contractions were induced by intra peritoneal injection of 0.6% acetic acid in a volume of 0.1 ml/10g body weight. The PV group was orally administrated with EPV at doses of 0.08g/kg and DEX group with dexamethasone at 0.02mg/kg 30 min before the acetic acid injection. The CON group was orally administrated saline at 8ml/kg. Writhes number was recorded during continuous observation for 15 min beginning from 5 min after the injection of acetic acid.
The effects of PV on the serum levels of IL-6, IL-8 and TNF-α in the model rats with pelvic inflammation
32 female SD rats, weighing 180–200g, were used in the test. 8 rats were randomly taken as the normal controls and the other 24 rats were used as the pelvic inflammatory model rats. The pelvic inflammation models were induced by injecting 0.08ml of 20% phenol mucilage into the right uterus of rats. 20% phenol mucilage was prepared by combining 8ml of phenol with 32ml of 1% carboxymethylcellulose sodium mucilage. 15 days after the model rats were established, they were randomly divided into four groups with ten rats in each group. The PV group was orally administrated EPV at 0.55g/kg and DEX group was orally administrated dexamethasone at 0.02mg/kg for 28 days respectively. The CON group was orally administrated saline at 8 ml/kg for 28 days. The normal control group (NOR group) was orally administrated saline at 8ml/kg for 28 days. After the treatment ended, all the rats were anesthetized with sodium pentobarbital (40 mg/kg, i.p.). The serum samples were taken from caudal vein and Enzyme-Linked ImmunoSorbent Assay (ELISA) kit, provided by Shanghai Hushang Biotechnology Co., Ltd, China, were used to detect the levels of IL-6, IL-8 and TNF-α in the serum.
Statistical analysis
Results were analyzed by an independent statistician using computer software, namely, Statistical Package for Social Sciences (SPSS 13.0 for Windows). Analysis of variance (ANOVA) was employed. A 5% significance level (P<0.05) and two-tailed tests were used for all hypothesis tests.
Results and Discussion
Ear edema test in mice
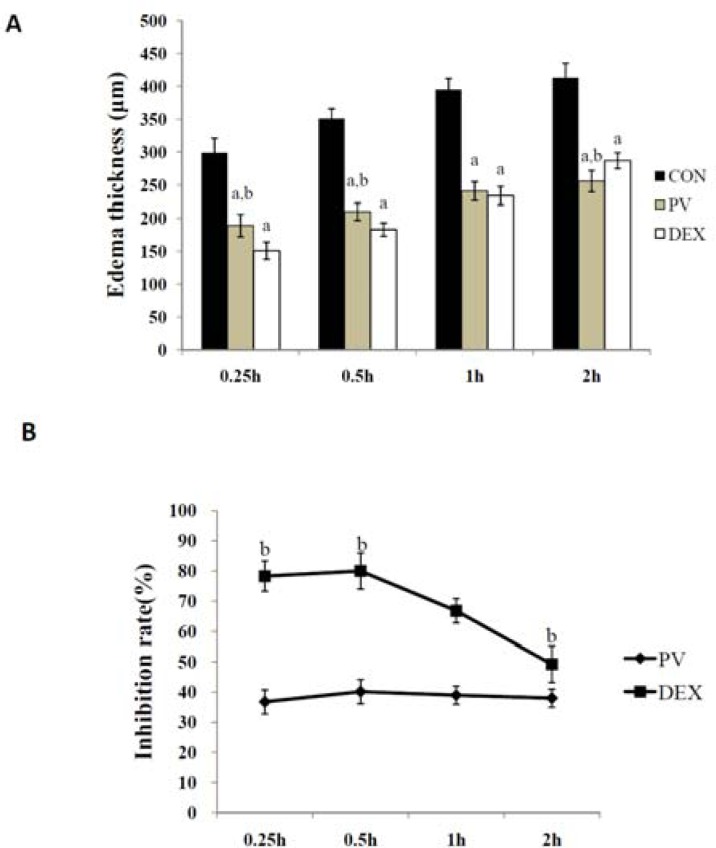
Both of DEX and EPV showed potently inhibitory effects in the ear edema test (Figure 1). During the first 0.5h, compared with the DEX group, PV had significant anti-inflammatory effects with a inhibition percentage of 78±4%, 80±4% respectively at 0.25h and 0.5h. Although after 0.5h there was no significant difference between DEX and PV groups, the inhibition rate of PV group is higher than that of DEX group.
Figure 1.
Results of ear edema test. (A) Edema thickness (µm) (B) Inhibition rate (%)
Notes: The significant difference was set at ap < 0.05, compared with the CON group; b p <0.05, compared with the DEX group.
Carrageenan-induced rat paw edema test
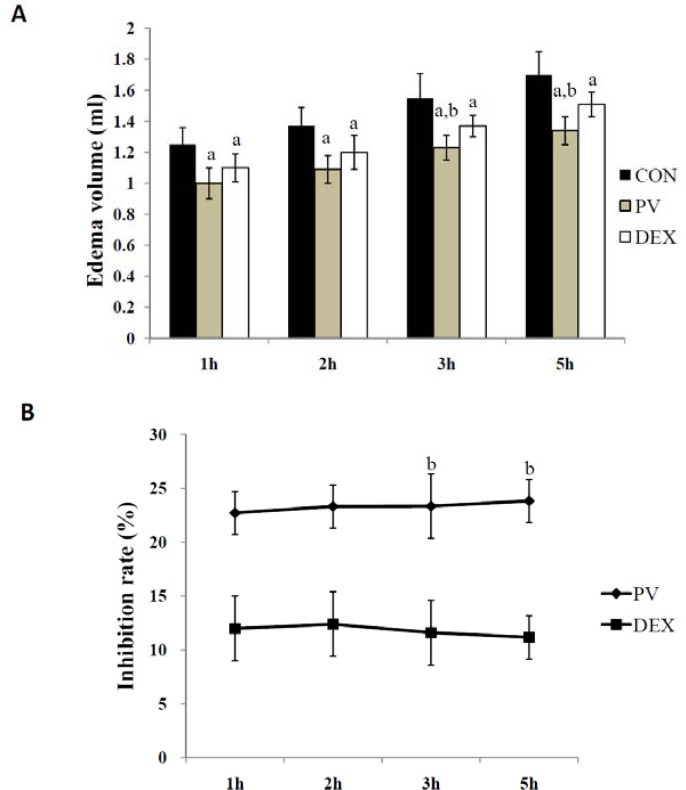
PV group showed a significant reduction of the carrageenan-induced paw edema compared with the control group (Figure 2). There was no significant difference between PV and DEX group. From 3h to 5h, the inhibition rate of DEX group decreased, while the inhibition rate of PV group increased.
Figure 2.
Results of carrageenin-induced paw edema test.
(A) Edema volume (ml) (B) Inhibition rate (%)
Notes: The significant difference was set at ap < 0.05, compared with the CON group; b p <0.05, compared with the DEX group.
Cotton pellet induced granuloma test
Both of PV and DEX demonstrated significantly inhibitory activities on the granuloma formation and PV had more eminent effects than DEX (Table 1).
Table 1.
Results of cotton pellet induced granuloma
| Group | weight of granuloma (mg) | Inhibition rate (%) |
| CON | 5.80±0.12 | - |
| PV | 3.23±0.10a,b | 44 b |
| DEX | 3.86±0.11a | 37 |
Notes: The significant difference was set at
p < 0.05, compared with the CON group;
p <0.05, compared with the DEX group.
Acetic acid induced writhing test
Both of DEX and EPV showed significantly inhibitory effects on the writhing numbers and PV had more eminent effects than DEX (Table 2)
Table 2.
Results of acetic acid induced writhing tests
| Group | Number of writhes | Inhibition of writhing response (%) |
| CON | 49.4±6.6 | - |
| PV | 25.0±4.5a,b | 48 b |
| DXS | 32.3±5.2a | 58 |
Notes: The significant difference was set at
p < 0.05, compared with the CON group;
p <0.05, compared with the DEX group.
Comparison of the serum levels of TNF-α, IL-6 and IL-8
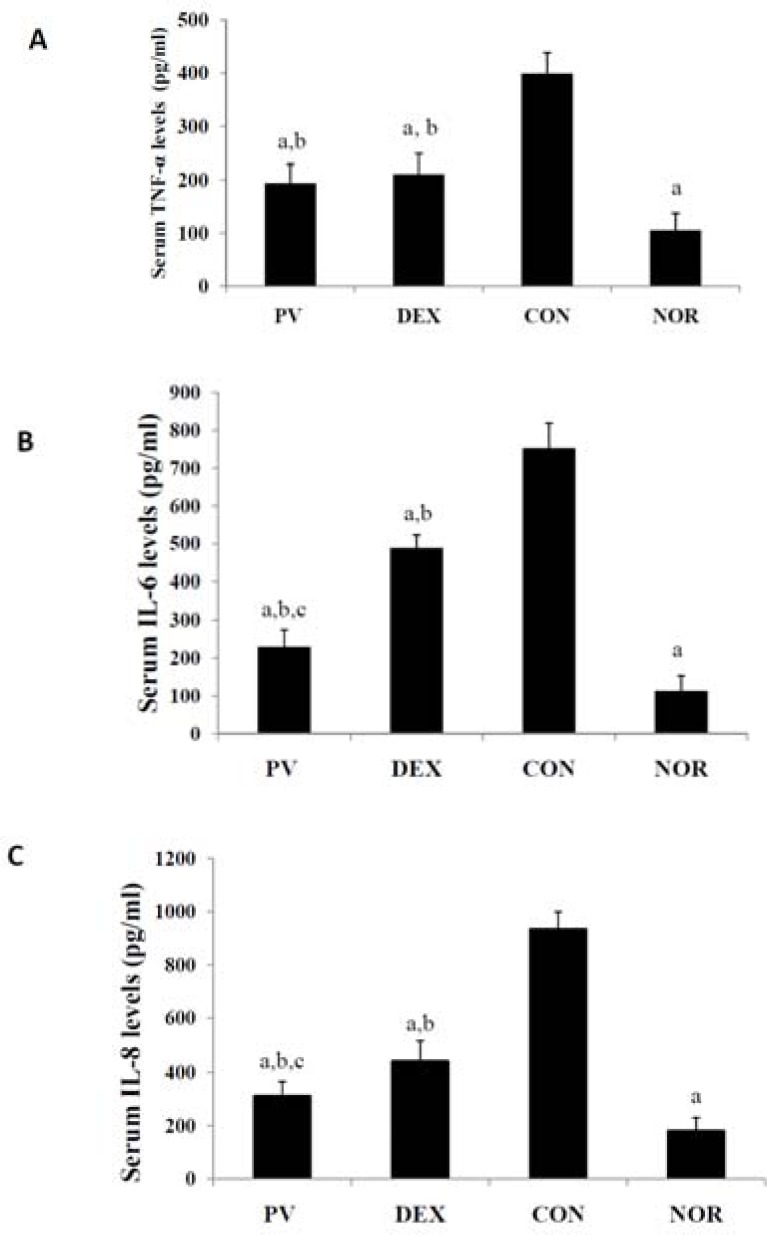
As shown in Figure 3A, the serum TNF-α levels of PV group, DEX group and NOR group were significantly lower than those of the CON group (P<0.05). The serum TNF-α levels of PV group and DEX group were significantly higher than those of NOR group (P<0.05), while there was no marked difference between PV group and DEX group (P>0.05). As shown in Figure 3B, the serum IL-6 levels of PV group, DEX group and NOR group were significantly lower than those of the CON group (P<0.05). The serum IL-6 levels of DEX group and PV group were significantly higher than those of NOR group (P<0.05), and the serum IL-6 levels of PV group were significantly lower than those of DEX group (P<0.05). As shown in Figure 3C, the serum IL-8 levels of PV group, DEX group and NOR group were significantly lower than those of the CON group (P<0.05). The serum IL-8 levels of DEX group and PV group were significantly higher than those of NOR group (P<0.05), and the serum IL-8 levels of PV group were significantly lower than those of DEX group (P<0.05).
Figure 3.
The serum levels of TNF-α, IL-6 and IL-8
(A) The serum TNF-α levels (B) The serum IL-6 levels (C) The serum IL-8 levels
Notes: The significant difference was set at ap < 0.05, compared with the CON group; b p <0.05, compared with the NOR group; c p <0.05, compared with the DEX group.
The main bioactive compounds of PV are saponins and volatile substances. Saponin extracted from PV (SEPV) (50 mg/kg and 100 mg/kg) was found to effectively reduce the weight of U14 cervical tumor (35.1% and 57.1%, respectively), and the mechanism involved is associated with inhibition of tumor cells in G0/G1 phase, inducing apoptosis and inhibiting the expression of PCNA, mutant P53 and Bcl-2 protein(Zhang et al., 2008). The supercritical CO2 fluid extraction (SFE-CO2) was used to extract the volatiles from PV(Fan et al., 2008). The chemical compositions identified by gas chromatography-mass spectrometry showed that the total volatile of one extract consisted hydrocarbon (49.65%), aldehyde (16.66%), fatty acid (22.38%), terpene (9.04%) and little alcoholic(Fan et al., 2008). From the other extracts, 32 compounds were identified, in which hydrocarbon, aldehyde, fatty acid and terpene possessed 58.21%, 5.97%, 13.19% and 21.79%, respectively(Fan et al., 2008). A Chinese medicinal formula containing PV was used to treat persistant inflammatory pain and hyperalgesia in a previous study(Wei et al., 1999). In the study, the inflammation was induced by injecting complete Freund's adjuvant (CFA) into one hindpaw of each rat and the formula significantly attenuated CFA-induced hyperalgesia at 2 hours and facilitated the recovery from hyperalgesia, when compared to saline-treated rats.
In the present study, the results of the ear edema test, carrageenin-induced paw edema test, cotton pellet-induced granuloma formation test, and acetic acid-induced writhing test all showed that PV had strong anti-inflammatory and anti-nociceptive effects. The dosage of PV for all the activities evaluated is based on the results of the preliminary experiments and the standard clinical dosage. In the experiment using model rats with pelvic inflammation, we found that the serum levels of TNF-α, IL-6, and IL-8 in PV and DEX group were all significantly lower than those of the CON group, and the serum levels of IL-6 and IL-8 in PV group were significantly lower than those of the DEX group. The present study demonstrated that EPV with its strong anti-inflammatory and anti-nociceptive activities, can be used to treat pelvic inflammation and to relieve the associated pain. However, more should be done on the involved mechanism in the future.
Abbreviations
- PV
Patrinia villosa
- IL-6
interleukin-6
- IL-8
interleukin-8
- TNF-α
tumor necrosis factor-alpha
References
- 1.Brattsand R, Roempke K, Johansson U, Havu N, Hasselgren M. An Improved Model for Testing the Topical Anti-Inflammatory Activity of Drugs on Rat Ear Edema. Acta Pharmaceutica Suecica. 1983;20:37–38. [Google Scholar]
- 2.But PPH, Chang H-M. Pharmacology and applications of Chinese materia medica. Singapore ; Philadelphia, PA, USA: World Scientific; 1986. [Google Scholar]
- 3.Collier PN. The synthesis of 3-aryl-3-azetidinyl acetic acid esters by rhodium(I)-catalysed conjugate addition of organoboron reagents. Tetrahedron Letters. 2009;50:3909–3911. [Google Scholar]
- 4.Fan GR, Xie Y, Peng JY, Wu YT. Chemical composition and antioxidant activity of volatiles from Patrinia Villosa Juss obtained by optimized supercritical fluid extraction. Journal of Pharmaceutical and Biomedical Analysis. 2008;48:796–801. doi: 10.1016/j.jpba.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Gazvani R, Smith L, Fowler PA. Effect of interleukin-8 (IL-8), anti-IL-8, and IL-12 on endometrial cell survival in combined endometrial gland and stromal cell cultures derived from women with and without endometriosis. Fertil Steril. 2002;77:62–67. doi: 10.1016/s0015-0282(01)02954-5. [DOI] [PubMed] [Google Scholar]
- 6.Gionchetti P, Campieri M, Belluzzi A, Bertinelli E, Ferretti M, Brignola C, Poggioli G, Miglioli M, Barbara L. Mucosal concentrations of interleukin-1 beta, interleukin-6, interleukin-8, and tumor necrosis factor-alpha in pelvic ileal pouches. Dig Dis Sci. 1994;39:1525–1531. doi: 10.1007/BF02088059. [DOI] [PubMed] [Google Scholar]
- 7.Harada T, Iwabe T, Terakawa N. Role of cytokines in endometriosis. Fertil Steril. 2001;76:1–10. doi: 10.1016/s0015-0282(01)01816-7. [DOI] [PubMed] [Google Scholar]
- 8.Hu S, Cai W, Ye J, Qian Z, Sun Z. Influence of medicinal herbs on phagocytosis by bovine neutrophils. Zentralbl Veterinarmed A. 1992;39:593–599. doi: 10.1111/j.1439-0442.1992.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 9.Kuraishi Y, Tohda C, Kakihara Y, Komatsu K. Inhibitory effects of methanol extracts of herbal medicines on substance P-induced itch-scratch response. Biological & Pharmaceutical Bulletin. 2000;23:599–601. doi: 10.1248/bpb.23.599. [DOI] [PubMed] [Google Scholar]
- 10.Lee SA, Tsai HT, Ou HC, Han CP, Tee YT, Chen YC, Wu MT, Chou MC, Wang PH, Yang SF. Plasma interleukin-1beta, -6, -8 and tumor necrosis factor-alpha as highly informative markers of pelvic inflammatory disease. Clin Chem Lab Med. 2008;46:997–1003. doi: 10.1515/CCLM.2008.196. [DOI] [PubMed] [Google Scholar]
- 11.Li SS, Li HY, Piao YA, Liu DL, Tian WJ, Dong YM. [The anti-respiratory syncytial virus effect of an active compound (AP3) from a Chinese medicinal herb-Herba patriniae in vitro] Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25:150–153. [PubMed] [Google Scholar]
- 12.Li Y, Li J, Fang C. Inhibitory effects of anti-SARS traditional Chinese medicines on the UV irradiation of lambda-lysogen. Am J Chin Med. 2006;34:147–155. doi: 10.1142/S0192415X06003710. [DOI] [PubMed] [Google Scholar]
- 13.Maggi M, Fibbi B, Penna G, Morelli A, Adorini L. Chronic inflammation in the pathogenesis of benign prostatic hyperplasia. International Journal of Andrology. 2010;33:475–488. doi: 10.1111/j.1365-2605.2009.00972.x. [DOI] [PubMed] [Google Scholar]
- 14.Trnavsky K. Effect of Formaldehyde-Induced Periarthritis Upon the Composition of Cotton-Pellet Granuloma in Rats. J Pharm Pharmacol. 1965;17:261–262. doi: 10.1111/j.2042-7158.1965.tb07665.x. [DOI] [PubMed] [Google Scholar]
- 15.Wei F, Zou S, Young A, Dubner R, Ren K. Effects of four herbal extracts on adjuvant-induced inflammation and hyperalgesia in rats. J Altern Complement Med. 1999;5:429–436. doi: 10.1089/acm.1999.5.429. [DOI] [PubMed] [Google Scholar]
- 16.Winter CA, Risley EA, Nuss GW. Carrageenin-Induced Edema in Hind Paw of Rat as an Assay for Antiinflammatory Drugs. Proceedings of the Society for Experimental Biology and Medicine. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 17.Young JM, Deyoung LM. Tachyphylaxis to Phorbol Ester Induction of Mouse Ear Edema and Ornithine Decarboxylase. Journal of Investigative Dermatology. 1981;76:314–314. [Google Scholar]
- 18.Zhang T, Li Q, Li K, Li Y, Li J, Wang G, Zhou S. Antitumor effects of saponin extract from Patrinia villosa (Thunb.) Juss on mice bearing U14 cervical cancer. Phytotherapy Research. 2008;22:640–645. doi: 10.1002/ptr.2354. [DOI] [PubMed] [Google Scholar]