Abstract
Gentamicin and vitamin C have been proposed as nephrotoxic and antioxidant, respectively. This study involved biochemical and histopathologic investigation showing protection and treatment of gentamicin-induced nephrotoxicity in rabbits using vitamin C for 26 days hypothesizing that whether vitamin C would inhibit or decrease the raised serum urea and creatinine levels. This study was conducted on 25 healthy male albino rabbits (average weight 1.5±0.2 kg), classified into 5 groups: group A, B, C, D and E for nephrocurative (study-I) and nephroprotective (study-II) studies. Control group of rabbits (group A) received only the vehicle of gentamicin ampoule. In study-I, gentamicin sulphate (GS 80 mg/kg, i.m.) was administered to group B and C rabbits for ten days, then group C rabbits received vitamin C 250 mg/Kg for remaining 16 days. Group D and E received GS 80 mg/kg and GS 80 mg/kg i.m.-vitamin C 250 mg/kg orally, respectively during whole period (26 days) of study-II. After 26 days, various biochemical parameters, i.e. serum creatinine, blood urea nitrogen (BUN), and serum antioxidant activity, and histopathologic investigations were made. Nephrotoxicity was observed in rabbit groups B, C and D as evident from significant (p<0.05) high levels of serum creatinine and BUN and low serum antioxidant levels as compared to the levels of control group. Decrease in the levels of serum creatinine and BUN along with the increase in serum antioxidant activity was observed after vitamin C treatment in group C. While, renal-protective role of vitamin C was seen in group E as compared to the control. In conclusion, Gentamicin induced nephrotoxicity can be attenuated or treated using vitamin C.
Keywords: Vitamin C, Gentamicin, Nephroprotective and nephrocurative activities, Serum creatinine, Blood urea nitrogen, Serum antioxidant activity
Introduction
Gentamicin is an aminoglycoside and is used for treating gram-negative bacillary infections (Goodman and Gillman, 2005). Side effects of gentamicin usage involve nephrotoxicity that is typically illustrated by following observations: decrease in glomerular filtration rate, diminished urine concentrating capacity and proteinuria (Cojocel et al., 1984). A dose of gentamicin 80 mg or greter/kg body weight of rats for more than seven days is found to be the most common origin of nephrotoxicity (Ajith et al., 2007).
It has been observed that gentamicin causes renal toxicity by disturbing the proximal tubular cells (Kadkhodaee et al., 2004). The mode of renal toxicity by gentamicin involves its binding to the cell wall phospholipids resulting in the blockage of chain reactions of phosphatidyl inositol, and ultimately impairment of cell integrity (Kadkhodaee et al., 2004).
One of the previous studies presents that free radicals like increase with gentamicin treatment (Varzi et al., 2007). Gentamicin-induced renal toxicity arises as a result of membrane lipid peroxidation due to the stimulation of reactive oxygen entities like H2O2 and O2 which potentially induce the contraction of mesangial cells. As a result, filtration surface area and ultrafiltration coefficient changes, which causes the retardation in glomerular filtration rate (Mingeot-Leclercq and Tulkens, 1999; Derakhshanfar et al., 2007; Mashhadian and Rakhshandeh, 2005).
Vitamin C is a hydrophilic substance (Akturk et al., 2006; Harapanhalli et al., 1996) that acts as a nutritional supplement and is considered to be very essential in preventing scurvy (Belin et al., 2009). It is known to be an outstanding chain-breaking antioxidant as well as a very good free radical scavenger (Premila, 2005). In a lead-induced oxidative stress, an increase in the levels of antioxidant enzymes along with the reduction in the levels of lipid peroxide after administering vitamin C due to its antioxidant activity (Kadkhodaee et al., 2004; Patra et al., 2001). The reactive oxygen species like hydroxyl radicals possess an unpaired electron that renders the specie quite reactive towards the nucleic acid, proteins and lipids leading to the cellular oxidative damages (Carcamo et al., 2004). Mechanism of antioxidant activity involves the conversion of vitamin C into its oxidized form (dehydro-ascorbic acid) by donating two electrons to reactive oxygen species (Fischer et al., 2004) while the oxidized forms of ascorbate are relatively stable and unreactive and do not cause cellular damage (Carcamo et al., 2004) however, these reactive oxygen species are then reduced to water.
Present study was aimed to assess various biochemical and histopathological parameters for investigating the nephroprotective and nephrocurative activity of vitamin C against gentamicin-induced renal toxicity in rabbits.
Material and Methods
Chemicals and plant material
Gentamicin sulphate intramuscular injection (80 mg/2ml, GS80 i.m.) ampoules were procured from Care Pharmacy, Lahore, Pakistan. Analytical grade chemicals i.e. vitamin C, sodium phosphate, sodium benzoate, sodium hydroxide, EDTA, Fe(NH4)2SO4, H2O2, acetic acid, thiobarbituric acid and uric acid were purchased from Merck, Germany. The Randox Standard Urea Kit and Randox Standard Creatinine Kit were obtained from Pak Chemicals, Lahore, Pakistan.
Study animals
This study was conducted on 25 healthy male albino rabbits (average weight 1.5±0.2 Kg) purchased from local animal market and were kept in stainless steel cages placed in the animal house of the University of the Punjab, Lahore, Pakistan. Water and green fodder were given as feed to the rabbits ad libatum. This study (2008-89/PU/MPhil.) was approved by the Board of Advanced Sciences and Research, the University of the Punjab, Lahore, Pakistan and was conducted in accordance with good clinical practice and international guidelines for animal use in experimentation.
Experimental design
The rabbits were classified into 5 groups: group A, B, C, D and E for nephrocurative (study-I) and nephroprotective (study-II) studies. Control group of rabbits (group A) received only the vehicle of gentamicin ampoule. Group B and C rabbits for nephrocurative and group D and E rabbits for nephroprotective activity were used. In study-I, gentamicin sulphate (GS 80 mg/kg, i.m.) was administered to group B and C rabbits for ten days, then group C rabbits received vitamin C 250 mg/Kg for remaining 16 days. Group D and E received GS 80 mg/kg and GS 80 mg/kg i.m.-vitamin C 250 mg/kg orally, respectively during whole period (26 days) of study-II. After 26 days, various biochemical parameters i.e. serum creatinine, blood urea nitrogen (BUN), and serum antioxidant activity, and histopathologic investigations were made.
Collection of blood samples
To determine nephrotoxicity by evaluating serum levels of creatinine, urea, and antioxidant activity in treated rabbits, blood samples were collected at first day before treatment, 10th, 14th, 18th, 22nd and 26th day during the treatment. To collect blood sample, marginal vein of shaved ear of rabbits were punctured with the help of sterilized disposable needle followed by the collection of 2 ml of blood in glass tubes. After coagulation, blood samples were centrifuged at 1000 × g for 15 min to obtain the serum which was used for determining antioxidant activity, urea, and creatinine levels.
Biochemical evaluation
To determine nephrotoxicity, various biochemical parameters like serum creatinine level (SCL), blood urea nitrogen (BUN) level and serum antioxidant activity (SAA) were assessed during the study. Nephrotoxicity involves an increase in SCL and BUN level along with the diminished SAA. The colorimetric method was used for the evaluation of SCL and BUN using Randox Standard Creatinine Kit and Randox Standard Urea Kit. The SAA was assessed by adopting spectrophotometeric method for the measurement of antioxidant activity in human fluids given by Koracevic et al., (2001). For the measurement of antioxidant activity in human fluids, firstly following solutions were prepared: sodium phosphate buffer (100 mmol/L, pH 7.4), sodium benzoate (10 mmol/L), sodium hydroxide (50 mmol/L) and EDTA (2 mmol/L in phosphate buffer), Fe(NH4)2SO4 (2 mmol/litre), Fe-EDTA complex (freshly prepared by mixing EDTA and Fe(NH4)2SO4 solution in equal volumes and was allowed to stand for 60 min at room temperature), H2O2 (10 mmol/L), acetic acid (20%), thiobarbituric acid [0.8% (w/v) in 50 mmol/L NaOH] and uric acid (1 mmol/L in 5 mmol/L NaOH) were freshly prepared just before use. The analytical procedure was as: control (Ao, blank sample) for each sample (A1) was prepared. For each series of analysis, a negative control (K1 and Ko) was also prepared (in triplicate) which contained the same reagents as A1 or Ao, except that serum was replaced with phosphate buffer. 1 mmol/litre uric acid (UA1 and UAo) was used as standard for calibration. Tubes were then incubated for 60 min at 37°C followed by the addition of specific quantities of acetic acid and thiobarbituric acid was added. Tubes were again incubated for 10 min at 100°C (in a boiling water bath). Then the tubes were cooled by placing them in an ice bath. Absorbance was measured at 532 nm using UV/Vis spectrophotometer (Shimadzu1601, Japan) against deionised water. Antioxidant activity was calculated using following formula:
Where, K = absorbance of control (K1 − K0), A = absorbance of sample (A1 − A0), UA = absorbance of uric acid solution (UA1 − UA0) and CUA = concentration of uric acid (in mmol/L).
Histopathological evaluation
To determine histopathological alterations in treated kidneys, kidney tissues of sacrificed rabbits were washed, fixed in 10% formalin and processed using different percentages of ethanol. After embeddig in paraffin wax for 6 hr, processed tissues were subjected to microtoming. Thin slices of kidney tissues, obtained after microtoming, were fixed on glass slides using gelatin and were placed in oven for 12 h at 58°C. After staining these slides with hematoxylin and eosin, the prepared slides were observed for histopathological alterations in kidney.
Statistical analysis
The results obtained from 5 replicates (n = 5) of each experiment are presented as mean ± standard deviation. Analysis of Variance (ANOVA) using SPSS version 13.0 was employed to express the significance of difference between results with p-values set at 0.05.
Results and Discussion
During study-I, no rabbit died while one rabbit from group D died at the 17th day. During study-I, there was nonsignificant (p>0.05) reduction in body weights of rabbits at the 10th day of GS80 i.m. injection however, subsequently group C rabbits regained their body weights considerably (Figure 1) till the 26th day. During study-II, group D rabbits also showed a non-significant (p>0.05) decline in their body weights as compared to the weights of group E rabbits (Figure 1).
Figure 1.
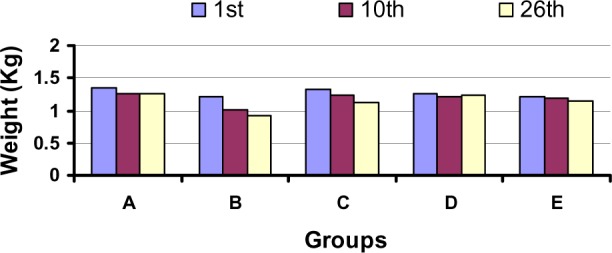
Variation in body weights (kg) among different groups of rabbits under study-I and -II (All the results are expressed as Mean ± SD, n = 5)
During study-I, there was a significant (p<0.05) increase in SCL in group B and C rabbits (Figure 2) at day 10 when compared to that of the control group. However, there was a significant (p<0.05) decrease in SCL in group C rabbits at day 18 with values 2.86±0.83, when compared to that of the control group (4.52±1.04). Further decrease in SCL (2.69±0.55) as compared that of the control group (4.38±0.66) observed at day 22. During study-I, there was a significant (p<0.05) increase in BUN level at day 10 in group B and C rabbits when compared that of the control group. Group C rabbits showed BUN level with values (15.37±0.97) at 10th day followed by a significant (p<0.05) decrease in BUN level when compared to that of the control group at 14th, 18th and 22nd day. Group C rabbits exhibited significantly (p<0.05) reduced BUN level with value 11.04±2.09 at day 26 as compared to that of the control group (15.52±2.20) (Figure 3). During study-I, group B rabbits showed significant (p<0.05) decrease in SAA at day 10 and 26 when compared to the levels of first day and control group (Figure 4), however group C showed an increase in SAA upto day 26 after an initial fall.
Figure 2.
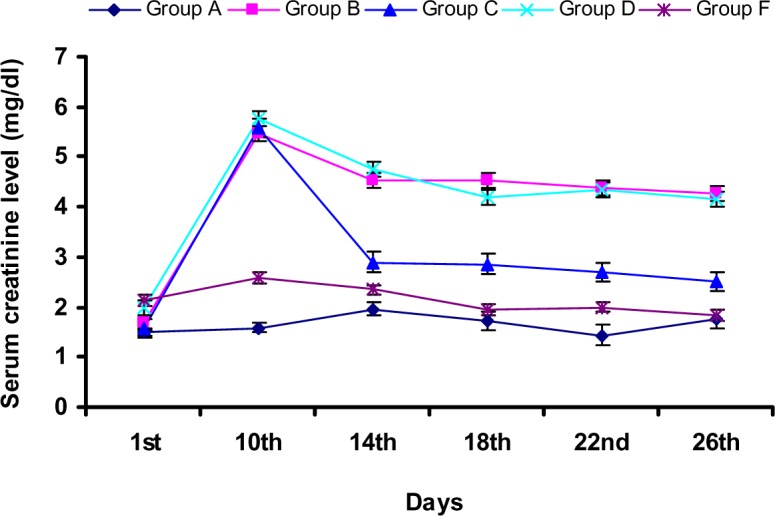
Variation in serum creatinine in rabbits during study-I and -II (All the results are expressed as Mean ± SD, n = 5)
Figure 3.
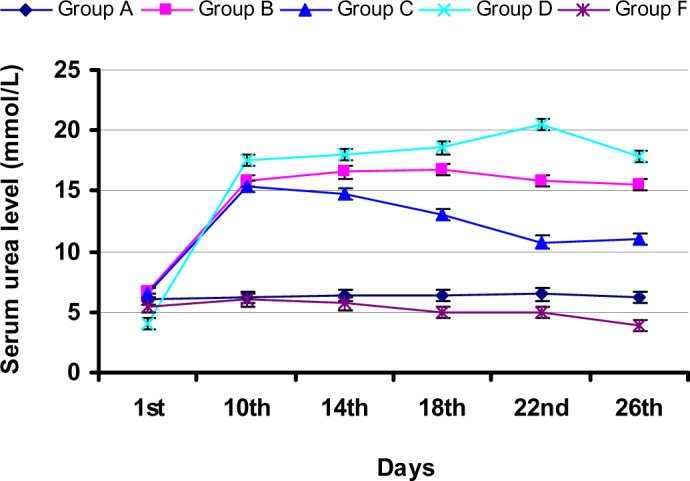
Variation in serum urea level in rabbits during study-I and -II (All the results are expressed as Mean ± SD, n = 5)
Figure 4.
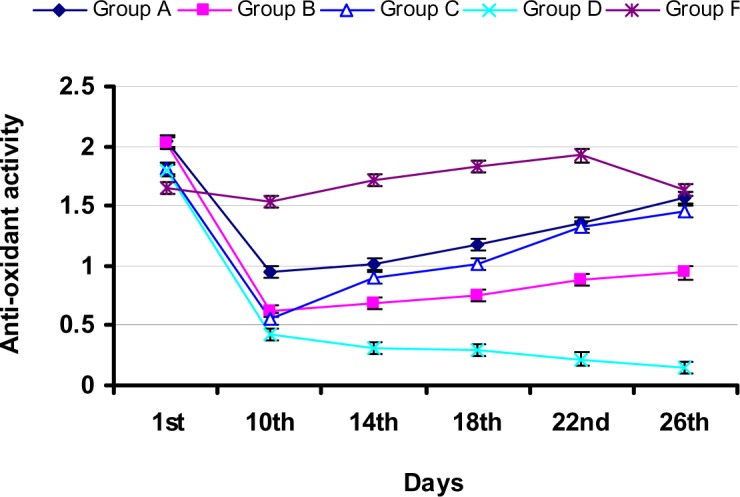
Variation in serum anti-oxidant activity in rabbits during study-I and -II (All the results are expressed as Mean ± SD, n = 5)
During study-II, group D rabbits showed significant (p<0.05) increase in SCL (5.78±1.17) at day 10 when compared to that of the control group (Figure 2). However, there was a significant (p<0.05) decrease in SCL in group E rabbits at day 14 with values, when compared to that of the control group. Further decrease in SCL (1.95±0.63) in group E rabbits as compared that of the control group (4.20±1.38) observed at day 18. At 26th day, group E rabbits exhibited significantly (p<0.05) decreased SCL (1.83±0.67) when compared to the gentamicin-treated group (4.16±0.49). During study-II, there was a significant (p<0.05) increase in BUN level at day 10 in group D when compared that of the control group. Increased value of BUN (18.05±4.43) in group D was also observed at day 14. At day 14, Group E rabbits exhibited the lowest BUN level (5.69±1.22). Further decrease in the BUN level of group E rabbits was observed at day 18, when compared to that of the control group. At day 22, group D rabbits showed the highest BUN value however, group E rabbits showed no increase in BUN level at this day. Group E rabbits exhibited significantly (p<0.05) reduced BUN level with value 3.84±0.82 at day 26 as compared to that of the control group (17.90±1.50) (Figure 3). During study-II, group D rabbits showed significant (p<0.05) decrease in SAA at day 10 and 26 when compared to the levels of first day and control group (Figure 4), however group E showed unchanged SAA upto day 26.
During study-I, histopathologic examination of the kidney tissues showed an increase in the amount of cellular infiltrations, sloughing of cells in the tubular lumen and renal hemorrhage in all groups after receiving GS80 i.m. treatment. However, reversing in the condition was seen in group C rabbits treated with vitamin C. During study-II, no major histopathologic changes were observed in the kidney tissues of group E rabbits. However, histopathologic study of group D rabbit's kidney tissues exhibited an acute tubular necrosis, damaged basolateral membranes, dilated tubules and sloughed cells in the tubular lumen (Figure 5).
Figure 5.
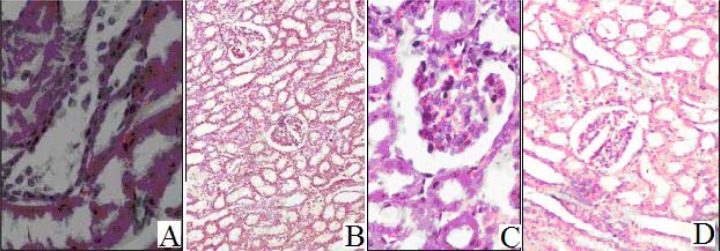
A: Damaged glumerulus and tubular necrosis, along with sloughing of cells in tubular lumen and celluler infiltration, after receiving gentamicin (Group B), B: Improved renal picture of animals treated with Nigella sativa oil (Group C), C: insulted glumerulus. D: Protective effect of vitamin C in group E
This study states the nephrotoxicity induced by administering challengingly high doses (80 mg/kg) of GS through intramuscular route in rabbits for 10 consecutive days. The nephrotoxicity was concluded from the raised levels of blood urea nitrogen and serum creatinine while lowered total serum antioxidant activity. This increase in serum urea and creatinine levels substantiates previous study which described markedly increase in these biochemical parameters after receiving high doses of aminoglycosides (Varzi et al., 2007), thus exhibiting induction of nephrotoxicity. Nephrotoxicity due to GS treatment was further supported by the histopathological evaluation treated kidneys which exhibited the morphological changes in glumerulus and tubular structures due to the cellular infiltrates and sloughing cells. These alterations in damaged kidneys can supported by a previous author (Prozialeck and Edwards, 2007) who proposes that the treatment of nephrons with toxic substances causes the cell disruption which results in the sloughing of these injured cells. Thus nephrotoxicity can be attributed to the buildup of drug in tubular epithelial cells followed by the production of reactive oxygen species (ROS). This condition produces the oxidative stress and causes a fall in renal function; due to which the diminished (as compared with the levels of control animals) level of total serum antioxidant activity in gentamicin-treated animal is detected.
The decline in total serum antioxidant activity (SAA) in gentamicin-treated dogs has also been demonstrated previously (Varzi et al., 2007). It has been found that total serum antioxidant activity is a crucial parameter in the determination of the potential of body antioxidant-defensive capability (Chung et al., 2007) as oxidative stress means an imbalance between ROS and antioxidants, thus body antioxidant activity decreases on the production of free radical species which is further augmented by increasing the antioxidant levels in the body. Vitamin C has been proposed as an agent that is capable to uptake the ROS in plasma and thus play a role in the prevention of their entrance and subsequently damaging the membrane (Ajith et al., 2007).
Conclusion
High doses of intramuscularly administered gentamicin i.e. 80 mg/kg can cause nephrotoxicity within 10 days of treatment and recovery of renal damage by gentamicin induced nephrotoxicity, without any therapy takes longer time if left untreated. In addition, vitamin C is a potential nephroprotective and nephrocurative agent as it normalizes SCL, BUN levels and SAA.
References
- 1.Ajith TA, Usha S, Nivitha V. Ascorbic acid and α-tocopherol protect anticancer drug cisplatin induced nephrotoxicity in mice: a comparative study. Clin Chim Acta. 2007;375:82–86. doi: 10.1016/j.cca.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Akturk O, Demirin H, Sutcu R, Yilmaz N, Koylu H, Altuntas I. The effects of diazinon on lipid peroxidation and antioxidant enzymes in rat heart and ameliorating role of vitamin E and vitamin C. Cell Biol Toxicol. 2006;22:455–461. doi: 10.1007/s10565-006-0138-5. [DOI] [PubMed] [Google Scholar]
- 3.Belin S, Kaya F, Duisit G, Giacometti S, Ciccolini J, Fontés M. Antiproliferative Effect of Ascorbic Acid Is Associated with the Inhibition of Genes Necessary to Cell Cycle Progression. PLoS ONE. 2009;4:e4409–e4413. doi: 10.1371/journal.pone.0004409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cárcamo MJ, Pedraza A, Bórquez-Ojeda O, Zhang B, Sanchez R, Golde DW. Vitamin C Is a Kinase Inhibitor: Dehydroascorbic Acid Inhibits IκBα Kinase β. Mol Cell Biol. 2004;24:6645–6652. doi: 10.1128/MCB.24.15.6645-6652.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung CH, Lee EY, Lee MY, Hong SW, Hong SY. Blockade of Oxidative Stress by Vitamin C Ameliorates Albuminuria and Renal Sclerosis in Experimental Diabetic Rats. Yonsei Med J. 2007;48:847–855. doi: 10.3349/ymj.2007.48.5.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cojocel C, Docius N, Maita K, Smith JH, Hook JB. Renal ultrastructural and biochemical injuries induced by aminoglycoside. Env Health Perspect. 1984;57:298–299. doi: 10.1289/ehp.8457293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derakhshanfar A, Bidadkosh A, Kazeminia S. Vitamin E protection against gentamicin-induced nephrotoxicity in rats: a biochemical and histopathologic study. Iranian J Vet. 2007;8:231–238. [Google Scholar]
- 8.Fischer H, Schwarzer C, Illek B. Vitamin C controls the cystic fibrosis transmembrane conductance regulator chloride channel. Proc Natl Acad Sci USA. 2004;101:3691–3696. doi: 10.1073/pnas.0308393100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodman LS, Gillman AG. Goodman and Gillman's The pharmacological basis of therapeutics. 10th Ed. London, UK: Elsevier; 2005. [Google Scholar]
- 10.Harapanhalli RS, Yaghmai V, Giuliani D, Howell RW, Rao DV. Antioxidant effects of vitamin C in mice following X-irradiation. Res Commun Mol Pathol Pharmacol. 1996;94:271–287. [PubMed] [Google Scholar]
- 11.Kadkhodaee M, Khastar H, Faghihi M, Ghaznavi R, Zahmatkesh M. Effects of cosupplementation of vitamins E and C on gentamicin-induced nephrotoxicity in rat. Exp Physiol. 2004;90:571–576. doi: 10.1113/expphysiol.2004.029728. [DOI] [PubMed] [Google Scholar]
- 12.Kadkhodaee M, Khastar H, Faghihi M, Ghaznavi R, Zahmatkesh M. Effects of cosupplementation of vitamins E and C on gentamicin-induced nephrotoxicity in rat. Exp Physiol. 2004;90:571–576. doi: 10.1113/expphysiol.2004.029728. [DOI] [PubMed] [Google Scholar]
- 13.Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. Method for the measurement of antioxidant activity in human fluids. J Clin Pathol. 2001;54:356–361. doi: 10.1136/jcp.54.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mashhadian NV, Rakhshandeh H. Antibacterial and antifungal effects of Nigella sativa extracts against S. aureus, P. aeroginosa and C. albicans. Pak J Med Sci. 2005;21:47–52. [Google Scholar]
- 15.Mingeot-Leclercq MP, Tulkens PM. Aminoglycosides: Nephrotoxicity. Antimicrob Agents Chemother. 1999;43:1003–1012. doi: 10.1128/aac.43.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patra RC, Swarup D, Dwivedi SK. Antioxidant effect of α tocopherol, ascorbic acid and L-methionine on lead induced oxidative stress to the liver, kidney and brain in rats. Toxicol. 2001;162:81–88. doi: 10.1016/s0300-483x(01)00345-6. [DOI] [PubMed] [Google Scholar]
- 17.Premila A. Vitamin C may be beneficial in the prevention of paracetamol-induced renal damage. Clin Exp Nephrol. 2005;9:24–30. doi: 10.1007/s10157-004-0335-6. [DOI] [PubMed] [Google Scholar]
- 18.Prozialeck WC, Edwards JR. Cell Adhesion Molecules in Chemically-Induced Renal Injury. Pharmacol Ther. 2007;114:74–93. doi: 10.1016/j.pharmthera.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varzi HN, Esmailzadeh S, Morovvati H, Avizeh R, Shahriari A, Givi ME. Effect of silymarin and vitamin E on gentamicin-induced nephrotoxicity in dogs. J Vet Pharmacol Therap. 2007;30:477–481. doi: 10.1111/j.1365-2885.2007.00901.x. [DOI] [PubMed] [Google Scholar]


