Abstract
The aim of this present study is to investigate the mucositis caused by methotrexate (MTX), as well as whether the application of royal jelly (RJ) has a protective effect on oxidative stress. This present study included six groups each consisted of 12 Wistar rats. Distilled water (po: peroral) was given to the 1st group as placebo for 10 days and MTX (20 mg/kg, intraperitoneal: ip) on the 7th day. The 2nd group received RJ (50mg/kg, po) for 10 days and normal saline (NS) instead of MTX. RJ (50mg/kg) was given to the 3rd group for 10 days and MTX on the 7th day. The 4th group received RJ (100 mg/kg, po) for 10 days and NS was given intraperitoneally. RJ (100mg/kg) was given to the 5th group for 10 days and a single dose of MTX. Distilled water was given to the 6th (control) group for 10 days and intraperitoneal NS on the 7th day. Malondialdehyde (MDA), glutathione peroxidase and superoxide dismutase were analyzed in blood samples on the 11th day. Morphological and histopathological changes were examined in the intestinal tissue samples. Villus length and mucosal thickness, as well as the villus length/crypt ratio, were significantly decreased with MTX administration, and the semi-quantitative histological evaluation (SQHE) score was measured high (p<0.001). In addition, a decrease in the antioxidant parameters and an increase in the MDA levels were identified. The villus length and SQHE were significantly different in the groups receiving RJ (p<0.001) as compared to the MTX group. Although RJ addition had no effect on the decreased mucosal thickness and villus/crypt ratio in MTX groups, it caused an improvement in the antioxidant levels and a remarkable decrease in MDA levels. Adding RJ has a decreasing effect on the MTX-induced intestinal damage and it has a suppressive effect on MTX-induced oxidative stress by means of increasing antioxidant enzyme activity and decreasing lipid peroxidation.
Keywords: Rat, methotrexate, mucositis, royal jelly, oxidative stress
Introduction
The damage caused by the chemotherapy (CT) and radiotherapy (RT) on the intestinal system is named as mucositis (Sonis et al., 2002). The severity of the mucositis ranges from mild inflammation to deep ulcerations (Blijlevens et al., 2000). The beginning step in the development of mucositis is the formation of reactive oxygen species (ROS); i.e. oxidative stress. Moreover, inflammation and bacterial colonization as well play an important role on the development steps of mucositis (Blijlevens et al., 2007). It has been shown that effective removal of the ROS in the patients receiving CT/RT decreases the development of mucositis (Culy et al., 2001). Royal jelly (RJ) is a bee product secreted from the hypopharyngeal glands of the young worker bees (Inoue et al., 2003). It has been demonstrated both in vitro studies and in vivo experimental rat studies that RJ has immunomodulatory (regulating) (Sver et al., 1996), anti-inflammatory (Kohno et al., 2004), wound healing (Fujii et al., 1990), antioxidative (Jamnik et al., 2007) and antibacterial effects. These effects suggest that they might be beneficial in decreasing the development and treatment of mucositis.
Methotrexate (MTX) is a chemotherapeutic agent that acts as a folate antagonist and likely causes severe mucositis (Chabner et al., 2007). MTX suppresses the antioxidative system and enhances oxidative stress in many organs (Babiak et al., 1998). Therefore, RJ might have protective and decreasing effect on mucositis and oxidative stress. The present study was planned to investigate the effect of RJ on the methotrexate-induced mucositis and oxidative stress in rats.
Materials and Methods
This experimental study was conducted between September 2006 and September 2007 at the Hakan Çetinsaya Experimental and Clinical Research Center (ECRC) obtaining the approval of the ethical committee of Erciyes University School of Medicine.
Animals
Seventy-two male Wistar rats, each weighing 211–316 g, were included in the study. They were kept in special nests, each containing four rats, in the ventilated sunny rooms. Within seven days prior to the study, the rats were kept at room temperature (22±2°C) in 12-hour natural light-12-hour dark cycle. They were fed with standard diet and given tap water as much as required. Water in the troughs was daily refreshed and the nests were cleaned every other day. After the seven-day adaptation period, food restriction without water restriction was applied 12 hours prior to the study in order to provide similar metabolic condition.
The source and the chemical analysis of royal jelly
The royal jelly used in the study was obtained from Civan Arıcılık Co. The royal jelly was put in the deep-freezer immediately after being obtained, and stored at −18 °C until being used in the study. The study standards were prepared daily, just before the analysis. The free amino-acid content of the RJ was identified with liquid chromatograph-mass spectrometry (Table 1).
Table 1.
Free amino acid content of the royal jelly (mg/100 g royal jelly)
| Free amino acid |
Royal jelly (mg/100 g) |
Free amino acid |
Royal jelly (mg/100 g) |
| Aspartic acid | 17.33 | Cysteine | 1.29 |
| Serine | 1.39 | Glutamic acid | 2.99 |
| Glycine | 1.66 | Threonine | 1.15 |
| Lysine | 62.43 | Alanine | 1.14 |
| Proline | 58.76 | Tryptophan | - |
| Valine | 3.29 | Histidine | - |
| Methionine | - | Arginine | - |
| Tyrosine | 1.29 | Cystine | 21.76 |
| Phenylalanine | 1.49 | Leucine-Isoleucine | 1.51 |
| Hydroxyproline | 1.61 | Glutamine | 1.46 |
Creating study groups and the application of royal jelly and MTX
Totally 72 animals were randomly divided into six groups each consisting of 12 rats. Oral distilled water was given to the 1st group (MTX) for 10 days and a single dose of MTX (20 mg/kg, ip) on the 7th day. The 2nd group (RJ50) received oral RJ (50 mg/kg) for 10 days and intraperitoneal normal saline on the 7th day instead of MTX. The 3rd group (MTX-RJ50) received oral RJ (50 mg/kg) for 10 days and a single dose of MTX (20 mg/kg, ip) on the 7th day. Oral RJ (100 mg/kg) was given to the 4th group (RJ100) for 10 days and intraperitoneal normal saline on the 7th day instead of MTX. (MTX-RJ100) Oral RJ (100 mg/kg) was given to the 5th group for 10 days and a single dose of MTX (20 mg/kg, ip) on 7th day; and the 6th group (control) received placebo for 10 days and intraperitoneal normal saline on the 7th day instead of MTX. RJ solution was given per oral together with equal amount of placebo (distilled water) via 3-inch 16-gauge lavage tube everyday at the same time. Similar to the previous models of MTX-induced mucositis, MTX was given at a dose of 20 mg/kg (Cetin et al., 2008, Leung et al., 1997).
The way that the samples obtained and their storage
Since the maximum mucosal damage caused by MTX occurs after 72 hours (Yonei et al., 1997),, on the 11th day 72 hours after the MTX administration, all the rats were sacrificed under general anesthesia (50 mg/kg ketamine ip). Just before they were sacrificed, 4–5 cc blood samples were obtained from each rat by intracardiac puncture and centrifuged at 3000 rpm for 10 minutes and the plasma was separated. Thereafter, the samples were stored at –80 °C until the oxidative stress markers (malondialdehyde-MDA, glutathione peroxidase-GSH-Px, and superoxide dismutase-SOD) were studied. The intestines of the rats were removed for histological examination just after they have been sacrificed, and put in formalin after being washed with iced isotonic saline solution.
Intestinal mucosa examination
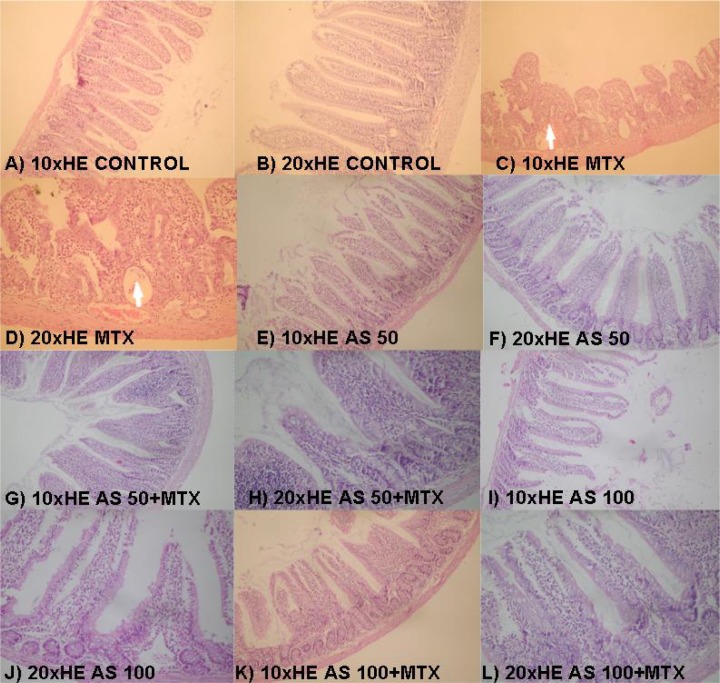
Duodenal tissues were cut and removed. On the histological examination performed after the routine tissue processing, villus length, mucosal thickness and the villus/crypt ratio were morphometrically evaluated analyzing the tissue sections stained with hematoxylin eosin (H&E) (Picture 1) (Carneıro-Fılho et el., 2004). The histopathological changes in the cells were examined via semi-quantitative histological evaluation (SQHE), which has been previously defined by Howarth et al. (Howarth et al., 1996). With this method, 11 parameters were evaluated including villus atrophy (blunting, fusion), superficial erythrocyte and brush-border destruction, decrease in the number of goblet cells, decrease in the number of mitotic figure, loss of crypt/structural deformation, destruction in crypt cells, crypt abscess, neutrophil infiltration, lymphocyte infiltration, lymphatic and capillary dilatation, thickening of submucosa and muscularis externa, and edema. For the duodenal tissue samples obtained from the rats, these histological variables were blindly scored from zero (normal) to three (maximum damage) by two pathologists considering the maximum value to be 33.
Picture 1.
The histopathological effect of RJ added in the diet on the intraperitoneal MTX-induced intestinal damage in rats. A–B (control group), E–F (RJ50 group), and G–H (RJ50-MTX group) are the sections that have normal morphological view; C and D (MTX group); branching and deepening of the villi is shown on C (arrow), crypt abscess and loss of goblet cells are shown on D (arrow). G,H (RJ50-MTX group) and K,L (RJ100-MTX group); less blunting of the villi and no remarkable loss of goblet cells were observed.
The analysis of oxidative parameters
Plasma MDA levels were measured using the method developed by Ohkawa et al. (Ohkawa et al., 1979). MDA reacts with thiobarbituric acid and produces fluorescent thiobarbituric acid reacting substances that are measured spectrophotometrically. The plasma SOD activity was identified with the method developed by Sun et al. (Sun et al., 1998), which is based on the measurement of the degree of nitroblue tetrazolium inhibition with xhantine-xhantine oxidase. The GSH-Px activity was identified in the plasma of the rats using the combined enzymatic method of Paglia et al (Paglia et al., 1967). The principle of the method is based on the measurement of the speed of GSH-Px reaction, which catalysis the oxidation of glutathione by H2O2, over NAPDH concentration that is consumed during the glutathione disulphide reductase (GSSG-Rd) reaction. MDA levels, SOD activity and GSH-Px activity were in turn represented as nmol/ml, U/mg and U/L.
Statistical analysis
Kruskal-Wallis One Way Analysis of Variance and Dunn's test, one of the multiple comparison tests (Post Hoc Tests), were used to compare the groups in terms of the mean values of villus length, villus length/crypt ratio and the total score of SQHE. One-way analysis of variance (One-Way ANOVA) was used to compare the mean values of mucosa thickness, plasma MDA, GSH-Px and SOD and the Tukey's and Dunnett's t-test were used for the multiple comparison. The data were represented as mean ± standard deviation (x̄ ± SD). A p value ≤0.05 was considered statistically significant. All data were analyzed using the Statistical Package for the Social Sciences (SPSS) computer program version 15.0.
Results
In the course of the study, two rats from the first group and one rat from the third group died 48 to 72 hours after the MTX administration. Diarrhea was observed in all rats in the first, third and fifth groups. Villus length, villus/crypt ratio and the mucosal thickness, morphometric measurements of the mucosa, and the results of SQHE, which were obtained via the morphometric analysis of the duodenal tissues of rats performed to evaluate the effect of RJ on the MTX-induced intestinal damage, are summarized in Table 2. The MDA levels and the data concerning GSH-Px and SOD activities are given oin Table 3.
Table 2.
Morphologic and histopathological intestinal parameters of the control and experiment groups
| Groups | Mucosal thickness (mm) x̄ ± SD |
Villus length (mm) x̄ ± SD |
Villus length/crypt ratio x̄ ± SD |
SQHE Total result x̄ ± SD |
| MTX (n=10) | 0.17±0.05a | 0.21±0.07a | 2.33±0.55a | 22.67±4.44a |
| RJ50+MTX(n=11) | 0.29±0.09ab | 0.20±0.17a | 3.16±1.99ab | 10.70±0.05b |
| RJ100+MTX (n=12) | 0.37±0.10b | 0.19±0.05a | 3.41±0.9b | 14.8±5.10b |
| RJ 50 (n=12) | 0.41±0.10b | 0.35±0.62b | 5.40±0.08c | 4.33±2.81c |
| RJ 100 (n=12) | 0.47±0.13b | 0.34±0.06b | 4.79±1.44cd | 3.00±1.55c |
| Control (n=12) | 0.42±0.15b | 0.40±0.82b | 4.39±0.89d | 2.83±1.474c |
| P | <0.05 | <0.001 | <0.05 | <0.001 |
MTX; Methotrexate, RJ; Royal Jelly, x̄ ± SD; mean ± standard deviation, SQHE; Semi-quantitative histological evaluation, Alphabetical superscripts; same letters indicate the absence of statistical significance between the groups, means that they are similar; different letters indicate the presence of statistically significant difference between the groups, means that they are distinct.
Table 3.
Plasma MDA, SOD, and GSH-Px activities in the control and experiment groups
| Groups | MDA (nmol/ml) x̄ ± SD |
SOD (U/mg) x̄ ± SD |
GPSHX (U/L) x̄ ± SD |
| MTX (n=10) | 5.35±0.92a | 14.98±1.23a | 298.76±63.98a |
| RJ50+MTX(n=11) | 4.23±0.55b | 17.15±1.17ab | 375.48±66.82ac |
| RJ100+MTX(n=12) | 4.06±0.54b | 18.36±1.41b | 413.01±62.37acd |
| RJ 50 (n=12) | 2.57±0.39c | 28.21±3.64c | 492.12±70.6bc |
| RJ 100 (n=12) | 2.06±0.24c | 32.98±3.48d | 601.33±92.39b |
| Control (n=12) | 2.19±0.39c | 26.85±3.09ce | 534.59±74.39bd |
| P | <0.001 | <0.001 | <0.05 |
MTX; Methotrexate, RJ; royal jelly, x̄ ± SS; mean ± standard deviation, MDA; Malondialdehide, SOD; Superoxide dismutase, GSH-Px; Glutathione peroxidase. Alphabetical superscripts; same letters indicate the absence of statistical significance between the groups, means that they are similar; different letters indicate the presence of statistically
Discussion
It has been definitely shown that MTX therapy causes oral and intestinal mucositis in patients. Rats as well, are suitable models for the MTX-induced gastrointestinal mucositis (GIM) (Howarth et al., 1996). In this present study, diarrhea was observed in all of the study groups that underwent MTX therapy. A possible diarrhea protecting effect of RJ in the RJ50-MTX and RJ100-MTX groups was negative.
Shortening in the villus length, as well as the decrease in the mucosal thickness and villus length/crypt ratio, is likely to occur in the MTX-induced mucositis models in rats (De Koning et al., 2007). Similarly, in this present study, mucosal thickness and villus length/crypt ratio were found decreased and the villus length was found shortened in the MTX groups as compared to the control group (p<0.001). In the multiple comparisons of the groups, no statistical difference was found in mucosal thickness between the RJ100-MTX group and the control group. The mean value of mucosal thickness in RJ50-MTX group was better the MTX group, but no difference was determined between RJ50-MTX group and the MTX group. This result suggests that dose of 100 mg/kg RJ is more effective than 50 mg/kg. RJ has dose dependent protective effect on the mucosal thickness.
The high total score that was measured by the semi-quantitative histological evaluation was significantly decreased in the groups in which RJ has been added to the MTX therapy.
The result of histological evaluations, show that, oral RJ administration with the dose used in the rats had no toxic effect on the intestinal mucosa, but had relieving effect on the MTX-induced mucositis particularly at a dose of 100mg. Initially in mucositis development, direct DNA damage caused by the CT and RT might lead to cellular death in the basal epithelium and submucosal cells, but clonal cell death occurs in only small part of the damaged cells. However, this is inadequate to explain the intensive damage, which is the clinical manifestation of the mucositis. More importantly, CT and RT lead to the formation of reactive oxygen species (ROS) during the therapy. ROS damage the connective tissue as well as DNA and cell membrane, stimulates macrophages, and triggers the critical biological mechanisms, molecules and the pathways (Sonis et al., 2007). In conclusion, CT, RT and ROS cause a biological formation that results in mucosal damage by directly or indirectly triggering too many signal pathways (Sonis et al., 2004). Release of the proinflammatory cytokines, increase in the epithelial and submucosal apoptosis, ulceration and bacterial transmission, and finally healing is the expected progression of this formation. Since the ROS are considered in such a central place in the development of mucositis, which occurs as the result of cancer therapy, they become the focus of the studies performed on the prevention and treatment of mucositis. Under normal conditions, there is a balance between the production and destruction of ROS. Oxidative stress is defined as the shift of the balance between the oxygen radicals and the protective antioxidant defense system in favor of radicals (Akkus et al., 1995).
In this present study, the plasma MDA levels were found significantly higher in the MTX group as compared to the control group. This corroborates the suggestion that MTX therapy leads to remarkably high rates of lipid peroxidation in the plasma. Although the MDA level was higher in the MTX group than the control group, it was found significantly lower in the RJ50-MTX and RJ100-MTX groups as compared to the MTX group. These results suggest that RJ has protective effect against lipid peroxidation in the MTX-induced systemic oxidative damage. Thus, it can be assumed that the increase in cellular permeability and inflammation, arachidonic acid release, and consequently organelle swelling and membrane rupture that are caused by the MDA and other lipid peroxidation products can be prevented.
The antioxidant efficacy of RJ has been evaluated in various studies. Both in the study performed by Kanbur et al. and in the study performed by El-Nekeety et al., it was reported that antioxidative enzymes are significantly higher in the groups given RJ as compared to the other study groups (El-Nekeety et al., 2007, Kanbur et al., 2009a, Kanbur et al., 2009b). In this present study, both enzyme activities were significantly decreased in the MTX group (Table 2). Although it was not found statistically significant, there was an increase in the activities of both enzymes in the RJ50-MTX group. The SOD activity was significantly higher in the RJ100-MTX group as compared to MTX group; the GSH-Px activity was higher as well, but this increase was not statistically significant. Interestingly, SOD and GSH-Px activities were found remarkably higher only in the RJ100 group as compared to the control group. Whereas the increase in SOD activity was statistically significant (p<0.001), the increase in GSH-Px activity was not (p>0.05). These results corroborate that RJ increases the activity of antioxidative enzymes both under normal physiological conditions and in the presence of oxidative stress. Increase in the enzyme activity becomes more conspicuous as the dose of RJ is increased. This suggests that increase in the activities of antioxidative enzymes would become more remarkable if high dose of RJ is applied together with MTX.
Taking all of these outcomes into consideration in the chain of the events that lead to MTX-induced mucositis in rats, in which oxidative stress plays an important role, it can be thought that RJ suppresses the oxidative stress and decreases the MTX-induced intestinal damage by means of increasing the activities of antioxidant enzymes and decreasing the lipid peroxidation. The results suggest that, better outcomes can be obtained with higher doses of RJ.
References
- 1.Akkuş İ. Serbest radikaller ve fizyopatolojik etkileri. Mimoza Basın Yayın Dağıtım. Konya. 1995:32–42. (Fre). [Google Scholar]
- 2.Babiak RM, Campello AP, Carnieri EG, Oliveira MB. Methotrexate: Pentose Cycle And Oxidative Stres. Cell Biochem Funct. 1998;6:283–293. doi: 10.1002/(SICI)1099-0844(1998120)16:4<283::AID-CBF801>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 3.Blijlevens NMA, Donnelly JP, De Pauw BE. Mucosal Barrier Injury: Biology, Pathology, Clinical Counterparts And Consequences Of Intensive Treatment For Haematological Malignancy: An Overview. Bone Mar Transplant. 2000;25:1269–1278. doi: 10.1038/sj.bmt.1702447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blijlevens N, Sonis S. Palifermin (Recombinant Keratinocyte Growth Factor-1): A Pleiotropic Growth Factor With Multiple Biological Activities In Preventing Chemotherapy- And Radiotherapy-Induced Mucositis. Ann Oncol. 2007;18:817–826. doi: 10.1093/annonc/mdl332. [DOI] [PubMed] [Google Scholar]
- 5.Carne±ro-F±lho BA, L±ma IPF, Araujo DH, et al. Intestinal Barrier Function And Secretion In Methotrexate-±nduced Rat Intestinal Mucositis. Dig Dis Sci. 2004;49:65–72. doi: 10.1023/b:ddas.0000011604.45531.2c. [DOI] [PubMed] [Google Scholar]
- 7.Chabner B, Wilson W, Supko J. Pharmacology And Toxicity Of Antineoplastic Drugs. In: Lichtman MA, Beutler B, Seligsohn U, Kipps TJ, Kaushansky K, editors. Williams Hematology. 7th Edition. United States: Mc Graw Hill Company; 2007. pp. 249–251. [Google Scholar]
- 8.Culy C, Spencer C. Amifostine: An Update On Its Clinical Status As A Cytoprotectant In Patients With Cancer Receiving Chemotherapy Or Radiotherapy And Its Potential Therapeutic Application. Drugs. 2001;61:641–684. doi: 10.2165/00003495-200161050-00012. [DOI] [PubMed] [Google Scholar]
- 9.De Koning BA, Sluis M, Lindenbergh-Kortleve DJ, et al. Methotrexate-Induced Mucositis In Mucin 2Deficient Mice. J Cell Physiol. 2007;210:144–152. doi: 10.1002/jcp.20822. [DOI] [PubMed] [Google Scholar]
- 10.El-Nekeety AA, El-Kholy W, Abbas NF, et al. Efficacy Of Royal Jelly Against The Oxidative Stress Of Fumonisin In Rats. Toxicon. 2007;50:256–269. doi: 10.1016/j.toxicon.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Inoue S, Koya-Miyata S, Ushio S, et al. Royal Jelly Prolongs The Life Span Of C3H/Hej Mice: Correlation With Reduced DNA Damage. Exp Gerontol. 2003;38:965–969. doi: 10.1016/s0531-5565(03)00165-7. [DOI] [PubMed] [Google Scholar]
- 14.Jamnik P, Goranovic D, Raspor P. Antioxidative Action Of Royal Jelly In The Yeast. Cell Exp Gerontol. 2007;42:594–600. doi: 10.1016/j.exger.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Kanbur M, Eraslan G, Beyaz L, et al. The Effects Of Royal Jelly On Liver Damage Induced By Paracetamol In Mice. Exp Toxicol Pathol. 2009;61:123–132. doi: 10.1016/j.etp.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Kohno K, Okamoto I, Sano O, et al. Royal Jelly Inhibits The Production Of Proinflammatory Cytokines By Activated Macrophages. Biosci Biotechnol Biochem. 2004;68:138–145. doi: 10.1271/bbb.68.138. [DOI] [PubMed] [Google Scholar]
- 18.Leung R, Ho A, Chan J, et al. Royal Jelly Consumption And Hypersensitivity In The Community. Clin Exp Allergy. 1997;27:333–336. [PubMed] [Google Scholar]
- 19.Ohkawa W, Ohishi N, Yagi K. Assay For Lipid Peroxides In Animal Tissues By Thiobarbituric Acid Reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 20.Paglia DE, Valentina WN. Studies On The Quantitative And Qunlitative Characterization Of Erytrocyte Glutathione Peroxidase. J Lab Clin Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 21.Sonis ST, Fey EG. Oral Complications Of Cancer Therapy. Oncology. 2002;16:680–686. [PubMed] [Google Scholar]
- 22.Sonis ST. Pathobiology Of Oral Mucositis: Novel Insights And Opportunities. J Support Oncol. 2007;5:3–11. [PubMed] [Google Scholar]
- 23.Sonis ST. The Pathophysiology Of Mucositis. Nature Reviews Cancer. 2004;4:277–284. doi: 10.1038/nrc1318. [DOI] [PubMed] [Google Scholar]
- 24.Sun Y, Oberley LW, Li Y. A Simple Method For Clinical Assay Of Superoxide Dismutase. Clin Chem. 1998;3:497–500. [PubMed] [Google Scholar]
- 25.Sver L, Orsolić N, Tadić Z, et al. A Royal Jelly As A New Potential Immunomodulator In Rats And Mice. Comp Immunol Microbiol Infect Dis. 1996;19:31–38. doi: 10.1016/0147-9571(95)00020-8. [DOI] [PubMed] [Google Scholar]
- 26.Yonei Y, Shibagaki K, Tsukada N, et al. Case Report: Haemorrhagic Colitis Associated With Royal Jelly Intake. J Gastroenterol Hepatol. 1997;12:495–499. doi: 10.1111/j.1440-1746.1997.tb00472.x. [DOI] [PubMed] [Google Scholar]