Abstract
Interleukin (IL)-2, a T-cell cytokine used to treat malignant melanoma, can induce profound depression. To determine whether pretreatment with the antidepressant escitalopram could reduce IL-2-induced neuroendocrine, immune, and neurobehavioral changes, 20 patients with Stage IV melanoma were randomized to either placebo or the serotonin reuptake inhibitor, escitalopram (ESC) 10–20 mg/day, 2 weeks before, and during IL-2 treatment (720 000 units/kg Q8 h × 5 days (1 cycle) every 3 weeks × 4 cycles). Generalized estimation equations were used to examine HPA axis activity (plasma ACTH and cortisol), immune activation (plasma IL-6), and depressive symptoms (Hamilton Depression Rating Scale (HDRS) score). Tolerance of IL-2 treatment (concomitant medications required) and adherence (number of IL-2 doses received) were also assessed. Both the groups (ESC (n=9), placebo (n=11)) exhibited significant IL-2-induced increases in plasma cortisol, IL-6, and depressive symptoms (p<0.05), as well as a temporal trend for increases in plasma ACTH (p=0.054); the effects of age and treatment were not significant. Higher plasma ACTH concentrations were associated with higher depressive symptoms during cycles 1–3 of IL-2 therapy (p<0.01). Although ESC had no significant effects on ACTH, cortisol, IL-6, tolerance of, or adherence to IL-2, ESC treatment was associated with lower depressive symptoms, ie, a maximal difference of ∼3 points on the HDRS, which, though not statistically significant (in part, due to small sample size), represents a clinically significant difference according to the National Institute for Health and Clinical Excellence guidelines. A larger sample size will establish whether antidepressant pretreatment can prevent IL-2-induced neurobehavioral changes.
Keywords: interleukin-2, escitalopram, depression, interleukin-6, adrenocorticotropin (ACTH), melanoma
INTRODUCTION
Patients treated with the innate immune cytokine interferon (IFN)-alpha suffer from marked neurobehavioral symptoms, such as depression, insomnia, and fatigue, which, in turn, contribute to reduced quality of life and decreased treatment adherence (Musselman et al, 2001). Patients administered the T-cell cytokine, interleukin (IL)-2, also exhibit marked behavioral alterations, including symptoms of depressed mood, anhedonia, fatigue, cognitive dysfunction, sleep impairment and, in some cases, psychosis and delirium (Nicolsen et al, 2006). The pathophysiology and treatment of these IL-2-induced neuropsychiatric symptoms have not been determined. In a previous study, we demonstrated that pretreatment with the serotonin reuptake inhibitor paroxetine was an effective strategy for minimizing depression induced by IFN-alpha in patients with malignant melanoma (Musselman et al, 2001). In this study, we used a similar double-blind, randomized, placebo-controlled design to determine whether the serotonin reuptake inhibitor escitalopram (ESC) would reduce the severity of neuropsychiatric symptoms during 12 weeks of intravenous (IV) IL-2 therapy in patients with malignant melanoma. In addition, we examined the impact of IL-2 and ESC on the activation of pathways related to: (a) the hypothalamic–pituitary–adrenocortical (HPA) system, ie by measuring the HPA axis hormones, ACTH and cortisol, and (b) the immune system, ie by measuring the cytokine IL-6. Lastly, we wished to determine whether ESC pretreatment reduced concomitant medications utilized by patients and increased adherence (number of IL-2 IV doses). ESC was chosen because of its ease of administration (once-daily dosing), absence of active metabolites, and favorable side-effect profile.
MATERIALS AND METHODS
Patients
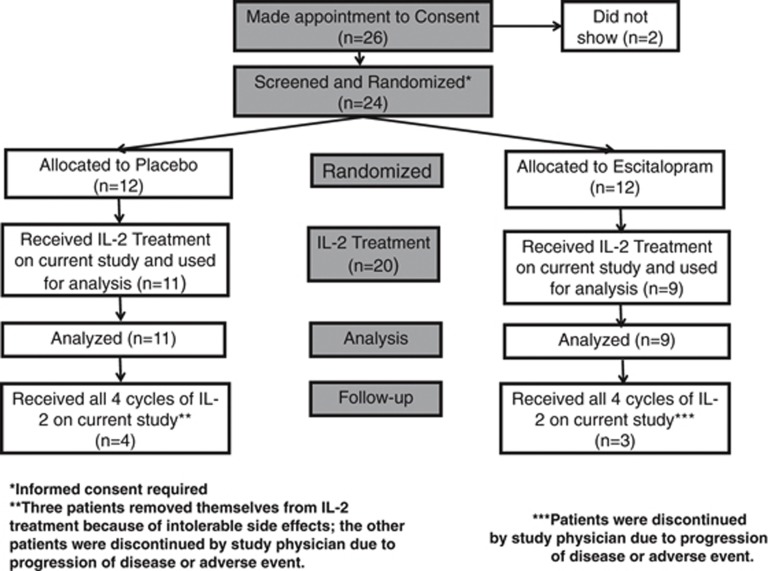
Sixty-seven patients referred with Stage III or Stage IV unresectable malignant melanoma were recruited and screened at the Winship Cancer Institute of Emory University School of Medicine between May 2005 and May 2010. Inclusion criteria for IL-2 therapy included those patients with ECOG performance status of 0 (fully active) or 1 (restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature), a normal cardiac stress test, and a FEV1 >70% of predicted. Study participants were also without infection, diabetes (type 1 or type 2), or major cardiac, pulmonary, or renal disease. Criteria for exclusion also included a score of <24 on the Mini-Mental State Examination (Folstein et al, 1975); current, effective treatment of depression with an antidepressant; a diagnosis of schizophrenia or bipolar disorder as determined by the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) (First et al, 1998); or unstable endocrine, hematological, hepatic, or neurological disease. The Emory University School of Medicine Institutional Review Board approved the study, and all patients gave written informed consent. Twenty-four patients fulfilled the criteria for eligibility, were randomized to study medication, and received IL-2 therapy (Figure 1).
Figure 1.
Flow diagram of the study.
Treatment and Follow-Up
Patients completed a screening visit (which occurred 1–2 weeks before IL-2 administration), and were randomly assigned in a double-blind fashion to begin 14 weeks of either ESC (Lexapro, Forest Pharmaceuticals, New York City, NY, USA) or placebo. They commenced treatment with study medication by ingesting one capsule of either ESC (10 mg) or placebo once per day. After 7 days, the dose was raised to two capsules daily as tolerated. Study participants were then re-evaluated 2 weeks after the screening evaluation and started on IL-2 therapy.
Patients received a 12-week treatment with IV IL-2 [720 000 units/kg Q8 h × 5 days (1 cycle) every 3 weeks × 4 cycles)]. They also received routine, algorithm-guided, symptomatic treatment of IL-2-induced symptoms; concomitant medications included temazepam or zolpidem for insomnia; lorazepam, prochlorperazine, granisetron, and ondansetron for nausea; and narcotics for pain. Psychotropic medications such as antidepressants or antipsychotics were not allowed during treatment.
Study participants were evaluated at screening and during days 1–3 of each cycle of IL-2 treatment, and 4 weeks after their last cycle of IL-2 treatment. Assessments included the 21-item, observer-rated Hamilton Depression Rating Scale (HDRS; Hamilton, 1960), which quantifies the severity of depressive symptoms, including depressed mood, loss of interest in usually pleasurable activities, insomnia, anorexia, fatigue, weight loss, and psychomotor retardation or agitation. A score of 0–6 on the HDRS indicates a normal state; a score of 7–17 indicates mild depression; a score of 18–24 indicates moderate depression; and a score of ⩾25 indicates severe depression (Schutte and Malouff, 1995). At each study visit, a psychiatrist determined whether the patient fulfilled DSM-IV criteria for major depression, which requires the presence and persistence for at least 2 weeks of a specified set of depressive symptoms (American Psychiatric Association, 1994). During the study, one or two capsules of study medication could be ingested per day based on depressive symptoms and tolerance. Study participants received study medication for 14 weeks in duration unless they dropped out or were terminated.
Laboratory Assays
At the screening assessment, blood samples were drawn for ACTH, cortisol, and IL-6. On the first day of each IL-2 cycle, blood samples were drawn just before the first dose of IL-2 (0600 hours) and again at 0900 hours. Additional blood samples were drawn at 0900 hours on days 2 and 3 of each IL-2 cycle. These sampling time points remained constant, even if doses of IL-2 were held (Capuron et al, 2000, 2001). Blood was collected in EDTA tubes, placed immediately on ice, and centrifuged at 4 °C for 10 min at 3000 r.p.m. Plasma was separated, coded, and stored at 80 °C until assayed.
Plasma concentrations of ACTH and cortisol were measured by radioimmunoassay according to the manufacture's protocol (Nichols Institute, San Juan Capistrano, California and ICN Biomedicals, respectively). Plasma cytokine levels for IL-6 were determined using sandwich ELISA according to the manufacturer's protocol (R & D Systems). Samples were assayed in duplicate, by laboratory personnel blinded to randomization. Quality control sera of low, medium, and high cytokine concentrations were included with every assay. The mean inter- and intra-assay coefficients of variation for control sera were reliably ⩽10%. For ACTH, the limit of quantification (LOQ) was 1.0 pg/ml, and for cortisol, the LOQ was 0.4 μg/dl. The mean minimum detectable dose (MDD) of the Il-6 immunoassay was 0.039 pg/ml. None of the samples had values below the LOQ or MDD.
Statistical Analyses
Wilcoxon's rank sum tests and signed-rank tests were used to perform comparisons between two independent samples and paired samples, respectively. McNemar's test was used to compare paired binary data. Generalized Estimating Equations (GEE) with the AR(1) correlation structure were used to analyze repeated-measures data. For subjects who dropped out of the study, the approach of the last observation carried forward (LOCF) was applied (Shao and Zhang, 2003). Mixed models were also used to confirm the results, when indicated. Two outcome variables were used for each neurobiological (ie, ACTH, cortisol, IL-6) or depression (ie, the HAM-D) variable, that is, the maximum value of the variable and the change in the variable relative to baseline at each IL-2 treatment cycle. Both outcomes for each variable were treated as continuous. Of note is that, within each cycle, the neurobiological variable or HAM-D score was measured for each of 3 days, and the maximum value over the 3 days was used. Time points were specified as Screening (1–2 weeks before Il-2 administration), and Cycles 1, 2, 3, and 4 (occurring at the initiation of IL-2 therapy, and then approximately 4, 7, and 10 weeks after the first cycle of IL-2). Age was included in the regression models as a potential confounder; gender was not included in the models, due to the small percentage of female study participants. For the percentage of patients using a class of medication during a cycle or per day, the outcome variable was treated as binary (ie, whether a subject used a medication within a given day or cycle), and GEE models for binary outcomes was applied.
RESULTS
Socio-Demographic Characteristics of the Study Sample
The characteristics of the 11 patients in the placebo-treated group and the 9 patients in the ESC-treated group were similar in terms of age, sex distribution, and proportions with previous antineoplastic treatments (Table 1).
Table 1. Characteristics of Patients with Malignant Melanoma at the Screening Visit.
| Characteristic | Placebo (n=11) | Escitalopram (n=9) | p-value |
|---|---|---|---|
| Age (year) | |||
| Min±SD | 45±16 | 51±8 | 0.16 |
| MIN, MAX, Range | 20, 68, 48 | 37, 62, 25 | |
| Sex | |||
| Male | 8 (73%) | 7 (78%) | 1.00 |
| Female | 3 (27%) | 2 (22%) | |
| Previous brain radiosurgery | 2 (18%) | 3 (33%) | 0.62 |
| Previous IFN-α therapy | 4 (36%) | 4 (44%) | 1.00 |
| Previous surgical removal | 10 (91%) | 8 (89%) | 1.00 |
| Previous chemotherapy | 0 (0%) | 2 (22%) | 0.19 |
Dropout
After the screening visit (Figure 1), of 24 patients, 4 study participants could not proceed to IL-2 therapy: 2 study subjects (1 randomized to placebo; 1 randomized to ESC) developed brain metastases, another patient (randomized to ESC) developed a small bowel intussuseption attributed to progression of melanoma; another study subject (randomized to ESC) fulfilled diagnostic criteria for major depression and was excluded from participating in the study. The other 20 patients received at least one dose of IL-2 therapy and were therefore considered in the statistical analyses. The attrition of the 20 IL-2-treated patients is as follows: 3 patients after 1 cycle of IL-2 therapy, 6 patients after 2 cycles of IL-2 therapy, 4 patients after 3 cycles of IL-2 therapy; 7 patients completed all 4 cycles of IL-2 therapy. The reasons for dropout after initiation of IL-2 therapy were: patient's choice (n=3 participants randomized to placebo), development of major depression (n=1 randomized to placebo), development of brain metastases and/or other progression of metastatic melanoma (n=3 randomized to placebo; n=5 randomized to ESC), and adverse cardiac effects associated with IL-2 administration (n=1 randomized to ESC). Thus, the dropout of study subjects resulted in 64% (7/11) and 67% (6/9) of the placebo-treated group and ESC-treated group, respectively, by the end of the study (p=0.63).
Neuroendocrine and Immune System Response in Placebo- and ESC-Treated Patients Receiving IL-2 Therapy
Mean ACTH plasma concentrations increased reaching a maximum for patients in the ESC-treated group by Cycle 2 and in the placebo-treated group by Cycle 3 (Figure 2; temporal trend, p=0.054). Both the groups exhibited significant IL-2-induced increases from screening to each cycle visit in plasma cortisol, and IL-6, with the greatest increases being observed in placebo- and ESC-treated groups at Cycle 3 (Figures 3 and 4); the effects of age and treatment were not significant. Although there was a drop of 80 pg of IL-6 in ESC-treated patients at Cycle 2, this was not significant (p=0.14), and is likely a spurious finding in this small sample. There were no significant differences in mean ACTH, cortisol, and IL-6 between the groups at any time point.
Figure 2.
Mean plasma concentrations of ACTH in placebo- and ESC-treated patients receiving IL-2 therapy (after last observation carried forward).
Figure 3.
Mean plasma concentrations of cortisol in placebo- and ESC-treated patients receiving IL-2 therapy (after last observation carried forward).
Figure 4.
Mean plasma concentrations of IL-6 in placebo- and ESC-treated patients receiving IL-2 therapy (after last observation carried forward).
Multiple regression models were used to determine the relationship between the biomarkers and changes in HAM-D scores over the course of the study. Of note is that the increases in plasma ACTH concentrations across time were significantly associated with increases of the HAM-D scores in the ESC-treated group (p<0.01). There was significant interaction of plasma ACTH concentrations and treatment assignment (p<0.01). No relationships were found between IL-6 or cortisol and HAM-D score in either group or the study population as a whole.
Concomitant Medications Utilized and the Number of IL-2 Doses Tolerated
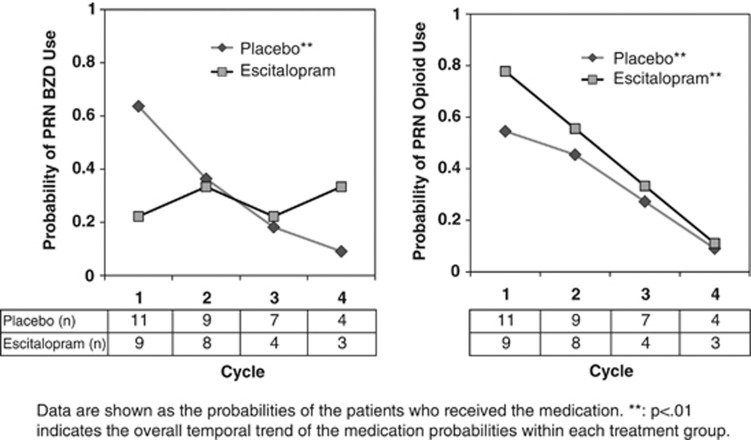
The need/usage of four classes of “as needed” or “PRN” medications over time was compared between the two treatment groups (ESC vs placebo) using either the daily value or the maximum value within each cycle (Figure 5). For the anti-emetics, narcotic/opioid analgesics, and sedative/hypnotic agents, the probability of utilizing these PRN medications significantly decreased over time (p<0.01; data for anti-emetics and tranquilizer medications not shown). For the benzodiazapines, placebo-treated study participants used significantly less PRN benzodiazepines (p=0.003), while ESC-treated patients did not significantly decrease their use of PRN benzodiazepines (p=0.7). Nevertheless, the differences in the use of these four classes of medications between the placebo- and ESC-treated groups were not statistically significant at any cycle.
Figure 5.
Utilization of ‘as needed' medications (after Last Observation Carried Forward).
After completion of Cycle 2, patients underwent cancer re-staging, per usual clinical protocol. Those without progression of their melanoma (four of the study participants treated with ESC vs seven of those treated with placebo) were allowed to proceed to Cycle 3. There was no significant difference between the groups in the average number of IL-2 doses tolerated (out of the possible 60 total doses): 18 doses (ESC-treated) vs 20 doses (placebo-treated).
Depressive Symptoms in Placebo- and ESC-Treated Patients
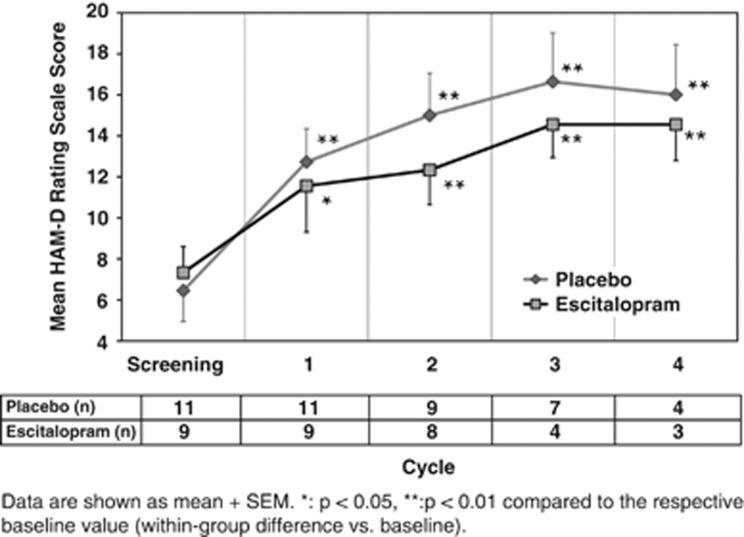
The mean HAM-D score significantly increased in both the groups with each cycle, reaching a maximum at Cycle 3, then decreasing by Cycle 4 (Figure 6). Using LOCF, the mean HAM-D score significantly increased over time (p=0.01). The mean HAM-D scores increased in the placebo-treated group from 6.5 at screening to a mean maximum of 16.6 (Cycle 3), or an increase of approximately 10 HAM-D points, over the course of IL-2 treatment compared with an increase of approximately 7 points for ESC-treated patients. The HAM-D scores for patients in the placebo-treated group were always higher than those for patients in the ESC group; however, ESC treatment did not have a statistically significant effect on HAM-D score, before and after adjustment by age. Benzodiazepine use by either treatment group had no significant effect on HAM-D scores (p=0.28).
Figure 6.
Mean HAM-D scores in placebo- and ESC-treated patients receiving IL-2 therapy (after LOCF).
DISCUSSION
There were four main findings of the present study. First, in response to IL-2 administration, over time, there appears to be an enhancement of ACTH response. This increasing ACTH secretion is congruent with previous preclinical and clinical studies showing that IL-2 administration is associated with activation of stress-responsive neuroendocrine pathways [corticotropin releasing hormone (CRH) and HPA axis] (Butler et al, 1989; Denicoff et al, 1987; Hanisch et al, 1994; Karanth and McCann, 1991; Katahira et al, 1998; Raab et al, 1999; Raber et al, 1995; Spina et al, 1994). We postulate that this enhanced ACTH response may be related to IL-2-induced dysregulation of glucocorticoid receptor function at the level of the pituitary, hypothalamus, and/or hippocampus (Raison and Miller, 2003a) and possibly due to interactions between glucocorticoid receptors and innate immune signaling pathways and/or activation of T-cell responses involving DNA binding of nuclear factor of activated T cells and activation protein-1. Relevant in this regard is that a number of studies have demonstrated that IL-2 has a profound negative effect on glucocorticoid receptor function (through activation of p38 mitogen-activated kinase and STAT 5 signaling) that is believed to contribute to steroid resistance in patients with asthma and other inflammatory disorders (Goleva et al, 2002; Irusen et al, 2002). Of note is that this is the only clinical study to confirm that chronic IL-2 administration results in persistent elevations in cortisol (Bindon et al, 1983; Chambrier et al, 1990; Spinazzé et al, 1991; Witzke et al, 2003).
Second, we noted a difference between the groups in terms of the cycle when ACTH plasma concentrations reached maximum: at Cycle 2 in ESC-treated patients (which was after 6 weeks of ESC treatment), compared with Cycle 3 in placebo-treated patients. After Cycle 2, ESC may have improved sensitivity to glucocorticoid receptor sensitivity and/or decreased CRH activity as reflected by the earlier decrease in ACTH plasma concentrations in ESC-treated individuals (Pariante and Miller, 2001). Though others have shown that antidepressants may decrease the production of proinflammatory cytokines (Pariante and Miller, 2001), patients receiving ESC or placebo exhibited similar secretion of cortisol from the adrenal cortex and IL-6 release into plasma from T-cells, macrophages, and other cell types. The similar plasma concentrations of cortisol between the two groups, despite the lower ACTH secretion in ESC-treated patients, may have been due to vasopressin stimulation after its release from hypothalamus and amygdala in response to IL-2 (Perraudin et al, 1993; Hillhouse, 1994; Raber and Bloom, 1994). The similar increases in IL-6 over time in both the treatment groups may also represent a more generalized immune response. Indeed, the equivalent initial IL-6 responses to IL-2 in both the groups support this notion (Capuron et al, 2003). Interestingly, over time, patients in both the groups requested less ‘as needed' medications, despite the marked systemic toxicities of IL-2, including capillary leak syndrome, which results in uncomfortable peripheral edema and weight gain (commonly between 5 and 10 kg per 5-day cycle), hypotension, and often renal insufficiency (Rosenberg et al, 1989). The decrement in use of ‘as needed' medications may have been due to the fact that patients intolerant of IL-2 toxicities had discontinued their IL-2 treatment or their knowledge of the transient nature of such symptoms, which generally reverse relatively quickly after the end of each IL-2 cycle (Rosenberg et al, 1989). Of note is that in the peripheral nervous system, through its binding to opioid μ-receptors, IL-2 may decrease pain (Jiang and Lu, 1998; Song et al, 2002), which may have also contributed to our study participants' decreasing use of opioid medications.
Third, irrespective of pretreatment with ESC or placebo, study participants exhibited significant IL-2-induced increases in depressive symptoms (p=0.02). Using either a LOCF or mixed model method, similar effects were found. As discussed above, activation of stress-responsive neuroendocrine pathways (CRH and HPA) likely contributed, given that ACTH plasma concentrations had a significant association with magnitude of HAM-D score. The release of proinflammatory cytokines implicated in mood regulation, including IL-6 and TNF-alpha (Butler et al, 1989; Raab et al, 1999; Saraya and Balkwill, 1993), during IL-2 therapy likely also contributed to the induction of depressive symptoms. Alterations in metabolism of certain monoamines (eg, serotonin, dopamine, acetylcholine) or receptor function (eg, NMDA) might also have been plausibly involved in IL-2's induction of depressive symptoms (Anisman and Merali, 1999; Lacosta et al, 2000). Indeed, repeated administration of IL-2 to mice (0.55–17.6 × 103 IU per mouse) has been found to increase the turnover of NE within the median eminence and hippocampus, alter 5HT levels in the hippocampus and prefrontal cortex, and reduce DA turnover in the caudate and substantia nigra (Lacosta et al, 2000), brain regions involved in symptoms relevant to depression. Moreover, IL-2 activates the indolamine 2,3 dioxygenase metabolic pathway that diminishes the availability of serotonin and increases kynurenine and its neuroactive metabolites kynurenic acid and quinolinic acid, the latter of which has been implicated in the development of depressive symptoms during IFN-alpha administration (Carlin et al, 1987; Raison et al, 2010).
Relevant in this regard is that, to our knowledge, only Capuron et al (2000) have utilized psychometric rating scales to characterize changes in mood induced by IL-2. They administered the clinician-administered Montgomery Asberg Depression Rating Scale (MADRS; Montgomery and Asberg, 1979) to a group of 20 patients during their first 5 days of daily 18 million IU of subcutaneously administered IL-2 for renal cell carcinoma (Sleijfer et al, 1992). Within 3 days of initiating IL-2 therapy, significant increases in depressive symptoms were apparent. By day 5 of treatment, their average MADRS score had significantly increased to an average of 11, indicating mild depressive symptoms. In this study, depressive symptoms of ESC- and placebo-treated patients reached a magnitude of mild severity (ie, a mean HAM-D score of 14.6 and 16.6 during Cycle 3, respectively), and only one of the study participants (treated with placebo) eventually fulfilled DSM-IV criteria for major depression. By contrast, during 12 weeks of high-dose IV treatment with the innate immune system cytokine, IFN-alpha, 45% of placebo-treated study participants developed an episode of major depressive disorder (Musselman et al, 2001).
The induction of mild depressive symptoms in our study population, similar to Capuron et al (2000), despite a markedly greater dose of IL-2 administered by IV, might have been due to the ‘rest periods' between the IL-2 cycles, thereby allowing for the resolution of neuropsychiatric side effects during the 3 weeks between each IL-2 cycle (Denicoff et al, 1987). Another possible contributing factor to the low incidence of major depression of our study patients was the exclusion of antidepressant-treated patients, who plausibly may be more susceptible to suffering IL-2-induced major depression. Also at issue was the potential effect of the drugs that were used to alleviate symptoms of the IL-2 treatment, and how these might have impacted the induction of neuropsychiatric symptoms. Administration of such medications, including temazepam for insomnia, lorazepam for nausea, and meperidine for rigors, was therefore analyzed as one of our study's outcomes.
We offer that the major reason for the lack of statistical significance between the two treatment groups with regard to HAM-D scores and dropout is that the study was underpowered. However, another possibility is that our hypothesis that the SSRI ESC would prevent IL-2-induced depressive symptoms and nonadherence was wrong, and thus was not supported by our study. Nevertheless, though the differences between the groups was not statistically significant, ESC-treated patients exhibited lower depressive symptoms (maximal difference of ∼3 points on the HDRS) than placebo-treated patients. Of note is that a 2–3-point average difference between the groups can translate to differences of 20–30% in terms of response and remission rates of depression, thus representing a clinically significant difference according to the National Institute for Health and Clinical Excellence guidelines (NICE, 2004; Gibbons et al, 2012). Moreover, the patients were not depressed at baseline, and given the typical 3–4 week onset of antidepressant effects of SSRIs, the modest drug–placebo difference might have been due to delayed onset of action by ESC. This would be consistent with Figure 6, in that the difference between drug and placebo on HAM-D scores began in Cycle 2. Interestingly, by Cycle 2, decrements in ACTH production were observed only in patients randomized to ESC (which was their sixth week of antidepressant therapy), congruent with other studies documenting that antidepressants can reduce elevated CRH activity (Pariante and Miller, 2001; Raison and Miller, 2003b).
Admittedly, recruitment of a control population would have been desirable; however, recruiting a comparable group of individuals with an equally life-threatening disease who chose not to proceed with IL-2 therapy would have been very difficult. Moreover, such a control population might plausibly have consisted of those with personality variables qualitatively different from patients who opt for more aggressive treatment strategies. As IL-2 causes sufficiently different changes in neurobehavioral symptoms/neuroimmune physiology (over baseline), based on available data, we offer that the changes we observed cannot be confused with the natural fluctuations in these parameters over time (as would been assessed in a control population).
CONCLUSION
In order to develop new strategies to help diagnose and manage depression in the medically ill study, model systems are needed to study the neurobiology and treatment of cytokine-induced depression. This study demonstrates that patients receiving the T-cell cytokine IL-2 experienced increased depressive symptoms, associated with IL-2's potent and chronic activation of the HPA axis and release of IL-6, a cytokine implicated in mood disorders. By contrast, in the IFN-alpha model of innate immune-induced depression, preclinical and clinical studies reveal that IFN-alpha is associated with an initial increase in plasma ACTH, cortisol, and IL-6 concentrations, which subsequently diminish with ongoing IFN-alpha treatment (Capuron et al, 2003; Felger et al, 2007). This study is also is the first, randomized, placebo-controlled trial examining the efficacy of an antidepressant in ameliorating the depressive symptoms experienced by patients undergoing IL-2 therapy. Certainly, a larger sample size is needed to establish whether antidepressant pretreatment can prevent IL-2-induced behavioral changes. In future studies, specific neuropsychiatric symptoms (including memory impairment and psychosis) can be more closely correlated with HPA axis and immune function both before and during IL-2 therapy. These studies will provide insights into differential mechanisms of symptom development and stimulate informed intervention strategies to ameliorate development of debilitating neurobehavioral syndromes during cytokine therapies.
Acknowledgments
We are indebted to Lukas Austin-Page, MD, James Ritchie, PhD, the 7E nursing and clinical pharmacy service of Emory University Hospital, members of the Winship Cancer Institute Melanoma (Theresa Gregerson, Necia Maynard, NP, Julie Leff, RN); the Emory Investigational Drug Service (Ms. Susan Rogers, PharmD), and to the study participants, their families, and friends. This work was supported by grants from the National Institute of Mental Health (MH64619, MH00680, MH071580, and MH60723). This work was also supported, in part, by PHS Grant UL1 RR02500) from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources.
DM has received research support from Forest Laboratories, Inc., lecture honoraria from the Florida Psychiatric Society, and reimbursement from the NIH for her efforts on study sections. AHM has served as a consultant for Abbott Laboratories, AstraZeneca, GlaxoSmithKline, Lundbeck Research USA, F. Hoffmann- La Roche Ltd, Schering-Plough Research Institute and Wyeth/Pfizer Inc. and has received research support from Centocor Inc., GlaxoSmithKline, and Schering-Plough Research Institute. DHL has served in compensated advisory roles for BMS, Merck, GSK, and Genzyme. Genzyme was an industrial sponsor for an ECOG trial of which he was PI (E4697). All the other authors declare no conflict of interests.
References
- American Psychiatric Association 1994Diagnostic and Statistical Manual of Mental Disorders4th edn.American Psychiatric Association: Washington, DC, USA [Google Scholar]
- Anisman H, Merali Z. Anhedonic and anxiogenic effects of cytokine exposure. Adv Exp Med Biol. 1999;461:199–233. doi: 10.1007/978-0-585-37970-8_12. [DOI] [PubMed] [Google Scholar]
- Bindon C, Czerniecki M, Ruell P, Edwards A, McCarthy WH, Harris R, et al. Clearance rates and systemic effects of intravenously administered interleukin 2 (IL-2) containing preparations in human subjects. Br J Cancer. 1983;47:123–133. doi: 10.1038/bjc.1983.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler LD, Mohler KM, Layman NK, Cain RL, Riedl PE, Puckett LD, et al. Interleukin-2 induced systemic toxicity: induction of mediators and immunopharmacologic intervention. Immunopharmacol Immunotoxicol. 1989;11:445–487. doi: 10.3109/08923978909005379. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Dantzer R. Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon alfa-2b therapy. J Clin Oncol. 2000;18:2143–2151. doi: 10.1200/JCO.2000.18.10.2143. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Dantzer R. Timing and specificity of the cognitive changes induced by interleukin-2 and interferon-alpha treatments in cancer patients. Psychom Med. 2001;63:376–386. doi: 10.1097/00006842-200105000-00007. [DOI] [PubMed] [Google Scholar]
- Capuron L, Raison CL, Musselman DL, Lawson DH, Nemeroff CB, Miller AH. An exaggerated HPA axis response to the initial injection of interferon-alpha is associated with the development of depression during interferon-alpha therapy. Am J Psychiatry. 2003;160:1342–1345. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- Carlin JM, Borden EC, Sondel PM, Byrne GI. Biologic-response-modifier-induced indoleamine 2,3-dioxygenase activity in human peripheral blood mononuclear cell cultures. J Immunol. 1987;139:2414–2418. [PubMed] [Google Scholar]
- Chambrier C, Mercatello A, Tognet E, Cottet-Emard JM, Cohen R, Blay JY, et al. Hormonal and metabolic effects of chronic interleukin-2 infusion in cancer patients. J Biol Response Modif. 1990;9:251–255. [PubMed] [Google Scholar]
- Denicoff KD, Ribinow DR, Papa MZ, Simpson C, Seippp CA, Lotze MT, et al. The neuroendocrine effects of interleukin-2 treatment. J Clin Endocrinol Metab. 1987;69:402–410. doi: 10.1210/jcem-69-2-402. [DOI] [PubMed] [Google Scholar]
- Felger JC, Alagbe O, Hu F, Mook D, Freeman AA, Sanchez MM, et al. Effects of interferon-alpha on rhesus monkeys: a non-human primate model of cytokine-induced depression. Biol Psychiatry. 2007;62:1324–1333. doi: 10.1016/j.biopsych.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders SCID I: Clinician Version 2.0. Rev. Biometrics Research Department, New York State Psychiatric Institute: New York, NY, USA; 1998. [Google Scholar]
- Folstein MF, Folstein SE, McHugh A. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Hur K, Brown CH, Davis JM, Mann JJ. Benefits from antidepressants: synthesis of 6-week patient-level outcomes from double-blind placebo-controlled randomized trials of fluoxetine and venlafaxine. Arch Gen Psychiatry. 2012;69:572–579. doi: 10.1001/archgenpsychiatry.2011.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goleva E, Kisich KO, Leung DY. A role for STAT5 in the pathogenesis of IL-2-induced glucocorticoid resistance. J Immunol. 2002;169:5934–5940. doi: 10.4049/jimmunol.169.10.5934. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK, Rowe W, Sharma S, Meaney MJ, Quirion R. Hypothalamic-pituitary-adrenal activity during chronic central administration of interleukin-2. Endocrinology. 1994;135:2465–2472. doi: 10.1210/endo.135.6.7988433. [DOI] [PubMed] [Google Scholar]
- Hillhouse EW. Interleukin-2 stimulates the secretion of arginine vasopressin but not corticotropin-releasing hormone from rat hypothalamic cells in vitro. Brain Res. 1994;650:323–325. doi: 10.1016/0006-8993(94)91799-x. [DOI] [PubMed] [Google Scholar]
- Irusen E, Matthews JG, Takahashi A, Barnes PJ, Chung KF, Adcock IM. p38 Mitogen-activated protein kinase-induced glucocorticoid receptor phosphorylation reduces its activity: role in steroid-insensitive asthma. J Allergy Clin Immunol. 2002;109:649–657. doi: 10.1067/mai.2002.122465. [DOI] [PubMed] [Google Scholar]
- Jiang C, Lu C. Interleukin-2 and its effects in the central nervous system. Biol Signals Recept. 1998;7:148–156. doi: 10.1159/000014541. [DOI] [PubMed] [Google Scholar]
- Karanth S, McCann SM. Anterior pituitary hormone control by interleukin-2. Proc Natl Acad Sci USA. 1991;88:2961–2965. doi: 10.1073/pnas.88.7.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katahira M, Iwasaki Y, Aoki Y, Oiso Y, Saito H. Cytokine regulation of the rat proopiomelanocortin gene expression in AtT-20 cells. Endocrinology. 1998;129:2414–2422. doi: 10.1210/endo.139.5.6005. [DOI] [PubMed] [Google Scholar]
- Lacosta S, Merali Z, Anisman H. Central monoamine activity following acute and repeated systemic interleukin-2 administration. Neuroimmunomodulation. 2000;8:83–90. doi: 10.1159/000026457. [DOI] [PubMed] [Google Scholar]
- Montgomery S, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Lawson DH, Gumnick JF, Penna S, Goodkin R, Greiner K, et al. Paroxetine for the prevention of the depression and neurotoxicity induced by high dose interferon-alpha therapy. N Engl J Med. 2001;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- National Institute for Clinical Excellence . Depression: Management of Depression in Primary and Secondary Care. National Institute for Clinical Excellence: London, England; 2004. [Google Scholar]
- Nicolson SE, Miller AH, Lawson D, Musselman DL. Neuropsychiatric effects of IL-2: mechanisms and treatment implications. Depression: Mind and Body. 2006;2:120–129. [Google Scholar]
- Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to the pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- Perraudin V, Delarue C, Lefebvre H, Contesse V, Kuhn JM, Vaudry H. Vasopressin stimulates cortisol secretion from human adrenocortical tissue through activation of V1 receptors. J Clin Endocrinol Metab. 1993;76:1522–1528. doi: 10.1210/jcem.76.6.7684742. [DOI] [PubMed] [Google Scholar]
- Raab C, Weidmann E, Schmidt A, Bregmann L, Badenhoop K, Usadel KH, et al. The effects of interleukin-2 treatment on endothelin and the activation of the hypothalamic-pituitary-adrenal axis. Clin Endocrinol (Oxf) 1999;50:37–44. doi: 10.1046/j.1365-2265.1999.00592.x. [DOI] [PubMed] [Google Scholar]
- Raber J, Bloom FE. IL-2 induces vasopressin release from the hypothalamus and the amygdala: role of nitric oxide-mediated signaling. J Neurosci. 1994;14:6187–6195. doi: 10.1523/JNEUROSCI.14-10-06187.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Koob GF, Bloom FE. Interleukin-2 (IL-2) induces corticotropin-releasing factor (CRF) release from the amygdala and involves a nitric oxide-mediated signaling: comparison with the hypothalamic response. J Pharmacol Exp Ther. 1995;272:815–824. [PubMed] [Google Scholar]
- Raison CL, Dantzer D, Kelley KW, Lawson MC, Woolwine BJ, Vogt G, et al. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry. 2010;15:393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003a;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. Depression in cancer: new developments regarding diagnosis and treatment. Biol Psychiatry. 2003b;54:283–294. doi: 10.1016/s0006-3223(03)00413-x. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, Lotze MT, Yang JC, Aebersold PM, Linehan WM, Seipp CA, et al. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989;210:474–485. doi: 10.1097/00000658-198910000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraya KA, Balkwill FR. Temporal sequence and cellular origin of interleukin-2 stimulated cytokine gene expression. Br J Cancer. 1993;67:514–521. doi: 10.1038/bjc.1993.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte NS, Malouff JM. Sourcebook of Adult Assessment Strategies. Plenum Press: New York, NY, USA; 1995. [Google Scholar]
- Shao J, Zhang B. Last observation carry-forward and last observation analysis. Stat Med. 2003;22:2429–2441. doi: 10.1002/sim.1519. [DOI] [PubMed] [Google Scholar]
- Sleijfer DT, Janssen RA, Buter J, de Vries EG, Willemse PH, Mulder NH. Phase II study of subcutaneous interleukin-2 in unselected patients with advanced renal cell cancer on an outpatient basis. J Clin Oncol. 1992;10:1119–1123. doi: 10.1200/JCO.1992.10.7.1119. [DOI] [PubMed] [Google Scholar]
- Song P, Lie-Cheng W, Wang GD, Zhou Z, Zhao ZQ. Interleukin-2 regulates membrane potentials and calcium channels via mu opioid receptors in rat dorsal root ganglion neurons. Neuropharmacology. 2002;43:1324–1329. doi: 10.1016/s0028-3908(02)00298-8. [DOI] [PubMed] [Google Scholar]
- Spina MP, Santi G, Poggiato M, Bacchioni G, Sacca G, Cerri A, et al. In vitro effects of IL-2 on human hypophyseal adenoma. Minerva Endocrinol. 1994;19:163–168. [PubMed] [Google Scholar]
- Spinazzé S, Viviani S, Bidoli P, Rovelli F, Palmer P, Franks CR, et al. Effect of prolonged subcutaneous administration of interleukin-2 on the circadian rhythms of cortisol and beta-endorphin in advanced small cell lung cancer patients. Tumori. 1991;77:496–499. doi: 10.1177/030089169107700609. [DOI] [PubMed] [Google Scholar]
- Witzke O, Winterhagen T, Kribben A, Philipp T, Mann K, Reinhardt W. Interleukin-2 given to asymptomatic HIV-infected individuals leads to an exaggerated response of the pituitary gland to the action of CRH. Clin Endocrinol. 2003;59:104–109. doi: 10.1046/j.1365-2265.2003.01803.x. [DOI] [PubMed] [Google Scholar]